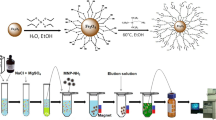
Abstract
We have prepared a highly selective and efficient sorbent for the simultaneous separation and preconcentration of lead and cadmium ions from milk and water samples. An ionic liquid was deposited on the surface of magnetic nanoparticles (IL-MNPs) and used for solid phase extraction of these ions. The IL-MNPs carrying the target metals were then separated from the sample solution by applying an external magnetic field. Lead and cadmium were almost quantitatively retained by the IL-MNPs, and then eluted with nitric acid. The effect of different variables on solid phase extraction was investigated. The calibration curve is linear in the range from 0.3 to 20 ng mL−1 of Cd(II), and from 5 to 330 ng mL−1 of Pb(II) in the initial solution. Under optimum conditions, the detection limits are 1.61 and 0.122 μg L-1 for Pb(II) and Cd(II) respectively. Relative standard deviations (n = 10) were 2.87 % and 1.45 % for 0.05 μg mL-1 and 0.2 μg mL-1 of Cd (II) and Pb (II) respectively. The preconcentration factor is 200 for both of ions.

A novel, highly selective and efficient sorbent, was prepared and applied for separation and preconcentration of lead and cadmium from real samples. Lead and cadmium could be quantitatively retained by ionic liquid-modified magnetite nanoparticles and then easily separated from the aqueous solution by applying an external magnetic field; so, no filtration or centrifugation was necessary.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The levels of heavy metals circulating in the environment have seriously increased due to human activity. The toxic heavy metals such as Cd(II) and Pb(II) are capable of causing ecological risk to aquatic organisms. The regular absorption of small amounts of Cd(II) and Pb(II) may cause serious effects especially on the health of growing children, including retardation of mental development and deficiencies in concentration, adverse effects on kidney function, blood chemistry and the cardiovascular system [1]. The main sources of heavy metals for humans and animals are water, food and atmosphere [2, 3]. Hence, reliable and sensitive analytical methods and procedures have an important role to evaluate the impacts of metal pollutants. However, the direct determination of these metal ions is often difficult because of their low concentrations and matrix interference occurring in real samples. Therefore, preliminary preconcentration-separation procedures are required.
In recent years, many methods have been developed for the preconcentration of trace metals from various samples, such as coprecipitation [4, 5], liquid–liquid extraction [6, 7], solid-phase extraction [8–10], and cloud point extraction [11, 12]. Among these techniques, SPE is widely used in preconcentration methodology because of its simplicity, achievement of high recoveries, high sorption capacity, minimal costs due to low consumption of reagents and low extraction time to sample preparation [13–15].
The choice of the sorbent is a key point in SPE [16], because it determines the analytical sensitivity, affinity, capacity and precision [17]. For this reason, the current researches are focused on the modify the surface of classical SPE adsorbents to increase the analytical sensitivity and precision [17]. Recently, magnetic nanoparticles have attracted substantial interest because of their special properties [18]. They possess a very large surface area, highly active surface sites and a short diffusion route, which result in high extraction capacity and rapid extraction of large-volume samples by employing an external magnetic field. These particles are superparamagnetic, which means that they can readily be attracted from sample solutions by the application of an external magnetic field, but retain no residual magnetism after the field is removed. Therefore, suspended superparamagnetic particles tagged to the target can be removed from a matrix quickly, by applying a magnetic field, but they do not agglomerate after removal of the field. However, pure magnetic nanoparticles suffer from some inherent limitations as they tent to agglomerate, thus altering magnetic properties in complex matrices [19, 20]. Moreover, these nanometer-sized metal oxides are not target-selective and are unsuitable for samples with complicated matrices [21]. Therefore, the modification of these magnetic nanoparticles is essential to overcome such limitations [22].
Room temperature ionic liquids (RTILs), as useful environment friendly green solvents, have recently attracted special attentions due to their unique chemical and physical properties such as nonvolatile (environmentally benign), excellent solvation qualities, non-flammable and high thermal stability [8, 23]. Since RTILs can dissolve many kinds of organic, organometallic and inorganic compounds [24], they are applied in many fields of analytical chemistry.
