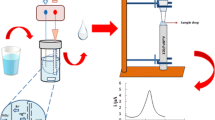
Abstract
Centri-voltammetry is a method for concentrating an analyte on an electrode with the aid of a centrifuge. It can be performed in the presence or the absence of a solid carrier/support. This is followed by a voltammetric (analytical) scan. Specifically, we describe here an application of the technique to the determination of glutathione (GSH). Silica gel is used as the carrier precipitate to which gold nanoparticles were added in order to improve accumulation as a result of their strong affinity for thiols. Voltammetry was performed with a carbon paste electrode modified with multi-wall carbon nanotubes. The response to GSH is linear in the 25 and 800 μM concentration range (the correlation coefficient being 0.9915) and the relative standard deviation is 3.40 % (at 250 μM of GSH and n = 6). The procedure was successfully applied to the determination of GSH in wine and in synthetic plasma using the standard addition method. The recoveries are 100.8 % and 100.0 %, respectively.

Centri-voltammetric GSH detection was conducted where silica gel and AuNP were used as carrier materials. As a result, sensitive, robust and practical method was developed for GSH detection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glutathione (GSH) is a tripeptide (γ-Glu-Cys-Gly) that contains a -SH group. It helps to prevent the damage of the cells by neutralizing harmful molecules generated during energy production. Recently, GSH has been encountered in many tumor tissues and altered levels of GSH in plasma have been implicated in a number of pathological conditions including macular degeneration, diabetes, Alzheimer’s, Parkinson’s and HIV diseases [1–7].
Sensitive and accurate determination of GSH is also important for food analysis. GSH prevents formation of free radicals and detoxifying cells. It also inhibits enzymatic and non-enzymatic mechanisms involved in browning of the fruit juice and other foods [8–12]. Additionally, GSH prevents the oxidation of phenolic compounds in wine and is claimed to play a major role in protecting volatile thiols during the aging of bottled white wines [13].
Several methods have been available for the detection of GSH such as high-performance liquid chromatography [14–17], spectrofluorimetry [18] and spectrophotometry [19, 20]. However, most of these techniques experience difficulties at the sample preparation step and the necessity of derivatization or lack of sensitivity limits their practical utility [21]. Electrochemical methods, on the other hand, provide a simple and sensitive alternative for GSH detection [22, 23]. The detection principle in these techniques is based on following the electrochemical oxidation reaction of GSH as shown in Eq. 1.
The main drawback of the method is the high background current resulted from the strong adsorption of the hydrosulfide group of GSH on the metallic electrodes like platinum, gold and silver [24, 25]. Modified electrodes like boron-doped diamond electrodes [26], carbon nanotube paste electrodes [4] and sol–gel-derived ceramic-carbon nanotube nanocomposite electrodes [27] have been utilized in order to facilitate the electrochemical oxidation of GSH. Gold nanoparticles (AuNPs) have also been employed for detection of thiol-containing amino acids based on the strong affinity of the -SH groups to gold [28, 29].
Centri-voltammetry on the other hand, is a method which combines the advantages of centrifugation and voltammetry. The method offers a practical way for the application of co-precipitation in the same cell allowing direct voltammetric scan afterwards. In this way, the loss of analyte is prevented which usually is the case for other preconcentration techniques. The first application of this method, developed by our group, includes sensitive heavy metal detection with good reproducibility [30, 31]. Ürkmez et al. [32] applied the method for anodic stripping analysis of mercuric ions at gold film electrode where sub ppb levels of mercury were detected.
More recently, the scope of the method was extended by applying centri-voltammetry for detection of biological compounds. The method was named as bio-centrivoltammetry [33, 34]. In our first work, we manage to construct a xanthine biosensor by immobilizing xanthine oxidase onto the working electrode of centri-voltammetric cell [33]. In the second bio-centrivoltammetric application, we manage to monitor the acethyl choline esterase enzyme activity biocentrivoltammetrically [34]. A survey including previous centri-voltammetric studies were summarized in Table 1. Growing demand for more selective and sensitive determinations of biologically important molecules encourages the usage of more selective carriers as well as new electrode materials adoptable to the centri-voltammetric cell.
