Abstract
In this work, we report an environment friendly core-shell material based on Carbon Dot core and Molecularly Imprinted Polymer shell as sensor for highly selective fluorescence detection of ketoprofen. The Carbon Dots (CDs) were prepared by a hydrothermal method and the polymer layer around the CDs core was synthesised by sol-gel polymerisation. The prepared material was characterized by Fluorescence Spectroscopy, FT-IR Spectroscopy and Transmission Electron Spectroscopy (TEM). Fluorescence from the Carbon Dots- Molecularly Imprinted Polymer (CDs-MIP) was found to quench selectively in the presence of ketoprofen and quenching effect was found to be greater than for Non-Imprinted Polymer (CDs-NIP) which indicated the potential of CDs-MIP as a fluorescence sensing material for ketoprofen. The imprinting factor was obtained to be 2.35. Under optimized conditions, a linear response was obtained in the concentration range from 0.039 to 3.9 μM with a detection limit of 0.01 μM. The correlation coefficient was 0.999. The developed sensor was applied to determination of ketoprofen in human serum and urine samples with good recoveries ranging from 96 to 104% indicating successful application of the proposed sensor in biological fluids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Molecular Imprinting Technique (MIT) involves the artificial creation of cavities in polymer matrices which are complementary to the template in their size, shape, and functionality. It is considered as an efficient and versatile tool capable of recognizing a wide variety of molecules, both biological and chemical [1, 2]. The Molecularly Imprinted Polymers (MIPs) are tailor-made materials having specific recognition abilities [3, 4]. Molecularly Imprinted Polymers have gained significance as materials of high sensitivity and affinity, which have huge potential in the field of sensor development [5,6,7]. Traditional MIPs, generally prepared by bulk synthesis suffer from many drawbacks such as non-uniform distribution of binding sites, deep embedded cavities, difficult extraction and poor rebinding etc. which can be largely overcome by use of core-shell MIPs [8, 9]. The special structure of core-shell MIPs results in concentration of recognition sites at or near the surface which results in faster removal and rebinding of the template molecules [10, 11]. Detection approaches using core-shell MIPs result in faster detection using lesser amount of samples. Sol-gel process is one of the efficient methods for core-shell polymerisation which results in formation of rigid inorganic framework around the template molecule. The method has certain advantages such that it is simpler, easier and needs no extreme temperatures for polymerization and it results in the formation of polymers having excellent permeability, good homogeneity and uniform porosity [12, 13]. Sol-gel process combined with surface imprinting technology results in core-shell MIPs with highly desirable characteristics [14]. Compared to traditional acrylic based MIPs, sol-gel silicas are more robust, inert, bio-compatible and exhibit faster diffusion kinetics [13, 15].
Fluorescent carbon dots have aroused much interest in researchers in recent years. The CDs are superior to quantum dots and fluorescent dyes as fluorescence probes owing to their easy and fast synthesis, low cost, high aqueous solubility, chemical inertness, good biocompatibility and low toxicity. As a result, they are being applied in diverse fields such as sensing, biological labelling, bio-imaging, drug delivery, catalysis etc. [16, 17]. During the recent years, a lot of research has been done in the field of synthesis and applications of CDs and their field of applications is fast expanding [18, 19]. Semiconductor quantum dots (SQDs) are generally used as core materials, however CDs offer an alternative approach for the formation of cores but they have been rarely used in core-shell MIPs. Another advantage of CDs is their ease of functionalization. Various groups can be easily grafted onto the surface of CDs which affect their optical and physico-chemical properties [20]. Modification of cores by some functionalizing reagent improves their efficiency towards polymerisation and result in better stability and biocompatibility [21, 22]. Combining the unique fluorescence properties of CDs with specific recognition properties of MIPs can result in highly functional materials which can perform selective recognition of analytes. In such systems, the change of fluorescence signal of CDs in response to specific binding of analytes to MIPs can be utilized for specific sensing of certain analytes. However, the potential of this sensing system has not been much exploited [23].