Ionic liquid-based extraction has some disadvantages, such as low rate of mass transfer, long equilibrium time, difficulty in separate-phase and entraining loss of IL to aqueous phase [25]. These limitations can be overcome by immobilizing ionic liquids on solid substrates. Some studies have been recently focused on the successful application of RTILs for extraction of metal ions based on various extraction techniques. However, There are relatively few reports for the application of supported ionic liquid phase for the preconcentration of trace metal ions [26].
To the best of our knowledge, there is no report on the use of ionic liquid coated magnetic NPs for the extraction and determination of trace metal ions. The purpose of this study is to establish a simple and fast method for the determination of Pb(II) and Cd(II) using ionic liquid-modified Fe3O4 NPs. In this work, the ionic liquid 1-hexyl-3-methylimidazolium hexafluorophosphate, [C6MIM][PF6] modified magnetite NPs was employed as a sorbent for SPE of Pb(II) and Cd(II) as 1-(2-pyridylazo)-2-naphthol (PAN) complex. The optimum conditions for sorption and enrichment of Pb(II) and Cd(II) were studied. In addition, the possible use of the developed method for analyzing Pb(II) and Cd(II) in food sample and water sample solutions was explored.
Experimental
Apparatus
The determination of Pb(II) and Cd(II) was carried out using a Varian Spectra AA-400 atomic absorption spectrometer (http://www.varianinc.com), equipped with a deuterium background correction and an air-acetylene burner. The lamp currents were set at 5 and 4 mA for Pb(II) and Cd(II), respectively. All Measurements were carried out in the peak height mode at 217.0 and 228.8 nm, using a spectral bandwidth of 1.0 nm and 0.5 nm for Pb(II) and Cd(II), respectively. The pH-meter Model 692 from Metrohm (http://www.metrohm-ag.com, Herisau, Switzerland) equipped with a glass combination electrode was used for the pH measurements. In addition, for magnetic separations a strong neodymium-iron-boron (Nd2Fe12B) magnet (1.31 T) was used. A Field emission scanning electron microscope (FESEM), model S-4160 (www.hitachi.com/procurement/network/japan) was used for preparation of SEM images. Besides, FTIR was recorded with one ABB Bomem MB100 IR spectrometer using KBr discs.
Reagents
All reagents used were of analytical-reagent grade and all aqueous solutions were prepared using doubly distilled water. The stock standard solutions of Cd(II) and Pb(II) (1,000 mg L−1) were prepared from appropriate amounts of their nitrate salts (Merck, Darmstadt, Germany, http://www.merck.de) in distilled water and normalized by titration with EDTA and working standard solutions were prepared by appropriate stepwise dilution of the stock standard solutions. Nano Fe3O4 was purchased from Sigma-Aldrich (http://www.sigmaaldrich.com, Fe3O4 spheres powder, <50 nm, purity >98 %). A solution of 10−3 mol L−1 of 1-(2-pyridylazo)-2-naphthol (PAN) from Merck (Darmstadt, Germany) was prepared by dissolving the appropriate amount of this reagent in pure ethanol. 1-hexyl-3-methylimidazolium hexafluorophosphate, [C6MIM][PF6] was purchased from Merck (Darmstadt, Germany) and used for the modification of sorbent. A buffer solution (pH = 5.5) was prepared by dissolving appropriate amounts of potassium hydrogen phthalate and Sodium hydroxide in deionized water. High purity HNO3 (65 %, Suprapur, Merck, Darmstadt, Germany), and hydrogen peroxide were used for digesting of milk samples throughout this work. The pipettes and vessels used were kept in 20 % (v/v) nitric acid for at least 24 h and then washed with ultra pure water.
Preparation of ILs-modified magnetite nanoparticles
The method was based on the physical adsorption of [C6MIM][PF6] hydrophobic ionic liquid on the surface of magnetic nanoparticles. In order to prepare magnetic nanoparticles supported ionic liquids 0.5 g of [C6MIM][PF6] was added to 15 mL of acetone extra pure (Merck), stirred and then 1.5 g of Fe3O4 powder was added gently to the solution and mixed thoroughly. After stirring for 2.5 h, the volatile components of the mixture were evaporated by water bath. Then washed thoroughly with methylene chloride and distilled water, and dried in an oven at 60 ◦C for 3 h. The total weight of the IL-silica is 1.8 g, so 0.3 g of ionic liquid was grafted on the surface of MNPs Fig. 1 shows the SEM image of the modified nanoparticles.
