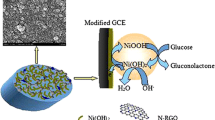
Abstract
We have developed a stable and sensitive nonenzymatic glucose sensor by modifying a glassy carbon electrode (GCE) with a composite incorporating nickel(II) oxides and reduced graphene. The oxides were generated by directly electrodepositing nickel on the GCE with a graphene modifier using a multi-potential pulse process, and then oxidizing nickel to nickel(II) oxides by potential cycling. In comparison to the conventional nickel(II) oxides-modified GCE, this new nickel(II) oxides-graphene modified GCE (NiO-GR/GCE) has an about 1.5 times larger current response toward the nonenzymatic oxidation of glucose in alkaline media. The response to glucose is linear in the 20 μM to 4.5 mM concentration range. The limit of detection is 5 μM (at a S/N of 3), and the response time is very short (<3 s). Other beneficial features include selectivity, reproducibility and stability. A comparison was performed on the determination of glucose in commercial red wines by high-performance liquid chromatography (HPLC) and revealed the promising aspects of this sensor with respect to the determination of glucose in real samples.

A stable and sensitive nonenzymatic glucose sensor is developed by preparing the nickel(II) oxides-reduced graphene nanocomposite modified glassy carbon electrode (NiO-GR/GCE), and then used to detect the glucose contents in the commercial red wines. This NiO-GR/GCE also has a high selectivity
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The qualitative and quantitative distribution of glucose in drinks and foodstuffs is closely related to their origin, ripeness degree and storing way, and determines their characteristic tastes [1]. The development of rapid, simple and reliable monitoring method for glucose is important in food control [2, 3]. By now, the electrochemical detection of glucose is usually based on the enzyme-modified electrodes or the enzyme-free sensors. Thereinto, the enzyme-modified electrodes are prepared by using glucose oxidase [4] or pyranose oxidase [5]. However, there are still several disadvantages including poor stability, high cost of enzymes, critical operational conditions and complicated immobilization procedure for the enzyme-modified electrodes. Moreover, the catalytic activity of enzyme is easily affected by environmental conditions such as temperature, pH value, humidity, ionic detergents and toxic chemicals [6]. Therefore, the nonenzymatic glucose sensor seems to be an attractively alternative technique free from the above-mentioned drawbacks [7].
Various nanomaterials have been investigated as the electrocatalysts to determine glucose without the use of enzymes. The previous studies have mainly focused on the use of noble metal such as Pt, Au, Pd, Ag and their metal alloy electrodes for developing nonenzymatic glucose sensors [8–12]. In practice, the high cost of the electrode using valuable metal materials may limit their commercial applications [13]. Thus, some researchers have concentrated on the use of low-cost metal oxide materials such as nickel oxides [14], copper oxide, and cobalt oxide for the preparation of nonenzymatic glucose sensors [15, 16]. Among these metal oxides, nickel oxides are of particular interest in their virtue of good electrochemical stability and electrocatalytic activity. Recently, Shamsipur et al. [17] reported the application of a nickel(II) oxide/multi-walled carbon nanotube modified glassy carbon electrode to detect glucose with high sensitivity and stability. Mu et al. [14] reported the fabrication of a novel nonenzymatic composite electrode based on directly immobilized nickel oxide microfibers onto the surface of fluorine tin oxide electrode by electrospinning, which shows a highly sensitive, selective and extraordinary stable response towards glucose.