Carbon nanotubes (CNTs) offer better sensitivity, fast response, good reversibility, enhanced electron transfer and easy immobilization of biological substances with retention of activity when used as an electrode material for some reagents [35–42].
On the other hand, silica gel is a stable non-swelling inorganic material which is defined as an ideal support for organic groups. It has also been used for accumulation of different substances as an adsorbent. This material has high mass exchange characteristics, fast adsorption kinetics, high surface area and very high thermal resistance [43]. In some studies silica gel was modified by suitable reagents and as a result, more effective adsorbents were obtained [43–47].
In this study, the GSH content of the solution was driven onto the multi-wall carbon nanotube (MWCNT) modified carbon paste electrode surface by means of a centrifugal force in the presence of silica gel. The preconcentration step was enriched by addition of AuNP which are known to have a strong affinity to the thiol groups. In centri-voltammetric techniques, the analyte, together with carrier precipitate and supporting electrolyte accumulate on the electrode surface as a thin film. In this manner, the insertion of AuNPs into this film would increase the conductivity of the film which resulted with enhanced activity of the electrode surface. The analyte preconcentrated as a thin film is then detected by applying voltammetric scan. After the working parameters like silica gel and AuNP amounts, adsorption time, centrifugation speed and time were optimized, the analytical characteristics were explored. The performance of the developed centri-voltammetric system was also tested by detecting GSH in wine and synthetically prepared plasma samples.
Experimental
Apparatus
Differential pulse voltammetric measurements were carried out with a FRA 2 μ-AUTOLAB Type III electrochemical measurement system from ECO CHEMIE Instruments B.V., (Netherlands, www. ecochemie.nl) controlled by GPES software. The experiments were conducted in a 10 mL centri-voltammetric cell, at room temperature (25 °C), using a three-electrode configuration. A platinum electrode served as an auxiliary electrode while an Ag/AgCl electrode as reference electrode, and 4 % MWCNT modified CPE was used as the working electrode. The auxiliary and reference electrodes were inserted into the cell through the Delrin cover while the working electrode was placed at the bottom of the cell. A Sigma 3-16PK centrifuge was used for centrifugation (www.sigma-zentrifugen.de). The home-made cell, made from a Delrin tube, was constructed to be compatible to both the centrifuge and voltammetry. The working electrode was placed at the bottom of the cell to provide a flat surface for the deposits (Fig. 1).
Reagents
Graphite powder (particle size <50 μm, Merck, www.merck.com) and mineral oil (Aldrich, www.sigmaaldrich.com) were purchased. MWCNT (diam. = 110–170 nm, length = 5–9 μm, 90+%) was obtained from Aldrich (www.sigmaaldrich.com). A 0.01 M GSH stock solution was prepared freshly by dissolving the appropriate amount of GSH (L-Glutathione reduced, γ-L-Glutamyl-glycine GSH, cell culture tested, Sigma, www.sigmaaldrich.com). The solution was then deaerated with N2 gas. The silica gel (Silica Gel 60, particle size ≤0.04 mm (≥400 mesh ASTM, for preparative column chromatography) was used as the carrier material, which was supplied from Fluka (www.sigmaaldrich.com) while gold colloid was obtained from Sigma (0.75A520 units/mL; concentration: ~0.01 % as HAuCl4, www.sigmaaldrich.com). Phosphate buffer solution KH2PO4, Merck/purity 99,995 %, 0.05 M, pH 7.0, www.merck.com) was used as the supporting electrolyte. Synthetic plasma samples were prepared by incorporating the reagents into the Tris–HCl including 140 mM NaCl (99.0–100.1 % supplied from Pancreac, (www.pancreac.es), 4.5 mM KCl, 2.5 mM CaCl2 (Calcium chloride dehydrate, ACS reagent, ≥99 % from Sigma-Aldrich, www.sigmaaldrich.com), 0.8 mM MgCl2 (magnesium chloride hexahydrate, GR for analysis 99.0–101.0 % obtained from Merck, www.merck.com), 2.5 mM urea (for molecular biology, powder used from Sigma, www.sigmaaldrich.com), and 4.7 mM glucose (D-(+) glucose monohydrate for microbiology from Merck, www.merck.com). 10 mM Tris–HCl (Trizma®hydrochloride, reagent grade, ≥99.0 % (titration), crystalline supplied from Sigma, www.sigmaaldrich.com) buffer was used as the background of the synthetic plasma solution. All solutions were prepared by using double distilled water and other chemicals were all of analytical grade.
