Abstract
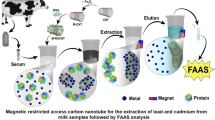
In this work, a new magnetic sorbent (cobalt ferrite nanoparticles modified with choline chloride: p-aminophenol deep eutectic solvent) was synthesized and used in magnetic dispersive solid phase extraction of heavy metals (Zn, Ni, Cu, Pb, Hg, and Cr) before their determination with inductively coupled plasma–optical emission spectrometry. In this method, firstly, the proteins of milk sample were precipitated by using trichloroacetic acid. Then, the magnetic sorbent was added into the clear supernatant phase obtained from pervious step and the mixture was vortexed. After isolating the sorbent particles in the presence of an external magnet, the supernatant phase was discarded and the sorbent was eluted using µL-level of ammonia solution. Finally, the eluent phase was injected into analytical instrument for the quantitative analysis. Under the optimized extraction conditions, satisfactory results including low limits quantification (1.4–2.1 ng mL−1) and detection (0.42–0.63 ng mL−1), high extraction recoveries (66–82%), and good precision with relative standard deviations ≤ 3.2 and 3.9% for intra– and inter day precisions, respectively) were obtained. In the last step, the suggested approach was used to determine the selected analytes in various milk samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since milk contains various nutritional materials such as vitamins, proteins, minerals, and enzymes that are necessary for having a healthy body, it is widely utilized all over the world [1]. Nevertheless, the milk samples can be polluted with various compounds such as heavy metals, pesticides, and antibiotics that can be threatful for the consumers [2,3,4]. Nowadays, food pollution with heavy metals is considered as a great concern considering the fact that their accumulation in living tissues can cause serious disorders and diseases (disturb the action of key organs such as liver, heart, and kidneys, affect central nervous system function, and DNA damage and cancer) [5]. The milk samples can be polluted with heavy metals by the polluted ingredients in the feeding of the cows. Considering the above–mentioned points, controlling the heavy metal residues in different foodstuffs especially the extensively used ones is crucial. To meet this goal, the development of a sensitive and efficient analytical method is the principal condition. Up to now, different analytical methods including flame atomic absorption spectrometry [6, 7], graphite furnace atomic absorption spectrometry [8, 9], inductively coupled plasma–atomic emission spectroscopy [10], and inductively coupled plasma–optical emission spectrometry (ICP–OES) [11, 12] have been employed for quantification of heavy metal. Despite the sufficient sensitivity of the mentioned instruments, a sample pretreatment step should be done to remove interfering compounds and preconcentration of the analytes prior to analysis the foodstuffs [13]. Over the years, various sample pretreatment methods like solid phase extraction (SPE) [14], dispersive solid phase extraction (DSPE) [15], magnetic dispersive solid phase extraction (MDSPE) [16], etc. have been developed to quench the thirsts for effective methods.
MDSPE is an efficient sample pretreatment method that has been developed to overcome the difficulties associated with SPE (being tedious and time–consuming and clogging the cartridges) and DSPE (the requirement of using centrifugation step for isolating the sorbent from sample solution) [17, 18]. In MDSPE process, a suitable magnetic sorbent is dispersed into a sample solution and after adsorption of the analytes onto the sorbent surface, the sorbent separation is done using an external magnet. After that, the analytes are eluted by a proper solvent to use in the following step 19, 20 and 21]. Efficiency of MDSPE procedure can significantly affected by the type of utilized sorbent. Cobalt ferrite (CoFe2O4) is an efficient magnetic sorbent which benefits from several advantages such as high physical and chemical stability, hardness, and suitable magnetization to be used in MSPE [22]. These properties provide the facile separation of the nanoparticles from sample solution via an external magnet. Also, extraction of target analytes can be performed by small amounts of the sorbent [23]. Despite the above-mentioned advantages of that CoFe2O4, limited interactions of the nanoparticles with analytes restricts their use in extraction methods without modifications. Thus, several materials like carbon nanotubes [24], graphene oxide [25], terephthalic acid [26], polyethyleneimine [27], etc. were used to enhance the selectivity and extraction capability of CoFe2O4. In recent years, the use of deep eutectic solvents (DESs) usage for modification of nanoparticles has attracted more attentions due to their more accessibility, easy preparation, inexpensiveness, and tunable properties [28]. DESs are new classes of ionic liquids which are prepared by mixing two or three substances. Hydrogen bonding interactions are formed between these substances and a stable, homogenous, and liquid solvents are obtained.