Besides, Fig 2 shows the FT-IR spectrums of ionic liquid-modified MNPs (a) and MNPs before modification (b). More details are placed in the Electronic Supplementary Material (ESM).
Procedure
An aliquot of 1.0 mL of 1 × 10−3 mol L−1 PAN and 40.0 mg of the modified MNPs was added to a tube containing 50 mL of an aqueous sample solution (pH = 5.5) of Pb(II) and Cd(II) and sonically agitated for 3 min to suspend thoroughly the NPs and allowed to complete the extraction process. Subsequently, a strong magnet was placed at the bottom of the tube to let IL-Fe3O4 NPs settle. After about 2 min, the solution became the limpid and supernatant was removed by decantation and the isolated NPs were eluted with 2.0 mL of (2.0 mol L−1) HNO3 to desorbe the metallic complexes. Finally, the obtained mixture was shaken and exposed on the magnet and the clear solution of eluent, containing the eluted metal ions, was transferred to the glass tube. The analytes in the eluted phase were determined by FAAS.
Sample preparation
Two kinds of certified reference materials, NIST-1640A (Natural water) NIM-GBW10011 (Wheat flour), were employed for testing the accuracy of the mentioned method. In addition, several corresponding real samples, milk (Milk sample was purchased from a local supermarket in Tehran, Iran), milk powder (Homana, Iran), sea water, mineral water and Tap water were analyzed. Details of the sample preparation are as follows.
Milk samples were digested according to established procedures, with a little modification. Briefly, an aliquot of each milk sample (100 mg of powdered milk and 1.0 mL for liquid sample) was weighed into a PTFE digestion vessel. Afterwards, 5.0 mL of HNO3 (65 %) and 2.0 mL of H2O2 (30 %) were added and the vessels were heated on a hot plate at a fairly low temperature, to avoid violent spurting. At the end of the digestion process, the vessels were cooled in the air to room temperature and the residues were dissolved in 1.0 mL of HNO3 0.1 M and 10 mL of triply distilled water, filtered through a filter paper and transferred into a 50 mL flask, adjusted to pH = 5.5, and diluted with highly purified deionized water. Then the general procedure was applied for preconcentration and determination of Cd(II) and Pb(II).
Results and discussion
In this research, solid phase extraction with ionic liquid modified magnetic nanoparticles was developed for the first time. Cadmium and lead were chosen as an example to study the possibility of this method. It is necessary to investigate the effect of all the parameters that can probably influence the extraction performance. The effect of various parameters on the performance of the method was investigated in order to achieve the highest recovery. In all optimization steps concentration of Cd(II) and Pb(II) were 0.05 and 0.2 μg mL−1 respectively.
Effect of pH
Identification of the optimum pH is very important because it not only influences on the surface active centers and charge of sorbent, but also affects on the degree of ionization and solubilization of sorbatein aqueous solutions. The effect of pH on the recovery of metal ions was studied with pH varying from 2.0 to 9.0. At pH values higher than 9, lead and cadmium ions form complexes and precipitates with OH− and their retention is changed and the recovery of the method is decreased. At lower pH values, ligand is protonated, so recoveries are decreased due to competition between hydronium ions and desired metal ions. The results were shown in Fig. 3. The extraction recovery is initially increased by rising pH to 5.0 and then after pH 6.0, the extraction recovery starts to decrease. Thus, pH 5.5 seems to be a proper choice for both complexation and extraction.