On the other hand, as a novel two-dimensional carbon material, graphene has also attracted considerable attention recently in the field of ultrasensitive sensors because of its excellent electronic and chemical properties [18, 19]. This tendency is well manifested by several recently published excellent reviews [20–22]. Considering the attractive properties of graphene, it is quite expected that graphene could provide an excellent support of the nickel(II) oxides for efficient electron transfer towards the oxidation of glucose. Very recently, Qiao et al. [23] developed a nonenzymatic glucose sensor based on a Ni(OH)2-GR nanocomposite modified glassy carbon electrode was fabricated by constant potential method. Lv et al. [24] prepared graphene nanosheet-NiO-DNA hybrids as the active material for high-performance non-enzymatic glucose sensors. In this paper, we developed a simple method to prepare nickel(II) oxides and graphene nanocomposite modified glassy carbon electrode (NiO-GR/GCE) for the nonenzymatic glucose detection, and then successfully applied the NiO-GR/GCE to the amperometric determination of the glucose concentration in red wines with high sensitivity and stability.
Experimental
Materials
Graphite powders (99 %, c.a.40 nm) was purchased from Aladdin Chemical Reagent Co. Ltd (Shanghai, China, http://www.aladdin-reagent.net). Glucose, ascorbic acid, sucrose, fructose, citric acid, acetic acid, ethanol and ethyl acetate were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China, http://www.reagebt.com.cn). All other reagents were of analytical grade and used without further purification. The electrochemical measurements were performed in 0.2 mol L−1 NaOH solution. The commercial red wine samples were obtained from a local supermarket.
The graphene oxide (GO) modifier was prepared using graphite powders as precursor through the modified Hummers’ method [25, 26]. The reduced graphene (GR) was prepared by using ascorbic acid as reduction agent. 10 mL GO aqueous solution (1 mg mL−1) and 0.1 mol L−1 ascorbic acid were mixed with a volume ratio of 1:1 and sonicated for 30 min at 60 °C, the reduction resultant was centrifuged at 104 rpm to remove the supernatant. Then, excess 30 % H2O2 was added to the black suspension to oxidize the remaining ascorbic acid under the sonicating condition for 30 min at 60 °C. The GR resultant was collected by centrifugation at 104 rpm, washed with ethanol and water three times and dried at 60 °C.
Preparation of modified electrodes
The as-synthesized GR was dispersing in N, N-dimethylformamide (DMF) at a concentration of 1 mg mL−1 with the aid of ultrasonic agitation for 1 h, resulting in a homogeneous black suspension. Prior to the surface modification, glassy carbon electrode (GCE) was polished with 0.05 μm alumina slurries, and then ultrasonically cleaned in water. The GR/GCE was prepared by casting a 2 μL of 1 mg mL−1 graphene/DMF suspension onto the surface of cleaned GCE and then evaporating the solvent under an infrared heat lamp. Afterwards, the GR/GCE was immersed into a NiSO4 solution (0.01 mol L−1), and a potentiostat was employed for the direct electrodeposition of Ni particles. In the first series of experiments, a pulse potential profile of 200 mV (500 ms) ~ −1,200 mV (100 ms) ~200 mV (500 ms) (vs. Ag/AgCl 3 mol L−1 KCl) was applied and 15 pulses were used for each deposition [27]. The electrode was then conditioned by potential cycling in a successive (0.2–0.5 V vs. Ag/AgCl) in 0.2 mol L−1 NaOH solution until a steady state voltammogram was obtained. The as-prepared modified NiO-GR/GCE was used for the study of glucose oxidation. A nickel(II) oxides modified glassy carbon electrode (NiO/GCE) was prepared in the same way, in the absence GR.
Analysis methods
All the electrochemical measurements were performed on a CHI 660B electrochemical workstation (CH Instruments, Shanghai Chenhua Instrument Corporation, China, http://chi.instrument.com.cn). The GCE or the modified electrode was used as working electrode,Ag/AgCl electrode (3 mol L−1 KCl) and Pt wire were used as reference and counter electrode, respectively. The morphology of NiO/GR composite was determined using field emission scanning electron microscopy (FE-SEM; Zeiss Ultra55, Germany) coupled with energy-dispersive X-ray (EDX) analysis attached to SEM. The high-performance liquid chromatography (HPLC, LC-10AT, Shimadzu, Kyoto, Japan) equipped with a C18 reverse phase column (5 μm, 4.6 × 250 mm) and an injection volume of 25 μL was employed as a comparative technique to test the correctness of the electrochemistry method.