In the present study, a DES–modified CoFe2O4 nanoparticles was employed in the MDSPE of some heavy metals from milk samples. Milk is a widely consumed food which is full of different nutrients. The nutrients of milk are vital for child growth and human health. However, the main way to attain the benefits of milk is assuring it is free of heavy metals. As a result, establishment of an efficient and reliable method for the analysis of these compounds in milk samples is an important issue. In the present study, DES–modified CoFe2O4 nanoparticles were used for extraction of six metal ions before their analysis by ICP–OES. For this purpose, the DES formed from combination of choline chloride (ChCl) and. p-aminophenol was coated onto the CoFe2O4 nanoparticles. The free −NH2 group of the DES can form complexes with the analytes without the need for further chelating agent. The method indicated high extraction efficiency for the analytes in a complex sample like milk.
Experimental
Chemicals and solutions
ChCl, hydrochloric acid (37%, w/w), sodium hydroxide, acetic acid, formic acid, sodium chloride, p–aminophenol, trichloroacetic acid (TCA), sodium dodecyl sulfate (SDS), ammonia, Fe(NO3)3.9H2O, FeCl3.6H2O, Co(NO3)2.6H2O, Zn(NO3)2.6H2O, Ni(NO3)2.6H2O, Cu(NO3)2.6H2O, Hg(NO3)2, Pb(NO3)2, and Cr(NO3)3.9H2O were prepared from Merck (Darmstadt, Germany). A mixture stock solution of Zn(II), Ni(II), Cu(II), Pb(II), Cr(III), and Hg(II) (50 mg L−1) was prepared by dissolving appropriate amounts of above mentioned salts in deionized water. Working standard solutions were prepared daily by diluting of the stock solution with deionized water.
Instrumentation
Quantitative analysis of the evaluated metals was done on an ICP–OES model 9000 (Shimadzu, Japan). The instrument was worked at the following conditions: operating power, 2.0 kW; plasma temperature, 7000–8500 K; observation height, 10 mm above the work coil; auxiliary gas flow rate (Ar), 1.1 L min−1; coolant gas flow rate (Ar), 15 L min−1; carrier gas flow rate, 1.1 L min−1; and sample flow rate, 1.0 mL min−1. Zn, Cu, Hg, Ni, Pb, and Cr were quantified at the wavelengths of 213.8, 324.3, 253.6, 290.8, 193.7, and 220.4 nm, respectively. A Tescan scanning electron microscope (SEM), energy–dispersive X–ray (EDX) spectroscope (Tescan, Czech), and a Bruker (Billerica, USA) Fourier transform infrared spectrophotometer (FTIR) were used to characterize the sorbent. A vibrating sample magnetometer (VSM) model Lake Shore 7404 Cryotronics (Westerville, Ohio, USA) was used in evaluating the magnetic property of the sorbent. An ultrasonic water bath (Falc, model LBS1–6, Treviglio, Italy), A vortex mixer (Labinco, model L46, Breda, Netherlands), and a refrigerated Eppendorf™ centrifuge (Hamburg, Germany) were applied in the extraction procedure.
Real samples
Ten milk samples from different brands were bought from local supermarkets (Tabriz, East Azarbaijan province, Iran), transferred into laboratory, and kept in a refrigerator at 4 °C before their analysis. Also, one another milk sample was purchased from a village (Lighvan, East Azerbaijan, Iran) and employed as a blank.
Preparation of ChCl: p–aminophenol DES
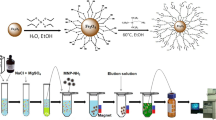
To prepare DES, ChCl (as HBA) and p–aminophenol (as HBD) were mixed with each other at a molar ratio of 1:1 in a glass test tube based on our previously reported paper [29]. In the following, this tube was heated in a water bath adjusted at 90 °C for 15 min (at intervals of 5–min, the tube was taken and vortexed for 1 min) to form DES.