Study of the concentration of PAN
PAN acts as a tridentate ligand and can form very stable complexes with metal ions through hydroxyl oxygen atom, nitrogen atom of pyridine and one of the azo group nitrogen atoms [27]. Metal ion complexes with PAN are sparingly soluble in water and can be extracted into organic solvents. The influence of PAN concentration on the extraction yields of Cd(II) and Pb(II) was studied in the concentration range of 2.0 × 10−6–4.0 × 10−5 mol L−1 of PAN (Fig. S1, ESM). As it can be seen, maximum absorbance was obtained at a concentration of 1.0 × 10−5 mol L−1 of the ligand and after that, absorbance approximately stays constant. Because of some other ions that could be present in real samples and probably react with PAN, a concentration of 2.0 × 10−5 mol L−1 was used in experiments. The extraction recovery was not affected by the addition of ligand excess. Therefore, the concentration of 2 × 10−5 mol L−1 was chosen for subsequent experiments.
Micro extraction time and amount of sorbent
The relationship between contact time and metal ions sorption onto newly modified magnetic nanoparticles was studied. The total extraction time relates to the time required for the complexation reaction and the transfer of hydrophobic complexes to modified magnetite through adsolubilization. Due to the relatively large surface area, the highly active surface sites lead to a very fast mass transfer process and generally offer a fast extraction process. The extraction recovery was studied at time intervals in the range of 2–10 min. It was observed that after 3 min of reaction time at room temperature the recoveries of the heavy metal ions exhibited no obvious variation. In the next step, the optimum amount of the sorbent for maximum take up was determined in the range of 10–100 mg. Accordingly, 40.0 mg of modified magnetite NPs were enough to preconcentrate the metal ions, at the studied concentrations, using 50 mL of sample. For higher amounts of modified magnetic NPs, the extraction efficiency was almost constant.
Desorption conditions
For selection of the best eluent, various concentrations of nitric acid and different organic solvents such as aceton, ethanol and ethanol (1 % HNO3) on the preconcentration yields of the metals were studied under the optimum conditions. The results are shown in Table S1 (ESM). Since the adsorption of Cd(II) and Pb(II) on modified nanoparticles at pH < 2 is found to be negligible, elution will be favored in acidic solution. Therefore it is not surprising the desorption ability of the nitric acid. As it can be seen, the nitric acid provided higher recovery efficiency compared to the other solutions. The minimum volume of nitric acid required for a quantitative elution of the retained analyte complexes was 2 mL. Desorption times were evaluated in the range of 1–15 min. The results showed that the time of 4 min is sufficient to quantitative desorption of the heavy metal ions by 2.0 mL of the 2.0 mol L−1 HNO3.
Effect of sample volume
Due to the low concentrations of trace metals in real samples, by using samples with large volumes, the trace metals in these volumes should be taken into smaller volumes for high preconcentration factor. Therefore, the maximum applicable sample volume was determined by increasing the dilution of metal ions solution. Fortunately, due to the magnetically assisted separation of the adsorbent, it is possible to collect the adsorbent from larger volumes of the sample solution. Thus the extraction of 2.5 μg and 10 μg of Cd(II) and Pb(II) ions respectively from different volumes of the water samples ranging from 100 to 600 mL were investigated (Fig. S2, ESM). The recovery was found to be stable until 400 mL and the adsorbed Cd(II) and Pb(II) could be eluted with 2.0 mL of eluent. Therefore the preconcentration factor 200 was achieved for both analytes.
Effect of ionic strength
The ionic strength of the working solution was varied from 0 % to 40 % (w/v) NaNO3. The rest of the experimental conditions were kept constant. No significant impact on the recovery was observed up to 5 % (w/v) NaNO3.
Sorption capacity
In order to determine the maximum amount of Pb(II) and Cd(II) ions retained on the modified magnetite nanoparticles, 60 mg of the adsorbent (in a beaker) was added to 50 mL of an aqueous solution containing 25 mg L−1 Pb(II) and 20 mg L−1 Cd(II) and 1 mL of PAN and buffer, and stirred it for 1 h and after decantation of the sorbent by applying an external magnetic field, the retained metal ions in the supernatant solution were determined by using FAAS. The maximum capacity was found to be 10.5 mg g−1 for Pb(II) and 9.8 mg g−1 for Cd(II).
