Abstract
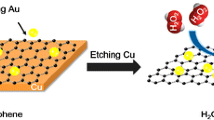
A nanocomposite consisting of silver nanoparticles (AgNPs), AlOOH and reduced graphene oxide (rGO) was prepared where γ-AlOOH was employed to modify rGO to obtain a platform for the growth of dispersed AgNPs. A glassy carbon electrode was modified with the nanocomposite in a chitosan matrix to obtain a nonenzymatic sensor for H2O2 with a working voltage of typically -0.3 V (vs. Ag/AgCl). The morphology and composition of the nanocomposites were characterized by transmission electron microscopy, Raman spectra, X-ray spectrometer and X-ray diffraction. Cyclic voltammetry revealed a wide linear range (5.0 μM to 4.2 mM), a sensitivity of 115.4 μA · mM−1 · cm−2 and a low detection limit (1.8 μM).

A nanocomposite containing silver nanoparticles, AlOOH and reduced graphene oxide was synthesized by employing AlOOH-reduced graphene oxide as support for silver nanoparticles. It was deposited on a glassy carbon electrode to give a nonenzymatic H2O2 sensor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous electrochemical [1] and optical [2] methods have been developed for the sensitive and selective detection of H2O2. The electrochemical method was given special attention owing to low cost, simplicity, and efficiency. Many papers have studied the electrochemical behavior of silver nanoparticles (AgNPs) towards H2O2 and these non-enzymatic H2O2 sensors based on AgNPs possess an excellent performance toward H2O2.[3–6]. However, it was found that aggregation of AgNPs prohibits extensive applications, thus the matrix for the preparation of highly dispersed AgNPs is very important. Reduced graphene oxide (rGO) is a kind of widely investigated nanomaterial, which was usually prepared by reducing graphene oxide (GO) through chemical [7], electrochemical [8] or hydrothermal methods [9]. RGO has been widely employed as the substrate material for the growth of metal nanoparticles due to its large specific surface area, excellent electrical and thermal properties [10]. However, it is well known that rGO tends to aggregate and there is less functional group on rGO, which make it very difficult to decorate nanoparticles on rGO sheets, therefore limiting the application of rGO to some extent [11]. Thus, it is still necessary to carry out some studies to develop the facile approach to the production of soluble rGO sheets and metal-graphene nanocomposites.
The modification of rGO with polymers obviously enhances the dispersibility of rGO and increases the number of functional group, making it easy to decorate nanoparticles on rGO sheets. For example, Liu [12] has reported that the stable aqueous dispersion of rGO and AgNPs/rGO can be obtained with the use of benzylamine as reducing and stabilizing agent. Because benzylamine contains a benzene ring and a polar -NH2 group. Therefore, rGO can bind strongly to benzylamine by π-interaction and -NH2 group can interact well with the polar solvent water. In addition, Sun [13] has reported that the noncovalent functionalization of rGO by aniline can make the dispersion of rGO very stable and it also makes the decoration of rGO with AgNPs very easy. Moreover, Guo [14] developed a facile approach to the synthesis of stable rGO and AgNP-rGO hybrids aqueous dispersions using chitosan as both the reducing and stabilizing agent. Although many water soluble polymers have been employed to modify rGO it is still necessary to study other materials to modify rGO sheets due to the limited stability of polymers at elevated temperature.
Aluminum oxyhydroxide (γ-AlOOH) was found to be a good adsorbent material due to that γ-AlOOH contains large number of hydroxyl groups (OH groups) for the adsorption of metal ions [15]. In addition, γ-AlOOH also has the advantages of good thermal stability, low cost and controllable synthesis process [16, 17]. From the above mentioned points, it was desirable to employ γ-AlOOH to modify rGO [18]. The modification of rGO with γ-AlOOH can prohibit rGO from aggregating and increase the active sites, making AlOOH-rGO an advanced support material for metal nanoparticles. However, few studies have managed to support metal nanoparticles on AlOOH-rGO. In addition, to the best of our knowledge, no attention has been paid to support AgNPs on AlOOH-rGO for H2O2 detection.