Results and discussion
Properties of modified electrode
The morphology of the resulting NiO-GR/GCE was characterized by FE-SEM, as shown in Fig. 1a. It can be observed that the typical crumpled and wrinkled graphene sheet structure on the surface of GCE. Clearly, the rough and stratified structure of the graphene can endowed the more effective active area for the nucleation of the nickel, and the edge plane of graphene is densely combined by nickel(II) oxides nanoparticles with an average diameter of about 30 nm. From the EDX image (Fig. 1b), the peak of Ni originating from the nickel(II) oxides on the modified film can be seen. The SEM and the EDX results indicate that the NiO/GR composite has been successfully prepared on GCE [28]. In addition, it was observed a pair of well-defined redox peaks with an anodic peak at 342 mV and a cathodic peak at 288 mV from the cyclic voltammograms of the NiO/GCE in 0.2 mol L−1 NaOH, corresponding to the Ni(II)/Ni(III) redox couple [29, 30]. At the NiO-GR/GCE, the anodic and cathodic peaks corresponding to Ni(II)/Ni(III) couple were observed at about 378 mV and 304 mV vs. Ag/AgCl, at a scan rate 50 mV s−1, respectively. Comparing with those of NiO/GCE, the cathodic and anodic peak currents in NiO-GR/GCE were increased significantly, which may be results from a larger surface area and excellent electrical conductivity of the GR.
Electrocatalytic oxidation of glucose at the modified electrode
The oxidation behaviors of glucose at the bare and modified GCEs were studied by cyclic voltammetry. As shown in Fig. 2a, no redox peak can be observed in the cyclic voltammogram of the bare GCE, suggesting that glucose cannot undergo the redox reaction in the potential range of interest. When in the case of GR/GCE (Fig. 2b), the oxidation of glucose requires a very high positive potential, leading to a poorly defined anodic wave involving very slow electrode kinetics. In contrast, the oxidation reaction of glucose at the NiO/GCE (Fig. 2c) and NiO-GR/GCE (Fig. 2d) shows much less positive potentials. At the same time, there is a great enhancement of the anodic peak current while the cathodic peak current obviously decreases.
Figure 3a shows the cyclic voltammograms of NiO-GR/GCE with different glucose concentrations at the scan rate of 50 mV s−1. It can be seen that the anodic peak currents are gradually enhanced with the increase of glucose concentrations, indicating that the NiO-GR/GCE has high sensitivity to glucose. The peak response currents obtained at different scan rates in the presence of 5 × 10−4 mol L−1 glucose in 0.2 mol L−1 NaOH are given in Fig. 3b. Obviously, the peak potential for the catalytic oxidation of glucose shifts to positive values with increasing scan rate, suggesting a kinetic limitation for the redox reaction of glucose in the NiO-GR/GCE. Thus, the possibility of a potential shift to more positive values due to higher ohmic drop is expected to be negligible. Meanwhile, the anodic peak current is proportional to the square root of the scan rate (Fig. 3b inset), indicating it is a typical mass transfer controlled reaction.