Effect of coexisting ions
Effects of several common potentially interfering ions in the natural water and food samples on the recovery of Pb(II) and Cd(II) were studied by spiking appropriate amounts of the relative ions to 50.0 mL of the solutions containing 0.2 μg mL−1 Pb(II) and 0.05 μg mL−1 Cd(II) and excess amounts of PAN (as chelating agent) and they were treated according to the recommended procedure. An ion was considered to interfere when its presence produced a variation of less than ±5 % in the recoveries. The effects of different ions are shown in Table S2 (ESM). The obtained results evidenced that the presence of major coexisting ions had no significant influence on the preconcentration of the Pb(II) and Cd(II) under the selected conditions. However, some of the species tried, such as Co2+, Cu2+ and Ni2+ interfered with the determination of ions.
Analytical figures of merit
To evaluate the practical applicability of the SPE technique for determination of Pb(II) and Cd(II) several analytical performance characteristics such as preconcentration factor (PF), linear range, limit of detection (LOD) and relative standard deviation (R.S.D.) were investigated under the optimized conditions. Table 1 summarizes the analytical characteristics of the optimized method. The limit of detection (LOD = 1.61 and 0.122 μg L−1 for Pb(II) and Cd(II) respectively), defined as LOD = 3Sb/m, where Sb is the standard deviation of seven replicates of blank signals and m is the slope of the calibration curve after preconcentration. The precocentration factor (PF = 200) for Pb(II) and Cd(II), defined as the ratio of the initial volume to the final volume. Precision of the method (RSD = 2.87 % and 1.45 % for Cd(II) and Pb(II) respectively) was determined by ten replicate measurements of 80 and 400 μg L−1 for Cd(II) and Pb(II) respectively on optimum conditions. A good correlation coefficient (r = 0.9991 and 0.9998 for Pb(II) and Cd(II) respectively) was obtained. The calibration curve was linear in the range of 5–330 and 0.3–20 ng mL−1 of lead and cadmium, respectively. The recoveries of Pb(II) and Cd(II) are quantitative.
Accuracy of the method
In order to establish the validity of the method, certified reference materials NIST-1640A (Natural water) and NIM-GBW10011 (Wheat flour), were analyzed. The results listed in Table 2. The results found were in good agreement with the certified values of CRM.
Analysis of water and milk samples
In order to validate the developed method, for samples with different matrices containing varying amounts of diverse ions, the method was applied to preconcentration and determination of Pb(II) and Cd(II) in different water samples (Mineral water, river water and sea water). Only the seawater sample was filtered through a 0.45 μm membrane filter immediately after sampling. The results were summarized in Table 3. To examine the reliability and accuracy of the method, different amounts of the investigated metal ions were spiked into 50 mL of the water samples at optimum conditions. The results indicated that the present method can be reliably used for the determination of Pb(II) and Cd(II) with good recoveries in the range of 96.2 and 104.0 %.
The method was also applied to the determination of Pb(II) and Cd(II) in milk and milk powder to evaluate applicability of the preconcentration/determination method. Accuracy was assessed by comparing results with those obtained by using electrothermal atomic absorption spectrometry (ETAAS). Applying paired t-test no significant difference at 95 % confidence level was observed. The data obtained with the proposed and ETAAS methods for real samples were summarized in Table 4. The results of analysis of samples showed that the development method can be reliably used for the determination of Pb(II) and Cd(II) in different matrices.
Comparison of analytical performance data with literatures
Table 5 compares the characteristic data of the present method with those reported in literature. It is evident that high PF and sorption capacity, low LOD and RSD are achieved with this method. These significant features which obtained with the SPE are comparable to or even better than some of other methods which use very sensitive detection method such as ICP-OES. These characteristics are of key interest for routine trace metal ion laboratory.