Ketoprofen is a nonsteroidal anti-inflammatory drug which serves to reduce inflammation and pain in the body and also have antipyretic activity. It is used for treatment of various kinds of inflammatory disorders such as those related to arthritis, vascular headaches and musculoskeletal pain. However, prolonged use and overdose can cause intestinal bleeding, ulceration, heart attack etc. [24, 25]. Due to widespread use of the drug, it is important to have rapid, simple and efficient methods for analysis of the drug. The reported methods for determination of ketoprofen include thin layer chromatography [26], high performance liquid chromatography [27, 28], gas chromatography [29], capillary electrophoresis [30], potentiometric [31], UV-visible spectrophotometry [32], flow injection chemiluminescence [33, 34], terbium sensitised luminescence [24] and quantum dot fluorescence [25]. The fluorescence analysis is simple, cost effective and sensitive technique for analytical method development making it suitable for trace analysis. However, the weakly fluorescent nature of ketoprofen limits its analysis by fluorimetric methods.
In this work, a highly selective surface molecularly imprinted polymer for ketoprofen was synthesized on the surface of silane functionalized CDs by a sol–gel process and a fluorescent sensor was developed for the determination of ketoprofen. In this sensing system, the fluorescence intensity of CDs varies as a result of specific binding of ketoprofen to the imprinted cavities. The variations in fluorescence intensity as a result of concentration of ketoprofen and applicability of the method were also evaluated.
Materials and Methods
Apparatus
The Fluorescence spectra were recorded on a Shimadzu RF-5301PC fluorescence spectrophotometer equipped with a quartz cell. The slit widths of the excitation and emission monochromators were both set at 10 nm. The UV-Vis absorption spectra were obtained by a Shimadzu UV-Vis 1600 spectrophotometer. Fourier transform infrared (FT-IR) measurements were carried out with an FT-IR spectrometer (Bruker Co., Germany). Particle sizes were determined using Hitachi TEM system (Hitachi, Japan).
Reagents and Solutions
The reagents used were of analytical grade and no further purification was done. Triply distilled de-ionized water was used for the preparation of all solutions. N- β-(aminoethyl)-γ-aminopropyltrimethoxysilane (AEAP), tetraethoxysilane (TEOS) and citric acid anhydrous were purchased from Merck. Ketoprofen was obtained from Sigma Aldrich. A stock standard solution of 1.0 × 10−3 mol L−1 ketoprofen was prepared in ethanol and stored at 4 °C. Working solutions were prepared by further dilution of the standard stock solution with water as and when required. For preparing Britton-Robinson buffer, phosphoric acid (0.1 M), acetic acid (0.1 M) and boric acid (0.1 M) were mixed together. The desired pH was obtained by making necessary adjustments with 0.1 M NaOH.
Synthesis of Silane Functionalized Fluorescent CDs
Fluorescent CDs were prepared by a reported method [35] with some modifications. For the synthesis of CDs, 0.5 g citric acid was dissolved in 10 mL of deionized water and the solution was degassed with nitrogen for about 10 min. Then 2.0 mL of AEAP was added and stirring was done for about 10 min. The resultant solution was taken in a 50 mL Teflon lined autoclave and kept in oven at 200 °C for 2 h for hydrothermal treatment. At the end of 2 h, fluorescent solids were obtained from a colorless solution.
Synthesis of MIP and NIP Coated CDs
The imprinted polymer was fabricated on fluorescent CDs via sol-gel polymerisation. For synthesis, 0.03 g of ketoprofen (template) was dissolved in 20 mL ethanol and mixed with 0.05 mL of AEAP. Then 0.5 g of silane functionalized CDs were added and stirred for about 10 min. To the mixture, 0.1 mL of TEOS and 0.1 mL of ammonia solution were added and the solution was stirred for about 24 h in dark. The resultant solution was centrifuged and MIP coated CDs were separated. Washing with ethanol was then carried out till no template could be detected by UV-Vis spectrophotometry. The prepared MIP coated CDs were dispersed in water and stored at 4 °C. The CDs coated with non imprinted polymer were prepared in similar manner without the addition of the template.