Electrode preparation
MWCNT modified CPE was prepared by using previously optimized ratios which is 66:30:4 % (w/w) graphite powder/mineral oil/MWCNT [35]. A portion of the resulting paste was then packed firmly into the electrode cavity (3.0 mm diameter and 5.0 mm depth) of a cell made of Delrin where electrical contact was established via a copper wire. The surface of the paste electrode was smoothed with a weighing paper and rinsed carefully with double distilled water.
Procedure
As can be followed from Fig. 1, the GSH solution was placed in a beaker and 1.0 mg of silica gel and 30 μL of AuNP solution were added. The stirred was switched on and the mixture was allowed to complete adsorption process for 7 min. Then, the mixture was placed into the centri-voltammetric cell and centrifuged for 4 min at 4000 rpm. After that the cell was carefully placed in the voltammetric stand where reference and counter electrodes were immersed into the working solution. Differential pulse voltammograms were recorded in the potential range between 0 V and +1.0 V in 50.0 mM phosphate buffer (pH 7.0) medium. The electrode surface was renewed prior to each use.
Sample application
The procedure was applied for the detection of GSH in food and clinical sample to show the versatility of the method developed. Synthetically prepared plasma sample and the red wine sample from local market were analyzed using the standard addition method. The wine sample was diluted in 1:100 ratios with 50 mM pH 7.0 phosphate buffer solutions and spiked with 100 μM GSH standard. The synthetic plasma sample was prepared according to the procedure reported before [48]. Synthetic plasma solution was comprised of 140 mM NaCl, 4.5 mM KCl, 2.5 mM CaCl2, 0.8 mM MgCl2, 2.5 mM urea, and 4.7 mM glucose. 10 mM Tris–HCl buffer was used as the background of the synthetic plasma solution. The final pH of the solution was adjusted to 7.3 with addition of proper amount of 1 M HCl. The standard solutions were prepared from using the synthetic plasma electrolyte as a background solution and then proper amount of analytes was spiked to the phosphate buffer solution (pH 7.0). The recovery values were calculated upon three successive additions of standard GSH solution into the mixtures.
Results and discussion
Electrochemical detection of GSH by centri-voltammetry
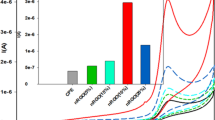
The effective side of the suggested method can easily be seen from Fig. 2 where 400 μM GSH was used and centrifugation was applied at 4000 rpm prior to voltammetric scan. With MWCNT-CPE, wide and small peak of GSH at 0.6 V was obtained when there is no silica-gel and AuNP in the medium (Fig. 2b). After the addition of silica gel, a little more significant but still wide peak was obtained (Fig. 2c). It has been believed that this process produces a thin film on the electrode surface along with the GSH content of the solution. As a result, the analyte is enriched in this film at a certain extent which results with an improvement at the signal. Actual improvement was observed when 30 μL of AuNP solution was added into the cell as described in the procedure. As a result, GSH peak has doubled (Fig. 2d). Here, AuNP serves as a carrier reagent and a modifier. Due to strong affinity of these nanoparticles to the thiol groups, it has been believed that the accumulation process is strengthened and as a result more significant GSH peak was obtained.