Conclusions
Magnetite nanoparticle modified with 1-hexyl-3-methylimidazolium hexafluorophosphate has been introduced as a novel and effective adsorbent for preconcentration of Cd(II) and Pb(II) in various real samples. The method was proved to be simple, selective, fast and environment-friendly and was able to treat large-volume samples in a short period avoiding the time-consuming column passing and filtration steps. This method gives good accuracy, low limit of detection, high preconcentration factor and excellent precision and high kinetic sorption for the target analytes due to magnetically assisted separation of the adsorbent, which show its potentiality to analysis trace heavy metals in various samples with various matrixes. This method was successfully applied for determination of lead and cadmium at low concentrations in water and food samples. In this method sample preparation time was minimized by the fact that no centrifugation is required for phase separation and collection. Comparison of analytical features of this method with those of some other preconcentration-FAAS techniques (Table 5) indicates that the linear range and LOD of this method are better than or comparable with most of the other methods.
References
Ataro A, McCrindle RI, Botha BM, McCrindle CME, Ndibewu PP (2008) Quantification of trace elements in raw cow’s milk by inductively coupled plasma mass spectrometry (ICP-MS). Food Chem 111:243
Tuzen M, Parlar K, Soylak M (2005) Enrichment/separation of cadmium(II) and lead(II) in environmental samples by solid phase extraction. J Hazard Mater 121:79
Viard B, Pihan F, Promeyrat S, Pihan J-C (2004) Integrated assessment of heavy metal (Pb, Zn, Cd) highway pollution: bioaccumulation in soil. Graminaceae and land snails. Chemosphere 55:1349
Prasad K, Gopikrishna P, Kala R, Rao TP, Naidu GRK (2006) Solid phase extraction vis-a-vis coprecipitation preconcentration of cadmium and lead from soils onto 5,7-dibromoquinoline-8-ol embedded benzophenone and determination by FAAS. Talanta 69:938
Kagaya S, Sagisaka T, Miwa S, Morioka K, Hasegawa K (2006) Rapid coprecipitation technique with hybrid hydroxide system using ytterbium(III), gallium(III), and magnesium(II) for simultaneous concentration of 13 elements in concentrated salt solution prior to their inductively coupled plasma atomic emission spectrometric determination. Bull Chem Soc Jpn 79:717
Saito K, Taninaka I, Yamamoto Y, Murakami S, Muromatsu A (2000) Liquid–liquid extraction of platinum(II) with cyclic tetrathioethers. Talanta 51:913
Jia XY, Han Y, Liu XL, Duan TC, Chen HT (2010) Dispersive liquid–liquid microextraction combined with flow injection inductively coupled plasma mass spectrometry for simultaneous determination of cadmium, lead and bismuth in water samples. Microchim Acta 171:49
Ayata S, Bozkurt SS, Ocakoglu K (2011) Separation and preconcentration of Pb(II) using ionic liquid-modified silica and its determination by flame atomic absorption spectrometry. Talanta 84:212
Marahel F, Ghaedi M, Montazerozohori M, Nejati Biyareh M, Nasiri Kokhdan S, Soylak M (2011) Solid-phase extraction and determination of trace amount of some metal ions on Duolite XAD 761 modified with a new Schiff base as chelating agent in some food samples. Food Chem Toxicol 49:208
Malekpour A, Hajialigol S, Taher MA (2009) Study on solid-phase extraction and flame atomic absorption spectrometry for the selective determination of cadmium in water and plant samples with modified clinoptilolite. J Hazard Mater 172:229
Sombra L, Luconi M, Silva MF, Olsina RA, Fernandez L (2001) Spectrophotometric determination of trace aluminium content in parenteral solutions by combined cloud point preconcentration-flow injection analysis. Analyst 126:1172
Panahi HA, Mottaghinejad E, Badr AR, Moniri E (2011) Synthesis, characterization, and application of amberlite XAD-2-salicylic acid-iminodiacetic acid for lead removal from human plasma and environmental samples. J Appl Polym Sci 121:1127
Parham H, Pourreza N, Rahbar N (2009) Solid phase extraction of lead and cadmium using solid sulfur as a new metal extractor prior to determination by flame atomic absorption spectrometry. J Hazard Mater 163:588