a Cyclic voltammograms of the NiO-GR/GCE with different glucose concentrations at a scan rate of 50 mV s−1, the inset shows the plots of peak current vs. glucose concentrations; b Cyclic voltammograms of the NiO-GR/GCE at different scan rates with the presence of 5.0 × 10−4 mol L−1 glucose, the inset shows the plots of peak current vs. v 1/2 (v denotes scan rate)
Amperometric response of glucose and calibration curve
Herein, +0.35 V was chosen as the applied potential of current-time measurements. The current response to glucose at the NiO-GR/GCE was studied, as shown in Fig. 4a. When glucose was added into the NaOH (0.2 mol L−1) solution, the modified electrode could achieve the steady-state current within less than 3 s, which is a very rapid response to glucose in this system. In Fig. 4b, the current response is linear with glucose concentration in the range from 2.0 × 10−5 mol L−1 to 1.12 × 10−2 mol L−1. The linear regression equation and correlation coefficient are: I(μA) = 15.94 c (10−3 mol L−1) + 3.89, r = 0.998 and I(μA) = 7.569 c (10−3 mol L−1) + 22.51, r = 0.999, respectively. Here, this phenomenon of the two linear regions may be due to the adsorption of intermediates [31]. At low concentration (from 0.02 to 2.0 × 10−3 mol L−1), the calibration plot is steeper than that at high concentration (from 2.0 to 11.2 × 10−3 mol L−1), indicating the NiO-GR/GCE is more sensitive to glucose at low concentration. Based on the signal-to-noise ratio of 3 (S/N), a detection limit of 5 × 10−6 mol L−1 can be obtained. The comparison of NiO-GR/GCE with other electrodes for the determination of glucose is listed in Table 1. It can be seen that the NiO-GR/GCE offers a reasonable linear range and a lower detection limit than some noble metal modified electrode.
Interference effect and real sample analysis
To evaluate the selectivity of the fabricated electrode, the influence of some potentially interfering substances with glucose in red wine were examined in NaOH (0.2 mol L−1) solution containing 2.0 × 10−4 mol L−1 glucose. As shown in Fig. 5, a well-defined glucose response can be obtained, and the interference effects of 5.0 × 10−4 mol L−1 sucrose, 5.0 × 10−4 mol L−1 fructose, 5.0 × 10−4 mol L−1 citric acid, 5.0 × 10−4 mol L−1acetic acid, 2.0 × 10−4 mol L−1 ethanol, 5.0 × 10−4 mol L−1 ethyl acetate are all below 5 % of the glucose response. It can be concluded that small amount of such carbohydrate compounds can be neglected, suggesting that the prepared sensor has a high selectivity toward the nonenzymatic oxidation of glucose. However,the interfering effect of ascorbic acid could be observed only when a comparative ascorbic acid was coexisted in glucose solution, but the interfering effects could be neglected under a low concentration.
The contents of glucose in real samples, i.e., two commercial red wines, were analyzed using the as-prepared NiO-GR/GCE. The amperometric detection was carried out at the applied potential of 0.35 V in 10 mL 0.2 mol L−1 NaOH solution under stirring condition with the injection of 10 μL red wine and the final concentration was diluted to 0.02–2.0 × 10−3 mol L−1, for which is in the linear range of the proposed sensor and can be detected sensitively. The quantitative determination of red wine samples was performed using the standard addition method and the results are shown in Table 2. As comparative data, the results measured by HPLC technique are also listed in Table 2. The results based on this proposed method are in good agreement with those determined by the HPLC technique and the recovery values are in the range of 98.6–101.9 (±5 %), indicating that the proposed method is reliable for the determination of glucose in the commercial red wines.
Reproducibility and stability of the NiO-GR/GCE
The reproducibility and stability of the sensor were also evaluated. Five NiO-GR/GCEs were investigated at +0.35 V to compare their amperometric current responses. The relative standard deviation (RSD) was 3.2 %, confirming that the preparation of the NiO-GR/GCE was highly reproducible. In addition, nine successive measurements of glucose on one NiO-GR/GCE yielded an RSD of 2.5 %, indicating that the sensor was stable. The stability of NiO-GR/GCE was also examined by recording its 30 consecutive CV curves in NaOH solution at a scan rate of 50 mV s−1. No obvious peak current change is found during 30 consecutive CV curves, implying that the NiO/GR was firmly modified onto the surface of GCE. The long-term stability of the sensor was evaluated by measuring its current response to glucose within a 25 days period. The sensor was exposed to air and its sensitivity was tested every 2 days. The current response of the NiO-GR/GCE was approximately 92 % of its original counterpart, which can be mainly attributed to its chemical stability in basic solution.