Differential pulse voltammetric responses of the 4 % MWCNT/CPE with centri-voltammetry a) background, b) 400 μM GSH, c) 400 μM GSH including silica gel, d) 400 μM GSH including silica gel and AuNP, in 50 mM phosphate buffer solution (pH 7.0), working potential: 0 V/+1.0 V. Inset: A comparison of a) bare CPE and b) 4 % MWCNT modified CPE for 400 μM GSH including silica gel and AuNP
In order to observe the effect of MWCNT, the procedure was performed with and without MWCNT modified CPE. It can be seen that when the 4 % MWCNT was incorporated into the CPE, a significant and well-defined peak obtained relating to electrochemical oxidation of GSH with centri-voltammetry whilst no distinct peak current was obtained with bare CPE (Fig. 2 inset). The voltammograms show that the plain CPE had a current value of 0.0047 μA while the MWCNT including CPE had current value of 0.087 μA. The significant increase in current value and the decrease in peak potential (as in the case of MWCNT) can be attributed to the electrocatalytic activity of CNT, which can mediate the electron transfer reactions [35].
Optimization of the experimental parameters
The experimental parameters including silica gel and AuNP amounts, pH, adsorption time and centrifugation parameters were optimized before the examination of analytical characteristics.
Effect of silica gel amount
Figure 3(a) demonstrates the effect of silica particle amounts (0.5, 1.0, 1.5, 2.0, 2.5 mg) on the current response of 250 μM GSH. As can clearly be seen from the figure, the peak current increases when the silica gel amount was increased from 0.5 to 1.0 mg and then a slight decrease was obtained for amounts greater than 1.0 mg silica gel. This can be attributed to the altered surface conductivity of the electrode due to the thickening of the film. Therefore, 1.0 mg of silica gel was chosen as optimum amount of carrier material and used for further studies
Influence of AuNP amount
In order to examine the effect of AuNP amount, the commercially available gold colloid solution was used for this purpose which was applied before in our previous works [24, 49, 50]. Similarly, various amounts of AuNP (10, 20, 30, 40, and 50 μL) were tested to examine their effect on the current response of 250 μM GSH (Fig. 3 (b)). As can clearly be seen from the Figure, best results were obtained with 30 μL of AuNP solution. The decrease in the peak current for higher amounts of AuNP can be assigned to the thickening of the film which may have caused a change on the surface characteristics.
Influence of pH
The medium pH is usually regarded as a one of the most important factors in electrochemical measurements. The electrochemical behavior of GSH in 50 mM phosphate buffer solution was studied in a pH range of 6.0 to 8.0 (Fig. 4 (a)) and best results were obtained at pH 7.0. Then a gradual decrease was obtained as the pH value increases. In accordance with previous studies on GSH [14, 24, 51, 52] this decrease can be attributed to structural effect that provides the increment of electronic density, facilitating the oxidation of the species formed in the chemical step [14].
Influence of the adsorption time
The time elapsed in adsorption process is of great importance allowing the maturation of the precipitate and an effective enrichment of the analyte. The interaction of GSH with AuNPs added into 250 μM GSH solution was examined by allowing the mixture for adsorption of 1–20 min. It is evident from Fig. 4(b) that best peak current value was obtained when 7 min. was applied as adsorption period. The decline in the response observed for longer adsorption times can be attributed to the agglomeration in the solution at some extent which may cause an alteration in nanoparticle behavior. Therefore, 7 min was selected as the optimum time and longer adsorption times were avoided.
Centrifugation parameters
The centrifugation parameters are vital for a sensitive and reproducible determination in centri-voltammetry. Figure 5(a) demonstrates the influence of the centrifugation time on centri-voltammetric results for 250 μM GSH. An increase in the current was observed from 1 to 4 min. and then, a sharp decrease was observed. Longer times might cause dispersion of deposited analyte on the electrode surface that result with decrease in current values [32]. As a result, 4 min. was taken as optimum working potential and used for further studies.