General Procedure
To a 4.0-mL standard cuvette, 0.5 mL of stock solution of CDs-MIP, 0.2 mL of Britton Robinson buffer solution (pH = 6, 0.1 M), and appropriate amounts of ketoprofen solution were added and the volume was made up to 3.5 mL with deionized water. After incubation for 5 min, the fluorescence intensity was measured at 450 nm with an excitation wavelength of 360 nm.
Analysis of Real Samples
Human serum samples were collected from a local hospital. Urine samples were collected from healthy persons. A 1.0 mL volume of the urine sample or serum sample was placed in centrifugation tube and spiked by appropriate amounts of ketoprofen standard solution. In order to precipitate the proteins, 1.5 mL of ZnSO4 (0.1 M) solution and 2 mL of Ba(OH)2 (0.1 M) solution were added in the tube and the solution was subjected to centrifugation for 15 min when the proteins coagulated and settled at the bottom. After centrifugation, the clear solution obtained was diluted to 20 mL and analyzed according to the general procedure.
Results and Discussion
Preparation and Characterisation of CDs, CDs-MIP and CDs-NIP
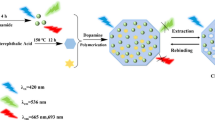
Highly luminescent CDs were prepared by one-step hydrothermal method using AEAP as functionalization reagent as shown in Scheme 1. The preparation of CDs involved pyrolysis and decomposition of anhydrous citric acid. AEAP used during the synthesis of CDs acted as coordinating solvent and also resulted in passivation of the surface of CDs. The surface functionalization of CDs with silane is known to increase their stability and improved their efficiency towards polymerisation [35]. MIP layer around the CDs was prepared by sol–gel polymerisation reaction. AEAP served as functionalization agent as well as functional monomer for the polymerisation reaction. The mixture was further copolymerized with functionalized CDs by the condensation and hydrolysis reaction of TEOS and AEAP in presence of aqueous ammonia as the catalyst as shown in scheme 2. Template molecules were removed from CDs-MIP by washing with ethanol and distilled water and specific recognition cavities of ketoprofen were obtained in the polymer matrix. The complete removal of the template from CDs-MIP was confirmed by absorption spectroscopy through monitoring of the absorption peak at 254 nm. The synthesized CDs, CDs-MIP and CDs-NIP were characterized with FT-IR, fluorescence and TEM measurements. The FT-IR spectra of CDs, CDs-MIP and CDs-NIP are shown in Fig. 1. The acylation reaction between carboxylic groups present on the surface of citric acid derived CDs and the primary amine group of AEAP was confirmed by FT-IR spectroscopy. The spectrum of silane functionalized CDs exhibited peaks at 1587.5 cm−1 and 1655.8 cm−1 corresponding to secondary amide N-H bending and secondary amide C=O stretching vibrations correspondingly. A broad peak around 3275.7 cm−1 corresponding to N-H stretching was also observed. This confirmed the formation of amide group during passivation reaction. The stretching vibration of Si–O-CH (1080 cm−1) and the asymmetric stretch of Si-CH2 (747.9 cm−1) and the bands near 2932.6 cm−1 corresponding to C-H stretching gave evidence of the presence of aminoalkyl group arising from AEAP bonding on the surface of the CDs. The FTIR spectra of both CDs-MIP and CDs-NIP show strong peak at 1078 cm−1 due to Si-O-Si asymmetric stretching and peaks at 755 cm−1 and 417 cm−1 due to Si-O vibrations. The presence of bands at 2922 cm−1 and 2965 cm−1 (aliphatic C-H stretching) and at 3452 cm−1 and 1591 cm−1 (N-H stretching) indicate presence of aminoalkyl groups. All these provide evidence for the formation of polymer layer around CDs core. The similar characteristic peaks in the FT-IR spectra of CDs-MIP and CDs-NIP also suggest complete removal of the template and similar compositions. The transmission electron microscope (TEM) images of the prepared CDs and CDs-MIP were also obtained in order to know about size and distribution of particles as shown in Fig. 2. The TEM image of CDs revealed a size distribution in the range of 7–10 nm. From the TEM image of CDs-MIP it could be verified that the CDs-MIP obtained had a