In this paper, Ag-AlOOH-rGO nanocomposites were synthesized through a facile approach. Then the nanocomposites were employed to fabricate a novel nonenzymatic sensor of H2O2 and further the electrochemical performance of the sensor toward H2O2 was investigated.
Experimental
Reagents and materials
Graphite was purchased from Qingdao Tianhe Graphite Co., Ltd (Qingdao, China, http://www.thsm.com.cn/en/p06.html). Silver nitrate (AgNO3) and Urea (CO(NH2)2) was obtained from Shanghai Tangfang Biotechnology Co., Ltd (Shanghai, China, http://shtangfang.company.weiku.com/). 0.1 M phosphate buffered saline (PBS, pH = 7.0) was used as the supporting electrolyte. Doubly distilled water was used in experiments.
Apparatus
Transmission electron microscopic (TEM) images were carried out by Tecnai G2 F20 S-TWIN (FEI, USA). X ray diffraction (XRD) patterns of the samples were taken by D/MAX 3C (Rigaku, Japan). Surface elemental compositions of the synthesized samples were characterized by an energy-dispersive X-ray spectrometer (EDX). Raman spectra were recorded at ambient temperature on a Renishaw Raman system model 1000 spectrometer with a 200 mW argon-ion laser at an excitation wavelength of 514.5 nm. Electrochemical measurements were carried out in a conventional three-electrode electroanalysis system controlled by EC 550 electrochemical workstation (Gaoss Union Technology Co., Ltd., Wuhan, China) and CHI 660 electrochemical workstation (Shanghai CH Instrument Co. Ltd., China). A conventional three-electrode cell was used, including a glassy carbon electrode (GCE, geometric area = 0.07 cm2) as the working electrode, an Ag/AgCl (3 M KCl) electrode as the reference electrode and platinum foil as the counter electrode. All potentials given in this work were referred to the Ag/AgCl electrode.
Synthesis of silver nanoparticles-AlOOH-reduced graphene oxide nanocomposites
Preparation of AlOOH-reduced graphene oxide nanocomposites
GO was prepared using the modified Hummers [19]. Then, 1.24 g Al(NO3)3 · 9H2O and 0.59 g CO(NH2)2 were dissolved in 39 mL of 0.5 mg · mL−1 GO aqueous solution under stirring. Moreover, the mixture was transferred into a 50 mL Teflon-lined autoclave and heated at 180 °C for a period of 10 h. rGO was prepared under the same condition except the addition of Al(NO3)3 · 9H2O and CO(NH2)2. After which, the nanocomposites were obtained from the solution by centrifugation, washed for three times by doubly distilled water and dried in a vacuum oven at 45 °C for 12 h.
Preparation of silver nanoparticles-AlOOH-reduced graphene oxide nanocomposites
0.01 g AlOOH/rGO was dissolved in 5 mL of distilled water. 0.6 mL of 35 mM freshly prepared [Ag(NH3)2]+ solution was added to this solution and stirred for 1 h. After which, the dispersion above was added into 20 mL of ethanol containing Polyvinylpyrrolidone (PVP) (0.004 g) in a 250-mL three-neck flask equipped with magnetic stirrer at 70 °C for around 7 h. Then, the nanocomposites were obtained from the solution by centrifugation, washed for three times by doubly distilled water and dried in a vacuum oven at 45 °C for 12 h.
Electrode modification
The glassy carbon electrode (GCE) was prepared by a simple casting method. Prior to use, GCE was polished with 1.0 and 0.3 μm alumina powder to obtain mirror like surface, respectively, and rinsed with doubly distilled water, followed by sonication in ethanol solution and doubly distilled water successively. Then, the GCE was allowed to dry in a stream of nitrogen. 1.0 mg of Ag-AlOOH-rGO were dispersed into chitosan (1 mL, 0.5 %) and sonicated for 5 minutes. The suspension (6 μL) was dropped onto the pre-polished mirror like surface of GCE and then dried in air at room temperature.