The effect of centrifugation speed on the centri-voltammetric results in the presence of 250 μM GSH, 50 mM phosphate buffer (pH 7.0) for 4 min with adsorption time of 7 min can be seen in Fig. 5(b). The peak current gives a maximum at 4000 rpm and the decrease for higher speed is observed probably due the thickening of the film with a reduced conductivity.
Analytical characteristics
After the optimization of experimental parameters, analytical characteristics were examined. The linear range was obtained between 25–800 μM where with the equation of \( \mathrm{y}=0.0002\mathrm{x}+0.0132 \) and correlation coefficient of R 2 = 0.9915 respectively (Fig. 6). R.S.D values were calculated for 250 μM GSH (n = 6) and found as 3.40 %. LOD value was also calculated from the calibration graph and found as 264.2 μM. These calculation was based on the formulation of 3 s/m (for LOD), where m is the slope of the calibration curve and s is the standard deviation of the blank current values.
On the other hand, the performance of the developed method was compared with other methods devoted for the determination of GSH in terms of analytical characteristics. The results were demonstrated in Table 2. As can clearly be seen from the Table, the overall performance of the developed sensor is in acceptable limits.
The interference effects of ascorbic acid and ʟ-cysteine were searched on the GSH peaks. For the same concentration of these analytes, the effect at pH 7.0 was calculated as 8.4 % and 9.4 %, respectively demonstrating that the similarity in the structure does not increase the interfering effect.
Sample application
In order to verify the applicability of the suggested method, the wine and synthetically plasma samples were analyzed as described in experimental section. Each experiment was employed for three times by applying standard addition method (n = 3). For wine samples the recovery of the analytical signal was calculated as 100.8 % while for synthetically prepared plasma sample recovery value was found as 100.0 %.
Conclusion
Present paper describes the utility of centri-voltammetry as an efficient method for organic molecules as well as the metallic ions. Here, the GSH content of a solution was driven onto the MWCNT modified carbon paste electrode surface by means of a centrifugal force in the presence of silica gel. The addition of AuNP co-precipitating along with the analyte constitutes the original part of this work. The insertion of AuNP into the film would provide enhanced activity of the electrode surface by increasing the conductivity of the film. The performance of developed centri-voltammetric system was also tested by detecting GSH in wine and synthetically prepared plasma samples without any need for sample preparation step. Recovery values demonstrate that it is possible to apply centri-voltammetry for GSH detection in real samples directly after dilution step, since the technique is not affected by the sample matrix and sample nature. On the other hand, ongoing works continue in our lab for application of centri-voltammetry at different areas.
References
Miao P, Liu L, Nie Y, Li G (2009) An electrochemical sensing strategy for ultrasensitive detection of glutathione by using two gold electrodes and two complementary oligonucleotides. Biosens Bioelectron 24:3347–3351
Zheng ZB, Zhu GZ, Tak H, Joseph E, Eiseman JL, Creighton DJ (2005) N-(2-hydroxypropyl)methacrylamide copolymers of a glutathione (GSH)-activated glyoxalase i inhibitor and DNA alkylating agent: synthesis, reaction kinetics with GSH, and in vitro antitumor activities. Bioconjug Chem 16:598–607