Conclusion
In this work, a novel nonenzymatic glucose sensor based on nickel(II) oxides and graphene modified glassy carbon electrode (NiO-GR/GCE) was fabricated without any adhesive. This NiO-GR/GCE showed high electrocatalytic activity for the nonenzymatic oxidation of glucose. A lower detection limit and higher sensitivity were obtained compared with those previously reported, and a good linear dependence of the amperometric response on the glucose concentration was achieved. More importantly, the interference from the oxidation of common interfering species including sucrose, fructose, citric acid, acetic acid, ethanol and ethyl acetate could be effectively avoided. In addition, this NiO-GR/GCE also had a high selectivity, reproducibility and stability, and a comparative sensitivity with HPLC for the amperometric determination of the glucose contents in the commercial red wines, showing its promising application in real samples analysis.
References
Ge L, Zhao YS, Mo T, Li JR, Li P (2012) Immobilization of glucose oxidase in electrospun nanofibrous membranes for food preservation. Food Control 26:188–193
Goriushkina TB, Soldatkin AP, Dzyadevych SV (2009) Application of amperometric biosensors for analysis of ethanol, glucose, and lactate in wine. J Agric Food Chem 57:6528–6535
Wei Q, Yan HX, Duan L, Cui Y, Yang Y, Li JB (2009) Glucose-sensitive microcapsules from glutaraldehyde cross-kinked hemoglobin and glucose oxidase. Biomacromolecules 10:1212–1216
Yang K, Shen GS, Wang H, Ou XM, Zhang XH, Lee CS, Lee ST (2009) ZnO nanotube arrays as biosensors for glucose. J Phys Chem C 113:20169–20172
Ozdemir C, Yeni F, Odaci D, Timur S (2010) Electrochemical glucose biosensing by pyranose oxidase immobilized in gold nanoparticle-polyaniline/AgCl/gelatin nanocomposite matrix. Food Chem 119:380–385
Wilson R, Turner APF (1992) Glucose oxidase: an ideal enzyme. Biosens Bioelectron 7:165–185
Lu LM, Zhang L, Qu FL, Lu HX, Zhang XB, Wu ZS, Huan SY, Wang QA, Shen GL, Yu RQ (2009) A nano-Ni based ultrasensitive nonenzymatic electrochemical sensor for glucose: enhancing sensitivity through a nanowire array strategy. Biosens Bioelectron 25:218–223
Vassilyev YB, Khozova OA, Nikolaeva NN (1985) Kinetics and mechanism of glucose electrooxidation on different electrode-catalysts. J Electroanal Chem 196:105–125
Zhou YG, Yang S, Qian QY, Xia XH (2009) Gold nanoparticles integrated in a nanotube array for electrochemical detection of glucose. Electrochem Commun 11:216–219
Meng L, Jin J, Yang GX, Lu TH, Zhang H, Cai CX (2009) Nonenzymatic electrochemical detection of glucose based on palladium-single-walled carbon nanotube hybrid nanostructures. Anal Chem 81:7271–7280
Tashkhourian J, Hormozi-Nezhad MR, Khodaveisi J, Dashti R (2011) A novel photometric glucose biosensor based on decolorizing of silver nanoparticles. Sens Actuators B 158:185–189
Lee YJ, Park JY (2011) A coral-like macroporous gold-platinum hybrid 3D electrode for enzyme-free glucose detection. Sens Actuators B 155:134–139
Cao F, Guo S, Ma H, Shan D, Yang S, Gong J (2011) Nickel oxide microfibers immobilized onto electrode by electrospinning and calcination for nonenzymatic glucose sensor and effect of calcination temperature on the performance. Biosens Bioelectron 26:2756–2760
Mu Y, Jia D, He Y, Miao Y, Wu HL (2011) Nano nickel oxide modified non-enzymatic glucose sensors with enhanced sensitivity through an electrochemical process strategy at high potential. Biosens Bioelectron 26:2948–2952