Results and discussion
Characterizations of silver nanoparticles-AlOOH-reduced graphene oxide nanocomposites
rGO possessed specific high surface area, exceptional electrical, mechanical, and thermal properties. AlOOH have large number of OH groups. Therefore, it is desired to employ AlOOH to modify rGO and the AlOOH-rGO nanocomposites can be used as an advanced support material for metal nanoparticles. AgNPs possessed the excellent catalytic activity to H2O2 reduction in PBS solution and AgNPs also exhibit the highest electrical and thermal conductivity among metals. Therefore, it is reasonable to expect that Ag-AlOOH-rGO nanocomposites would possess a good electrochemical activity toward the electrochemical sensing of H2O2.
AlOOH-rGO was synthesized by a green and facile hydrothermal method, in which AlOOH nanoplates were grown on the surface of graphene nanosheets along with the reduction of GO, therefore prohibiting rGO from aggregating. Then, AgNPs were decorated on AlOOH-rGO with the use of PVP as reducing agent. The morphologies and structures of GO and Ag-AlOOH-rGO nanocomposites were characterized by TEM. As shown in Fig. 1a and b, the TEM images of GO revealed a typical monolayer nanosheet structure with some wrinkles and folds. Figure 1c showed the TEM image of Ag-AlOOH-rGO and it can be seen that many dark spots decorated on the surface of AlOOH-rGO, in which AlOOH nanoplates were intercalated into the nanosheets of rGO. In order to further study the structures of the dark spots, a typical HRTEM was shown in Fig. 1d, which revealed clear lattice fringes with an interplanar distance of 0.23 nm and can be assigned to the (111) lattice space of AgNPs [20], therefore indicating that the dark spots were AgNPs.
Figure S1A in the supporting information showed the raman spectroscopy of (a) GO and (b) rGO. It can be seen that both GO and rGO show the G band around 1600 cm−1 and the D band around 1350 cm−1. However, rGO showed a relatively higher intensity of D to G bands (1.01) than that of GO (0.82), which can indicate a decrease in the size of the in-plane sp2 domains, the removal of the oxygen functional groups in the graphene oxide nanosheets, and the reestablishment of the conjugated graphene network. In addition, the chemical composition of the material was further determined by EDX. As shown in Figure S1B and C in the supporting information, the peak intensity of oxygen element decreased after hydrothermal reduction, indicating the removal of the oxygen functional groups in the graphene oxide nanosheets.
Further structural characterizations of AlOOH-rGO and Ag-AlOOH-rGO nanocomposites were carried out by XRD. The peaks at 14°, 27°, 38° and 49° in Fig. 2 (curve a) are assigned to orthorhombic γ-AlOOH (JCPDS no. 01-074-1895) [18]. In addition, the peaks (curve b) at 38°, 44° and 64° can be well indexed to (111), (200) and (220) planes of the face centered cubic phase of Ag [21], respectively. On the basis of results of TEM, Raman spectra, EDX and XRD characterizations, it can indicate that GO and Ag-AlOOH-rGO were successfully prepared.
Electrochemical properties of silver nanoparticles-AlOOH-reduced graphene oxide nanocomposites
Cyclic voltammograms (CVs) were recorded to investigate the electrocatalytic behavior of modified electrodes toward H2O2 and the results were shown in Fig. 3. As shown in Fig. 3a, the bare GCE (a), AlOOH-rGO/GCE (b) and Ag-AlOOH-rGO/GCE (c) exhibited almost no electrochemical response in the absence of H2O2. After adding 0.5 mM H2O2 into N2-saturated 0.1 M PBS (pH 7.0), it can be seen from Fig. 3b that the bare GCE (d) and AlOOH-rGO/GCE (e) still showed no significant current response, indicating that AlOOH-rGO possessed almost no catalytic performance for H2O2 reduction. However, Ag-AlOOH-rGO/GCE (f) displayed a remarkable catalytic current peak, which indicated that AgNPs contained in the nanocomposites exhibited a notable catalytic performance for H2O2 reduction [22].