White PC, Lawrence NS, Davis J, Compton RG (2002) Electrochemical determination of thiols: a perspective. Electroanalysis 14:89–98
Lawrence NS, Deo RP, Wang J (2004) Detection of homocysteine at carbon nanotube paste electrodes. Talanta 63:443–449
Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R et al (2004) Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem 50:3–32
Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH et al (2002) Plasma homocysteine as a risk factor for dementia and alzheimer’s disease. N Engl J Med 346:476–483
Kleinman WA, Richie JP (2000) Status of glutathione and other thiols and disulfides in human plasma. Biochem Pharmacol 60:19–29
Lavigne V, Pons A, Dubourdieu D (2007) Assay of glutathione in must and wines using capillary electrophoresis and laser-induced fluorescence detection: changes in concentration in dry white wines during alcoholic fermentation and aging. J Chromatogr A 1139:130–135
Friedman M (1994) Improvement in the safety of foods by sulfhydryl-containing amino acids and peptides. A review. J Agric Food Chem 42:3–20
Molnar-Perl I, Friedman M (1990) Inhibiton of browning by sulfur amino acids. 2. Fruit juices and protein-containing foods. J Agric Food Chem 38:1648–1651
Molnar-Perl I, Friedman M (1990) Inhibition of browning by sulfur amino acids. 3. Apples and potatoes. J Agric Food Chem 38:1652–1656
Friedman M (1996) Food browning and its prevention: an overview. J Agric Food Chem 44:631–653
Dubourdieu D, Lavigne-Cru’ege V (2002) 13th International Enology Symposium, Management and Wine Marketing, Montpellier, Proceedings, Trogus H, Gafner J, Sütterlin A (eds) International Association of Enology, Management and Wine Marketing, Breisach Germany, TS Verlag, Neuenberg a. Rhein pp 331–347
Lima PR, Santos WJR, Oliveira AB, Goulart MOF, Kubota LT (2008) Electrocatalytic activity of 4-nitrophthalonitrile-modified electrode for the l-glutathione detection. J Pharm Biomed Anal 47:758–764
Cereser C, Guichard J, Drai J, Bannier E, Garcia I et al (2001) Quantitation of reduced and total glutathione at the femtomole level by high-performance liquid chromatography with fluorescence detection: application to red blood cells and cultured fibroblasts. J Chromatogr B: Biomed Sci Appl 752:123–132
Zhang W, Wan F, Zhu W, Xu H, Ye X, Cheng R, Jin LT (2005) Determination of glutathione and glutathione disulfide in hepatocytes by liquid chromatography with an electrode modified with functionalized carbon nanotubes. J Chromatogr B 818:227–232
Shen Z, Wang H, Liang SC, Zhang ZM, Zhang HS (2002) Spectrofluorimetric determination of reduced glutathione in human blood using n-[p-(2-benzothiazoyl)-phenyl]maleimide. Anal Lett 35:2269–2278
Chen XP, Cross RF, Clark AG, Baker WL (1999) Analysis of reduced glutathione using a reaction with 2,41-dichloro-l-(naphthyl-4-ethoxy)-s-triazine (EDTN). Microchim Acta 130:225–231
Besada A, Tadros NB, Gawargious YA (1989) Copper(II)-neocuproine as colour reagent for some biologically active thiols: Spectrophotometric determination of cysteine, penicillamine, glutathione, and 6-mercaptopurine. Microchim Acta 99:143–146
Raggi MA, Nobile L, Giovannini AG (1991) Spectrophotometric determination of glutathione and of its oxidation product in pharmaceutical dosage forms. J Pharm Biomed Anal 9:1037–1040
Chwatko G, Bold E (2000) Determination of cysteine in human plasma by high-performance liquid chromatography and ultraviolet detection after pre-column derivatization with 2-chloro-1-methylpyridinium iodide. Talanta 52:509–515