Wang G, He X, Wang L, Gu A, Huang Y, Fang B, Geng B, Zhang X (2013) Non-enzymatic electrochemical sensing of glucose. Microchim Acta. doi:10.1007/s00604-012-0923-1
Jiang LC, Zhang WD (2010) A highly sensitive nonenzymatic glucose sensor based on CuO nanoparticles-modified carbon nanotube electrode. Biosens Bioelectron 25:1402–1407
Shamsipur M, Najafi M, Hosseini MRM (2010) Highly improved electrooxidation of glucose at a nickel(II) oxide/multi-walled carbon nanotube modified glassy carbon electrode. Bioelectrochemistry 77:120–124
Shan CS, Yang HF, Han DX, Zhang Q, Ivaska A, Niu L (2010) Graphene/AuNPs/chitosan nanocomposites film for glucose biosensing. Biosens Bioelectron 25:1070–1074
Li J, Guo S, Zhai Y, Wang E (2009) High-sensitivity determination of lead and cadmium based on the Nafion-graphene composite film. Anal Chim Acta 649:196–201
Gan T, Hu S (2011) Electrochemical sensors based on graphene materials. Microchim Acta 175:1–19
Liu YX, Dong XC, Chen P (2012) Biological and chemical sensors based on graphene materials. Chem Soc Rev 41:2283–2307
Pérez-López B, Merkoci A (2012) Carbon nanotubes and graphene in analytical sciences. Microchim Acta 179:1–16
Qiao N, Zheng J (2012) Nonenzymatic glucose sensor based on glassy carbon electrode modified with a nanocomposite composed of nickel hydroxide and graphene. Microchim Acta 177:103–109
Lv W, Jin FM, Guo Q, Yang QH, Kang F (2012) DNA-dispersed graphene/NiO hybrid materials for highly sensitive non-enzymatic glucose sensor. Electrochim Acta 73:129–135
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Kovtyukhova NI, Ollivier PJ, Martin BR, Mallouk TE, Chizhik SA, Buzaneva EV, Gorchinskiy AD (1999) Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem Mater 11:771–778
Chen XX, Eckhard K, Zhou M, Bron M, Schumann W (2009) Electrocatalytic activity of spots of electrodeposited nobel-metal catalysts on carbon nanotubes modified glassy carbon. Anal Chem 81:7597–7603
Nan J, Yang Y, Lin Z (2006) In situ photoelectrochemistry and Raman spectroscopic characterization on the surface oxide film of nickel electrode in 30 wt.% KOH solution. Electrochim Acta 51:4873–4879
Casella IG, Desimom E, Cataldi TRI (1991) Study of nickel-catalysed glassy carbon electrode for detection of carbohydrates in liquid chromatography and flow-injection analysis. Anal Chim Acta 248:117–125
Casella IG, Catald TRI, Salvi AM, Desimoni E (1993) Electrocatalytic oxidation and liquid chromatographic detection of aliphatic alcohols at nickel-based glassy carbon modified electrode. Anal Chem 85:3143–3150
Ndamanisha JC, Guo L (2009) Nonenzymatic glucose detection at ordered mesoporous carbon modified electrode. Bioelectrochemistry 77:60–63
Holt-Hindle P, Nigro S, Asmussen M, Chen A (2008) Amperometric glucose sensor based on platinum-iridium nanomaterials. Electrochem Commun 10:1438–1441
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21175047 and 21147003) and the Scientific Research Foundation of Graduate School of South China Normal University (2012kyjj217).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, X., Jiao, Q., Zhang, C. et al. Amperometric nonenzymatic determination of glucose based on a glassy carbon electrode modified with nickel(II) oxides and graphene. Microchim Acta 180, 477–483 (2013). https://doi.org/10.1007/s00604-013-0955-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-013-0955-1