Figure 4a showed CVs of Ag-AlOOH-rGO/GCE with different H2O2 concentrations in N2-saturated 0.1 M PBS (pH 7.0) at 100 mV/s and it can be seen that the peak current increased with the increase of H2O2 concentration. The CVs of Ag-AlOOH-rGO/GCE at different scan rates in N2-saturated 0.1 M PBS (pH 7.0) were shown in Fig. 4b. As can be seen, the cathodic peak current linearly increased with the square root of scan rates in the range of 20–200 mV/s, indicating that the catalytic process is diffusion-controlled [23].
(a) Cyclic voltammograms of Ag-AlOOH-rGO/GCE in N2-saturated 0.1 M PBS (pH 7.0) in the presence of H2O2 with different concentrations (from a to h: 0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5 mM) at a scan rate of 100 mV/s. (b) CVs of Ag-AlOOH-rGO/GCE in N2-saturated 0.1 M PBS (pH 7.0) containing 0.5 mM H2O2 at different scan rates (from a to j: 20, 40, 60, 80, 100, 120, 140, 160, 180 and 200 mV/s)
In order to investigate the sensing property of the electrochemical sensor, the amperometric current-time curve of Ag-AlOOH-rGO/GCE with successive addition of varying H2O2 concentrations in N2-saturated 0.1 M PBS (pH 7.0) were shown in Fig. 5. High operating potential can make the background current very high and also lead to more interference of other electroactive species in the solution. However, the low operating potential lead to very low response current. Therefore, to make compromises between high response current, low background current noise and less interference of other electroactive species [24], -0.3 V was chosen as the operating potential. As can be seen that the current increased with the increase of H2O2 concentration. Figure S2 in the supporting information displayed the linear relationship between the catalytic current and H2O2 concentration in the range of 5.0 μM to 4.2 mM. The linear regression equation for the H2O2 reduction was expressed as Ip (μA) = -0.203 + 8.084 · C (mM) with a correlation coefficient of 0.9935, a sensitivity of 115.4 μA · mM−1 · cm−2 and a detection limit of 1.8 μM at a signal-to-noise ratio of 3.
Table 1 was the comparison of Ag-AlOOH-rGO/GCE with several typical H2O2 sensors reported previously. The modified GCE displays high sensitivity, a wide linear range and a low detection limit. This is ascribed to the modification of rGO with γ-AlOOH which increases the number of active sites and prevents rGO from aggregating. This makes AlOOH-rGO a good platform for the growth of dispersed AgNPs while preserving their high catalytic performance. The large specific surface area can provide more electroative sites for H2O2 molecules to adsorb and react. Comparing with Cu nanoparticles and MnO2, Ag-AlOOH-rGO nanocomposites can exhibit an excellent performance to H2O2 in PBS solution (pH 7.0), not in the strong acid or alkali solution. This is benefit to its application in real life. In addition, the preparation of Ag-AlOOH-rGO nanocomposites were much cheaper and easier as compared with that of bio-based materials.
Interference study
Figure 6 showed the selectivity of Ag-AlOOH-rGO/GCE toward H2O2. It can be seen that 0.05 mM glucose (Glu), ethanol, dopamine (DA), uric acid (UA), acetaminophen (AP) and ascorbic acid (AA) have little interference for 0.005 mM H2O2, indicating the good selectivity. In addition, the relative standard deviation (RSD) for the cathodic peak current was less than 5.6 % for five modified GCE and Ag-AlOOH-rGO/GCE remained 83 % of its initial current response after three weeks, indicating the satisfactory reproducibility and stability. However, the nonenzymatic sensors modified with metal nanoparticles has the weak mechanical stability and durability of performance after a moth. These can be ascribed to the agglomeration, deformation, and collapse of metal nanoparticles for extended periods of time. Therefore, we will carry out some studies to improve the mechanical stability and durability of performance.