Calvo-Marzal P, Chumbimuni-Torres KY, Höehr NF, Kubota LT (2006) Determination of glutathione in hemolysed erythrocyte with amperometric sensor based on TTF-TCNQ. Clin Chim Acta 371:152–158
Chee SY, Flegel M, Pumera M (2011) Regulatory peptides desmopressin and glutathione voltammetric determination on nickel oxide modified electrodes. Electrochem Commun 13:963–965
Çubukçu M, Ertaş FN, Anık Ü (2012) Metal/metal oxide micro/nanostructured modified GCPE for GSH detection. Curr Anal Chem 8:351–357
Chen J, He Z, Liu H, Cha C (2006) Electrochemical determination of reduced glutathione (GSH) by applying the powder microelectrode technique. J Electroanal Chem 588:324–330
Terashima C, Rao TN, Sarada BV, Kubota Y, Fujishima A (2003) Direct electrochemical oxidation of disulfides at anodically pretreated boron-doped diamond electrodes. Anal Chem 75:1564–1572
Gong K, Zhang M, Yan Y, Su L, Mao L, Xiong S, Chen Y (2004) Sol–gel-derived ceramic-carbon nanotube nanocomposite electrodes: tunable electrode dimension and potential electrochemical applications. Anal Chem 76:6500–6505
Lim I-Im S, Mott D, Ip W, Njoki PN, Pan Y, Zhou S, Zhong CJ (2008) Interparticle interactions in glutathione mediated assembly of gold nanoparticles. Langmuir 24:8857–8863
Shang L, Yin J, Li J, Jin L, Dong S (2009) Gold nanoparticle-based near-infrared fluorescent detection of biological thiols in human plasma. Biosens Bioelectron 25:269–274
Anık-Kırgöz Ü, Tural H, Ertas FN (2004) A new procedure for voltammetric lead determination based on coprecipitation and centrifugation preconcentration. Electroanalysis 16:765–768
Anık-Kırgöz Ü, Tural H, Ertas FN (2005) Centri-voltammetric study with amberlite XAD-7 resin as a carrier system. Talanta 65:48–53
Ürkmez İ, Gökçel İ, Ertaş FN, Tural H (2009) Centrifugation: an efficient technique for preconcentration in anodic stripping voltammetric analysis of mercury using a gold film electrode. Microchim Acta 167:225–230
Anık Ü, Çevik S (2011) Centri-voltammetry for biosensing systems: biocentri-voltammetric xanthine detection. Microchim Acta 174:207–212
Çevik S, Timur S, Anık Ü (2012) Biocentri-voltammetry for the enzyme assay: a model study. RSC Adv 2:4299–4303
Anık Ü, Çubukçu M (2008) Examination of the electroanalytic performance of carbon nanotube (cnt) modified carbon paste electrodes as xanthine biosensor transducers. Turk J Chem 32:711–719
Anık Ü, Çevik S (2009) Double-walled carbon nanotube based carbon paste electrode as xanthine biosensor. Microchim Acta 166:209–213
Anık-Kırgöz Ü, Timur S, Odacı D, Perez B, Alegret S, Merkoçi A (2007) Carbon nanotube composite as novel platform for microbial biosensor. Electroanalysis 19:893–898
Timur S, Anık Ü, Odacı D, Gorton L (2007) Development of a microbial biosensor based on carbon nanotube (CNT) modified electrodes. Electrochem Commun 9:1810–1815
Anık Ü, Çevik S, Pumera M (2010) Effect of nitric acid “washing” procedure on electrochemical behavior of carbon nanotubes and glassy carbon μ-particles. Nanoscale Res Lett 5:846–852
Çevik S, Anık Ü (2010) Banana tissue-nanoparticle/nanotube based glassy carbon paste electrode biosensors for catechol Detection. Sens Lett 8:667–671
Merkoçi A, Pumera M, Llopis X, Pérez B, del Valle M, Alegret S (2005) New materials for electrochemical sensing VI: carbon nanotubes. Trends Anal Chem 24:826–838
Pérez B, Pumera M, del Valle M, Merkoçi A, Alegret S (2005) Glucose biosensor based on carbon nanotube epoxy composites. J Nanosci Nanotechnol 5:1694–1698
Fan H-T, Sun T, Xu H-B, Yang Y-J, Tang Q, Sun Y (2011) Removal of arsenic(V) from aqueous solutions using 3-[2-(2-aminoethylamino)ethylamino]propyl-trimethoxysilane functionalized silica gel adsorbent. Desalination 278:238–243