spherical structure with an average diameter of about 60 nm, thus indicating the formation of the material of desired shape and morphology. In order to obtain information on their fluorescence characteristics, the fluorescence spectra of CDs, CDs-MIP and CDs-NIP were also recorded. Silane functionalized CDs were found to exhibited strong fluorescence characteristics and their fluorescence emission was found to be dependent on excitation wavelength [36, 37] as shown in Fig. 3. The maximum fluorescence emission intensity was obtained at 450 nm when excitation was provided at 360 nm. The fluorescence emission spectra of CDs-MIP and CDs-NIP were also recorded to study the effect of MIP layer on the fluorescence properties of CDs. The CDs-MIP and CDs-NIP also exhibited emission maxima at 450 nm when excitation was done at 360 nm similar to silane modified CDs (Fig. 4). This revealed that silica coating does not restrict the fluorescence emission of functionalized CDs as silica is optically transparent material [38].
Method Development for Ketoprofen
The prepared CD-NIPs and CDs-MIP both exhibited a blue line emission wavelength at 450 nm when excitation was provided at 360 nm. However, the intensity of CDs-MIP is sharply quenched in the presence of template ketoprofen while removal of the template by extraction restores the intensity of CDs-MIP to about 90% of the value of CD-NIPs. This quenching of fluorescence of CD-MIPs by ketoprofen was found to be dependent on concentration and based on this an optical method was developed for the determination of ketoprofen. The fluorescence emission intensities of CDs-NIP, CDs-MIP in the presence and in the absence of template are shown Fig. 4. The various factors affecting the method were optimized.
Effect of pH
The effect of pH on the specific recognition of ketoprofen by CDs-MIP was studied in the pH range of 2–10 using Britton-Robinson buffer solutions of different pH values. The highest fluorescence change was observed at pH 6, therefore, this pH was selected for the further studies. The effect of pH on the fluorescence intensity of the system is given in Fig. 5.
Fluorescence Stability of CDs-MIP
The stability of the fluorescence emission of the developed sensor was investigated by measuring variations of fluorescence intensity of CDs-MIP with time. Measurements of the fluorescence intensity of a fixed amount of CDs-MIP were made every 5 min over a total time of 1 h (Fig. 6). The resulting plot indicates the excellent fluorescence stability of CDs-MIP. The relative standard deviation (R.S.D) of 3.91% was obtained by 12 repeated detections after every 5 min up to a total of 1 h in 1.9 μM ketoprofen CDs-MIP solution.
Response Time of the Sensor
The time response of fluorescence quenching of CDs-MIP by ketoprofen was also studied to know the optimum time required for the detection. The fluorescence intensity of CDs-MIP was found to decrease rapidly on the addition of a fixed amount of ketoprofen and stabilized quickly. A constant value was reached within 5 min. Therefore, 5 min was selected as a suitable response time for next experiments.
Ketoprofen Measurements and Calibration Curve
The fluorescence intensity of CDs-MIP was found to markedly quench with the increasing concentrations of ketoprofen (Fig. 7). This is due to specific recognition of ketoprofen by the polymer matrix as a result of imprinted cavities. However, the CDs-NIP did not exhibit much quenching with the addition of ketoprofen. Thus CDs-MIP turned out to be more sensitive for the ketoprofen determination. The relation between fluorescence intensity and the concentration of ketoprofen was found to obey Stern-Volmer equation:
where F0 and F are the fluorescence intensities of the CDs-MIP in the absence and presence of a given concentration of ketoprofen, respectively and KSV is the Stern–Volmer quenching constant. The Ster-Volmer plots are shown in Fig. 8. The CDs-MIP dots exhibited a linear response to ketoprofen in the range of 0.039–3.9 μM with a correlation coefficient (R2) of 0.999. The LOD, limit of detection (S/N = 3) was calculated to be 0.01 μM while LOQ, limit of quantification was calculated to be 0.33 μM.