Ngeontae W, Aeungmaitrepirom W, Tuntulani T (2007) Chemically modified silica gel with aminothioamidoanthraquinone for solid phase extraction and preconcentration of Pb(II), Cu(II), Ni(II), Co(II) and Cd(II). Talanta 71:1075
Xie F, Lin X, Wu X, Xie Z (2008) Solid phase extraction of lead (II), copper (II), cadmium (II) and nickel (II) using gallic acid-modified silica gel prior to determination by flame atomic absorption spectrometry. Talanta 74:836
Suleiman JS, Hu B, Peng H, Huang C (2009) Separation/preconcentration of trace amounts of Cr, Cu and Pb in environmental samples by magnetic solid-phase extraction with Bismuthiol-II-immobilized magnetic nanoparticles and their determination by ICP-OES. Talanta 77:1579
Tian M, Yan H, Row KH (2009) Solid-phase extraction of tanshinones from Salvia Miltiorrhiza Bunge using ionic liquid-modified silica sorbents. J Chromatogr B 877:738
Zhao XL, Shi YL, Ca YQ, Mou SF (2008) Cetyltrimethylammonium bromide-coated magnetic nanoparticles for the preconcentration of phenolic compounds from environmental water samples. Environ Sci Technol 42:1201
Zhao XL, Shi YL, Wang T, Cai YQ, Jiang GB (2008) Preparation of silica-magnetite nanoparticle mixed hemimicelle sorbents for extraction of several typical phenolic compounds from environmental water samples. J Chromatogr A 1188:140–147
Faraji M, Yamini Y, Rezaee M (2010) Magnetic nanoparticles: synthesis, stabilization, functionalization, characterization, and applications. J Iran Chem Soc 7:1
Pu XL, Jiang ZC, Hu B, Wang HB (2004) gamma-MPTMS modified nanometer-sized alumina micro-column separation and preconcentration of trace amounts of Hg, Cu, Au and Pd in biological, environmental and geological samples and their determination by inductively coupled plasma mass spectrometry. J Anal At Spectrom 19:984
Aboobakri SSE (2012) Magnetic nanoparticles with an imprinted polymer coating for the selective extraction of uranyl ions. Microchim Acta 178:89
Li J, Cai Y, Shi Y, Mou S, Jiang G (2008) Analysis of phthalates via HPLC-UV in environmental water samples after concentration by solid-phase extraction using ionic liquid mixed hemimicelles. Talanta 74:498
He C, Li S, Liu H, Li K, Liu F (2005) Extraction of testosterone and epitestosterone in human urine using aqueous two-phase systems of ionic liquid and salt. J Chromatogr A 1082:143
Liang P, Peng L (2010) Ionic liquid-modified silica as sorbent for preconcentration of cadmium prior to its determination by flame atomic absorption spectrometry in water samples. Talanta 81:673
Luo CZYHJ (2012) Ionic liquid-based hollow fiber supported liquid membrane extraction combined with thermospray flame furnace AAS for the determination of cadmium. Microchim Acta 177:53
Faraji M, Yamini Y, Saleh A, Rezaee M, Ghambarian M, Hassani R (2010) A nanoparticle-based solid-phase extraction procedure followed by flow injection inductively coupled plasma-optical emission spectrometry to determine some heavy metal ions in water samples. Anal Chim Acta 659:172
Tuzen M, Saygi KO, Soylak M (2008) Solid phase extraction of heavy metal ions in environmental samples on multiwalled carbon nanotubes. J Hazard Mater 152:632
Suleiman JS, Hu B, Huang C, Zhang N (2008) Determination of Cd, Co, Ni and Pb in biological samples by microcolumn packed with black stone (Pierre noire) online coupled with ICP-OES. J Hazard Mater 157:410
Mendil D, Tuzen M, Usta C, Soylak M (2008) Bacillus thuringiensis var. israelensis immobilized on Chromosorb 101: a new solid phase extractant for preconcentration of heavy metal ions in environmental samples. J Hazard Mater 150:357
Acknowledgements
Support of this investigation by The Research Council of University of Tehran through Grant is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 97 kb)
Rights and permissions
About this article
Cite this article
Davudabadi Farahani, M., Shemirani, F. Supported hydrophobic ionic liquid on magnetic nanoparticles as a new sorbent for separation and preconcentration of lead and cadmium in milk and water samples. Microchim Acta 179, 219–226 (2012). https://doi.org/10.1007/s00604-012-0885-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-012-0885-3