Conclusion
Ag-AlOOH-rGO nanocomposites have been successfully synthesized and the nonenzymatic H2O2 sensor based on the nanocomposites was fabricated. The sensor exhibited a wide linear range with a low detection limit and a high sensitivity. Considering the facile preparation route and excellent experimental results, the study demonstrated that Ag-AlOOH-rGO nanocomposite was a promising electrocatalytic material for constructing sensors. Furthermore, the studies to improve the weak mechanical stability and durability of performance are on our schedule.
References
Chen XM, Wu GH, Cai ZX (2014) Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim Acta 181:689–705
Michael S, Stephan S (2011) Luminescent probes for detection and imaging of hydrogen peroxide. Microchim Acta 174:1–18
Liu Y, Sun GZ, Jiang CB (2014) Highly sensitive detection of hydrogen peroxide at a carbon nanotube fiber microelectrode coated with palladium nanoparticles. Microchim Acta 181:63–70
Jiang BB, Wei XW, Wu FH (2014) A non-enzymatic hydrogen peroxide sensor based on a glassy carbon electrode modified with cuprous oxide and nitrogen-doped graphene in a nafion matrix. Microchim Acta 181:1463–1470
Cui X, Wu SN, Li YX (2015) Sensing hydrogen peroxide using a glassy carbon electrode modified with in-situ electrodeposited platinum-gold bimetallic nanoclusters on a graphene surface. Microchim Acta 182:265–272
Zöpfl A, Sisakthi M, Eroms J et al. (2015) Signal enhancement in amperometric peroxide detection by using graphene materials with low number of defects. Microchimica Acta 1-8
Sun ST, Wu PY (2011) Competitive surface-enhanced Raman scattering effects in noble metal nanoparticle-decorated graphene sheets. Phys Chem Chem Phys 13:21116–21120
Yang J, Deng SY, Lei JP, Ju HX (2011) Electrochemical synthesis of reduced graphene sheet–AuPd alloy nanoparticle composites for enzymatic biosensing. Biosens Bioelectron 29:159–166
Xu YX, Sheng KX, Li C, Shi GQ (2010) Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 4:4324–4330
Shen JF, Yan B, Shi M, Ma HW, Li N, Ye MX (2011) One step hydrothermal synthesis of TiO2-reduced graphene oxide sheets. J Mater Chem 21:3415–3421
Xu SJ, Yong L, Wu PY (2013) One-pot, green, rapid synthesis of flowerlike gold nanoparticles/reduced graphene oxide composite with regenerated silk fibroin as efficient oxygen reduction electrocatalysts. ACS Appl Mater Interfaces 5:654–662
Liu S, Tian JQ, Wang L, Sun XP (2011) A method for the production of reduced graphene oxide using benzylamine as a reducing and stabilizing agent and its subsequent decoration with Ag nanoparticles for enzymeless hydrogen peroxide detection. Carbon 49:3158–3164
Liu S, Wang L, Tian JQ, Luo YL, Zhang XX, Sun XP (2011) Aniline as a dispersing and stabilizing agent for reduced graphene oxide and its subsequent decoration with Ag nanoparticles for enzymeless hydrogen peroxide detection. J Colloid Interface Sci 363:615–619
Guo YQ, Sun XY, Liu Y, Wang W, Qiu HX, Guo JP (2012) One pot preparation of reduced graphene oxide (RGO) or Au (Ag) nanoparticle-RGO hybrids using chitosan as a reducing and stabilizing agent and their use in methanol electrooxidation. Carbon 50:2513–2523
Zhang YX, Yu XY, Jin Z, Jia Y, Xu WH, Luo T, Huang XJ (2011) Ultra high adsorption capacity of fried egg jellyfish-like γ-AlOOH (Boehmite) @ SiO2/Fe3O4 porous magnetic microspheres for aqueous Pb (II) removal. J Mater Chem 21:16550–16557
Shi T, Guo XZ, Yang H (2008) Preparation and characterization of transparent boehmite (γ-AlOOH) sol. Rare Metal Mat 37:73–75