The imprinting factor (IF) which is used to evaluate the selectivity of the imprinted materials, is given by the equation, IF = KSVMIP/KSVNIP and it could be calculated from the slopes of the linear equations obtained for CDs-MIP and CDs-NIP. Under the optimum conditions, the imprinting factor was calculated to be 2.35, indicating better selectivity of CDs-MIP as compared to CDs-NIP. The comparison of the present method with some other reported methods for ketoprofen is provided in Table 1. From the comparison, it can be concluded that the present method has comparable or better analytical performance than other reported methods.
The process of fluorescence quenching of CDs-MIP due to ketoprofen binding can be understood in light of the following facts. The fluorescence emission in the silane functionalized CDs is believed to mainly arise from radiative recombination of electron-hole pairs trapped on the surface. When there is no template around the CDs-MIP, a blue emission is generated by exciting at 360 nm. After adding the template, the amino groups (–NH2) of APES, in binding sites of CDs-MIP, can interact with the functional groups such as carbonyl in the template molecule to form a complex through hydrogen bonding. So, the quenching of MIP-CDs fluorescence can occur as a result of strong interaction between ketoprofen and MIP-CDs.
Selectivity Studies
To assess the ability of the developed sensor to analyze complex real samples, the effects of some species which could potentially interfere with the method, on the response of the CDs-MIP were investigated. For this, excess amounts of interfering substances were added to the sensing system and the resulting solutions were analyzed. The tolerance limit was taken as the concentration of the added species causing a variation of ±5% in the fluorescence signal. The obtained results are presented in Table 2. As can be seen from the Table, most of the potentially interfering substances do not interfere with determination of ketoprofen even when present at 100 folds higher concentration. Thus no interferences from these species are expected in determination of ketoprofen in serum and urine samples. The selectivity studies of the CDs-MIP were investigated with structurally related compounds including naproxen, ibuprofen and fenoprofen. Being similar in structure, these drugs do show some affinity for the molecularly imprinted polymer, resulting in some fluorescence quenching, however the fluorescence quenching of CDs-MIP was found to be strongest by ketoprofen as compared to other related drugs as shown in Fig. 9. This indicates the specific binding ability of the prepared CDs-MIP for ketoprofen because of imprinted sites specific for ketoprofen.
Applications
Low concentration and complexity of the real matrix make the determination of ketoprofen difficult. The proposed method can selectively determine ketoprofen in complex samples without interference. To validate the applicability of the sensor, the proposed method was employed for analysis of ketoprofen in real human urine samples. Recovery studies were carried out by spiking the samples with ketoprofen in the concentration range of 0.4 to 2 μM. A summary of the calculated mean ketoprofen concentration for each sample is shown in Table 3. The concentrations of ketoprofen in the spiked samples by the proposed method were in good agreement with those of ketoprofen added with recoveries ranging between 96.0% and 104%. The results indicate that the proposed method has a good accuracy. Therefore the proposed fluorescent sensor CDs-MIP can be successfully applied to the determination of ketoprofen in complex samples.
Conclusions
The present work involves development of a low cost, eco-friendly core shell material as fluorescent sensor for specific determination of ketoprofen. The fluorescent CDs were used as core and MIP shell was obtained from silica precursors by sol-gel process with surface imprinting technique resulting in robust, inert and non-toxic MIP material with high specificity and faster binding kinetics. The high sensitivity, selectivity and good linear range of the developed method enabled successful application of the developed method for the determination of ketoprofen in human serum and urine samples. The developed sensing system combines high selectivity of MIPs with unique fluorescence properties of CDs having many potential applications in analytical field.