Brasil JL, Martins LC, Ev RR, Dupont J, Dias SLP, Sales JAA, Airoldi C, Lima ÉC (2005) Factorial design for optimization of flow-injection preconcentration procedure for copper(II) determination in natural waters, using 2-aminomethylpyridine grafted silica gel as adsorbent and spectrophotometric detection. Intern J Environ Anal Chem 85:475–491
Puanngam M, Unob F (2008) Preparation and use of chemically modified MCM-41 and silica gel as selective adsorbents for Hg(II) ions. J Hazard Mater 154:578–587
Tso CY, Chao CYH (2012) Activated carbon, silica-gel and calcium chloride composite adsorbents for energy efficient solar adsorption cooling and dehumidification systems. Int J Refrig 35:1626–1638
Huang H, Oike T, Watanabe F, Osaka Y, Kobayashi N, Hasatani M (2010) Development research on composite adsorbents applied in adsorption heat pump. Appl Therm Eng 30:1193–1198
Çoldur F, Andaç M, Işıldak I (2010) Flow-injection potentiometric applications of solid state Li+ selective electrode in biological and pharmaceutical samples. J Solid State Electrochem 14:2241–2249
Çubukçu M, Timur S, Anık Ü (2007) Examination of performance of glassy carbon paste electrode modified with gold nanoparticle and xanthine oxidase for xanthine and hypoxanthine detection. Talanta 74:434–439
Anık Ü, Çubukçu M, Yavuz Y (2012) Nanomaterial-based composite biosensor for glucose detection in alcoholic beverages. Artificial Cells, Blood Substitutes and Biotechnology, (doi:10.3109/10731199.2012.696071, pages 1–5)
Raoof JB, Ojani R, Baghayeri M (2009) Simultaneous electrochemical determination of glutathione and tryptophan on a nano-TiO2/ferrocene carboxylic acid modified carbon paste electrode. Sensors Actuators B 143:261–269
Mao L, Yamamoto K (2000) Amperometric biosensor for glutathione based on osmium-polyvinylpyridine gel polymer and glutathione sulfhydryl oxidase. Electroanalysis 12:577–582
Zeng X, Zhanga X, Zhu B, Jia H, Yang W, Li Y, Xue J (2011) A colorimetric and ratiometric fluorescent probe for quantitative detection of GSH at physiologically relevant levels. Sensors Actuators B 159:142–147
Potesil D, Petrlova J, Adama V, Vacek J, Klejdus B, Zehnalek J, Trnkova L, Havel L, Kizek R (2005) Simultaneous femtomole determination of cysteine, reduced and oxidized glutathione, and phytochelatin in maize (Zea mays L.) kernels using high-performance liquid chromatography with electrochemical detection. J Chromatogr A 1084:134–144
Roušar T, Kucěra O, Lotkova H, Červinkova Z (2012) Assessment of reduced glutathione: comparison of an optimized fluorometric assay with enzymatic recycling method. Anal Biochem 423:236–240
Fracassetti D, Lawrence N, Tredoux AGJ, Tirelli A, Nieuwoudt HH, Du Toit WJ (2011) Quantification of glutathione, catechin and caffeic acid in grape juice and wine by a novel ultra-performance liquid chromatography method. Food Chem 128:1136–1142
Chailapakul O, Fujishima A, Tipthara P, Siriwongchai H (2001) Electroanalysis of glutathione and cephalexin using the boron-doped diamond thin-film electrode applied to flow ınjection analysis. Anal Sci 17:419–422
Acknowledgments
This work is supported by The Scientific and Technical Research Council of Turkiye (TUBITAK) (project no. 109T885). M.C. and F.N.E are gratefully acknowledged the grant from Ege University Project number 2011 FEN 088.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Çubukçu, M., Ertaş, F.N. & Anık, Ü. Centri-voltammetric determination of glutathione. Microchim Acta 180, 93–100 (2013). https://doi.org/10.1007/s00604-012-0910-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-012-0910-6