Yang H, Ji S, Liu X, Zhang D, Shi D (2014) Magnetically recyclable Pd/γ-AlOOH@Fe3O4 catalysts and their catalytic performance for the Heck coupling reaction. Sci Chin Chem 57:866–872
Gao C, Yu XY, Xu RX (2012) AlOOH-reduced graphene oxide nanocomposites: one-pot hydrothermal synthesis and their enhanced electrochemical activity for heavy metal ions. ACS Appl Mater Interfaces 4:4672–4682
Luo GQ, Jiang XJ, Li M (2013) Facile fabrication and enhanced photocatalytic performance of Ag/AgCl/rGO heterostructure photocatalyst. ACS Appl Mater Interfaces 5:2161–2168
Qin X, Lu W, Luo Y (2012) Green photocatalytic synthesis of Ag nanoparticle-decorated TiO2 nanowires for nonenzymatic amperometric H2O2 detection. Electrochim Acta 74:275–279
Tian Y, Wang F, Liu Y, Pang F, Zhang X (2014) Green synthesis of silver nanoparticles on nitrogen-doped graphene for hydrogen peroxide detection. Electrochim Acta 146:646–653
Li X, Liu Y, Zheng L (2013) A novel nonenzymatic hydrogen peroxide sensor based on silver nanoparticles and ionic liquid functionalized multiwalled carbon nanotube composite modified electrode. Electrochim Acta 113:170–175
Bai WS, Nie F, Zheng JB, Sheng QL (2014) Novel silver nanoparticle–manganese oxyhydroxide–graphene oxide nanocomposite prepared by modified silver mirror reaction and its application for electrochemical sensing. ACS Appl Mater Interfaces 6:5439–5449
Han Y, Zheng JB, Dong SY (2013) A novel nonenzymatic hydrogen peroxide sensor based on Ag–MnO2–MWCNTs nanocomposites. Electrochim Acta 90:35–43
Zhao HY, Zheng W, Meng ZX (2009) Bioelectrochemistry of hemoglobin immobilized on a sodium alginate-multiwall carbon nanotubes composite film. Biosens Bioelectron 24:2352–2357
Li LM, Du ZF, Liu S (2010) A novel nonenzymatic hydrogen peroxide sensor based on MnO2/graphene oxide nanocomposite. Talanta 82:1637–1641
Gao FX, Yuan R, Chai YQ (2007) Amperometric hydrogen peroxide biosensor based on the immobilization of HRP on nano-Au/Thi/poly (p-aminobenzene sulfonic acid)-modified glassy carbon electrode. J Biochem Biophys Methods 70:407–413
Babu KJ, Zahoor A, Nahm KS (2014) The influences of shape and structure of MnO2 nanomaterials over the non-enzymatic sensing ability of hydrogen peroxide. J Nanoparticle Res 16:1–10
Meng FH, Yan XL, Liu JG (2011) Nanoporous gold as non-enzymatic sensor for hydrogen peroxide. Electrochim Acta 56:4657–4662
Zhang MY, Sheng QL, Nie F (2014) Synthesis of Cu nanoparticles-loaded Fe3O4@carbon core–shell nanocomposite and its application for electrochemical sensing of hydrogen peroxide. J Electroanal Chem 730:10–15
Acknowledgments
The authors gratefully acknowledge the financial support of this project by the National Science Foundation of China (21575113, 21275116), Specialized Research Fund for the Doctoral Program of Higher Education (No. 20126101120023), the Natural Science Foundation of Shaanxi Province in China (2013KJXX-25 and 2012JM2013), the Scientific Research Foundation of Shaanxi Provincial Key Laboratory (2010JS088, 11JS080, 12JS087, 13JS097, 14JS094) and the Fund of Shaanxi Province Educational Committee of China (12JK0576).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1011 kb)
Rights and permissions
About this article
Cite this article
Yang, Z., Qi, C., Zheng, X. et al. Sensing hydrogen peroxide with a glassy carbon electrode modified with silver nanoparticles, AlOOH and reduced graphene oxide. Microchim Acta 183, 1131–1136 (2016). https://doi.org/10.1007/s00604-016-1743-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1743-5