References
Longo L, Vasapollo G (2008) Mini-Rev. Org Chem 5:163–170
Pichon V, Chapuis-Hugon F (2008) Role of molecularly imprinted polymers for selective determination of environmental pollutants—A review. Anal Chim Acta 622:48–61
Vasapollo G, Sole RD, Mergola L, Lazzoi MR, Scardino A, Scorrano S, Mele G (2011) Molecularly Imprinted Polymers: Present and Future Prospective. Int J Mol Sci 12:5908–5945
Morelli I, Chiono V, Vozzi G, Ciardelli G, Silvestri D, Giusti P (2010) Molecularly imprinted submicronspheres for applications in a novel model biosensor-film. Sens Actuators B Chem 150:394–401
Uzun L, Turner APF (2016) Molecularly-imprinted polymer sensors: realising their potential. Biosens Bioelectron 76:131–144
Shimizu KD, Stephenson CJ (2010) Molecularly imprinted polymer sensor arrays. Curr Opin Chem Biol 14:743–750
Erturk G, Mattiasson B (2017) Molecular Imprinting Techniques Used for the Preparation of Biosensors. Sensors (Basel) 17:288
W. Luo, L.H. Zhu, C. Yu, H.Q. Tang, H.X. Yu, X. Li, X. Zhang, Anal Chim Acta 2008 (618) 147–156
Liu JM, Wei SY, Liu HL, Fang GZ, Wang S (2017) Preparation and Evaluation of Core–Shell Magnetic Molecularly Imprinted Polymers for Solid-Phase Extraction and Determination of Sterigmatocystin in Food. Polymers 9:546–559
Zhang Z, Chen L, Fangfang Y, Li J (2014) Uniform core–shell molecularly imprinted polymers: a correlation study between shell thickness and binding capacity. RSC Adv 4:31507–31514
Tokonami S, Shiigi H, Nagaoka T (2009) Review: Micro- and nanosized molecularly imprinted polymers for high-throughput analytical applications. Anal Chim Acta 641:7–13
Yang Y, Fang G, Liu G, Pan M, Wang X, Kong L, He X, Wang S (2013) Electrochemical sensor based on molecularly imprinted polymer film via sol–gel technology and multi-walled carbon nanotubes-chitosan functional layer for sensitive determination of quinoxaline-2-carboxylic acid. Biosens Bioelectron 47:475–481
Zhu XB, Cui YM, Chang XJ, Zou XJ, Li ZH (2009) Selective solid-phase extraction of lead(II) from biological and natural water samples using surface-grafted lead(II)-imprinted polymers. Microchim Acta 164:125–132
Zahedi P, Ziaee M, Abdouss M, Farazin A, Mizaikoff B (2016) Biomacromolecule template-based molecularly imprinted polymers with an emphasis on their synthesis strategies: a review. Polym Adv Technol 27:1124–1142
Cummins W, Duggan P, McLoughlin P (2005) A comparative study of the potential of acrylic and sol–gel polymers for molecular imprinting. Anal Chim Acta 542:52–60
Baker SN, Baker GA (2010) Luminescent Carbon Nanodots: Emergent Nanolights. Angew Chem Int Ed Engl 49:6726–6744
Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T (2008) Quantum dots versus organic dyes as fluorescent labels. Nat Methods 5:763–775
Tuerhong M, Yang XU (2017) YIN Xue-Bo. Chin J Anal Chem 45:139–150
Lim SY, Shen W, Gao Z (2015) Carbon quantum dots and their applications. Chem Soc Rev 44:362–381
Dimos K (2016) Carbon Quantum Dots: Surface Passivation and Functionalization. Curr Org Chem 20:682–695
Wackerlig J, Lieberzeit PA (2015) Molecularly imprinted polymer nanoparticles in chemical sensing – Synthesis, characterisation and application. Sens. Actuators B Chem. 207:144–157
Moczko E, Guerreiro A, Piletska E, Piletsky S (2013) PEG-Stabilized Core–Shell Surface-Imprinted Nanoparticles. Langmuir 29:9891–9896
Mao Y, Bao Y, Han D, Li F, Niu L (2012) Efficient one-pot synthesis of molecularly imprinted silica nanospheres embedded carbon dots for fluorescent dopamine optosensing. Biosens Bioelectron 38:55–60
Al Kindy SMZ, Al Harasi Z, Suliman FEO, Al Hamadi A, Pillay A (2009) Terbium Sensitized Luminescence for the Determination of Ketoprofen in Pharmaceutical Formulations. J Fluoresc 19:249–255
Garcıa LM, Santos JLM, Medina AR, Martınez EJL (2013) Determination of ketoprofen based on its quenching effect in the fluorescence of quantum dots. J Food Drug Anal 21:426–431
Wankhede SB, Chitlange SS, Bhole RP, Zambare SS (2012) Anal. Chem Lett 20:301–308
Aguilar-Carrasco JC, Rodrıguez-Silverio J, Carrasco Portugal MC, Flores-Murrieta FJ (2011) Rapid and sensitive determination of ketoprofen in micro-whole blood samples by high-performance liquid chromatography and its application in a pharmacokinetic study in rats. J Liq Chrom Relat Technol 34:388–395
Garcia MTJ, Marchetti JM, Bentley MVLB (2001) Determination by hplc of ketoprofen in aqueous medium used forin vitroskin permeation studies. Anal Lett 34:1865–1874
Dowling G, Gallo P, Fabbrocino S, Serpe L, Regan L (2008) Determination of ibuprofen, ketoprofen, diclofenac and phenylbutazone in bovine milk by gas chromatography-tandem mass spectrometry. Food Add Contamin A 25:1497–1508
Glowka FK, Karazniewicz-Lada M (2008) CE Determination of Ketoprofen Enantiomers in Clinical Samples of Plasma, Synovial Fluid and Urine. Chromatographia 67:97–105
Kormosh Z, Hunka I, Bazel Y, Matviychuk O (2010) Potentiometric determination of ketoprofen and piroxicam at a new PVC electrode based on ion associates of Rhodamine 6G. Mater Sci Eng C 30:997–1002
C. Ozlu, H. Basan, E. Satana E, N. Ertas, N.G. Goger, J Pharm Biomed Anal 39 (2005) 606–611, Quantitative determination of ketoprofen in gels and ampules by using flow-injection UV spectrophotometry and HPLC
Zhuang Y, Song H (2007) Sensitive determination of ketoprofen using flow injection with chemiluminescence detection. J Pharm Biomed Anal 44:824–828
Kaczmarek M, Lis S (2012) Chemiluminescence determination of ibuprofen and ketoprofen using the Fenton system in the presence of europium(iii) ions. Anal Meth 4:1964–1967
Wang F, Xie Z, Zhang H, Liu CY, Zhang YG (2011) Highly Luminescent Organosilane-Functionalized Carbon Dots. Adv Funct Mater 21:1027–1031
Liu G, Chen Z, Jiang X, Feng DQ, Zhao J, Fan D, Wang W (2016) In-situ hydrothermal synthesis of molecularly imprinted polymers coated carbon dots for fluorescent detection of bisphenol A. Sens. Actuators B Chem. 228:302–307
Xu L, Fang G, Pan M, Wang X, Wang S (2016) One-pot synthesis of carbon dots-embedded molecularly imprinted polymer for specific recognition of sterigmatocystin in grains. Biosens Bioelectron 77:950–956
Sun J, Zhuang J, Guan S, Yang W (2008) Synthesis of robust water-soluble ZnS:Mn/SiO2 core/shell nanoparticles. J Nanopart Res 10:653–658
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhogal, S., Kaur, K., Maheshwari, S. et al. Surface Molecularly Imprinted Carbon Dots Based Core-Shell Material for Selective Fluorescence Sensing of Ketoprofen. J Fluoresc 29, 145–154 (2019). https://doi.org/10.1007/s10895-018-2322-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-018-2322-4