Preparation of CoFe2O4 nanoparticles
For preparation the magnetic cobalt ferrite nanoparticles, ferric chloride solution (40 mL) and cobalt nitrate solution (40 mL) at the concentrations of 1 and 2 M (prepared in 2 M HCl solution) were mixed. Then, the mixture was combined with NaOH solution (500 ml of 0.7 M). The mixture was vortexed for 5 min and heated for 30 min at 90 ˚C [30]. After reaching the solution to room temperature, the nanoparticles were collected by an external magnet and eluted by nitric acid and deionized water. After that, 40 mL Fe (NO3)3 solution at a concentration of 0.5 M (for 30 min). Then, solid particles was separated by centrifugation and washed with deionized water. The solid compound was dried by keeping them in an oven for 2 h.
Functionalization of CoFe2O4 NPs with ChCl: p–aminophenol DES
In order to functionalize the surface of NPs with ChCl: p–aminophenol DES a previously published method was used [31]. For this purpose, The CoFe2O4 nanoparticles (200 mg) were mixed with 2 mL SDS solution (10%, w/v) (to enhance the physical binding of ChCl: p–aminophenol DES onto the nanoparticles and non-agglomeration of the particles) and 2 mL ChCl: p–aminophenol DES. Then, the mixture was placed into a sonication bath for 60 min adjusted at 40 ˚C. Then, the magnetic NPs were separated in the presence of magnet and left to dry on a watch glass.
Extraction procedure
A 5 mL milk sample containing the studied heavy metals or real sample was taken and poured into a glass test tube. After that, the milk proteins were precipitated by adding TCA (150 mg) into the samples and vortexing the mixture for 2 min. After centrifugation at 5000 rpm (for 2 min), the supernatant phase was taken and added into another glass test tube. After dissolving 0.5 g (5%, w/v) NaCl in the solution, 20 mg of the prepared sorbent was added into the solution and the mixture was vortexed to enhance the contact area of the sorbent and sample solution. After extraction, the sorbent was collected via an external magnet, and the supernatant phase was discarded. The adsorbed metallic ions onto the sorbent surface were desorbed by 100 µL ammonia solution (10%, v/v) under vortex agitation for 2 min. The eluent was taken and injected into ICP–OES system for determination of the studied cations.
Results and discussion
Characterization of CoFe2O4 NPs modified with ChCl: p–aminophenol DES
In this step, the synthesized sorbent was characterized using FTIR, SEM, EDX, and VSM analysis. The FTIR spectra of sorbent is shown in Fig. 1a. The observed broad band between 3000 and 3500 cm−1 is associated to the absorbance band of -OH group of the synthesized ChCl: p–aminophenol DES in the sorbent surface. The absorption band at 1373 cm−1 is related to the ring stretching vibration of DES resulted from its p-aminophenol component. These points can verify the modification of the nanoparticles surface with the DES. The morphology of sorbent was investigated using SEM technique. The resulted SEM image of the CoFe2O4 nanoparticles and the DES coated sorbent are shown in Fig. 1b, c, respectively. The images show spherical nanoparticles are obvious in CoFe2O4 nanoparticles. In the DES-coated CoFe2O4 nanoparticles, the DES are occupied the sorbent pores and a relatively monolithic structure was obtained in accordance with a previously published method [31]. The EDX analysis of the synthesized sorbent (data not shown) reveals that the sorbent contains C, N, O, Cl, Fe, and Co with the weight percents of 29.80, 6.01, 6.32, 23.54, 24.31, and 10.02%, respectively (the existence of C, N, and Cl in the composition of sorbent verifies its modification with DES) exactly the same as previously published method [31]. The magnetic property of the sorbent was also investigated and the superparamagnetic property of the sorbent with the saturation magnetization of 15.4 emu g−1. The data was comparable with the results of previously published method [31].
Optimization of MDSPE procedure
Optimization of TCA amount
The presence of different proteins in milk sample can restrict the analytes extraction. As a result, precipitating of milk proteins before performing an extraction process is necessary. In the present work, TCA was used as a chemical agent to precipate the proteins through decreasing their solubility in the sample. In the current step, the amount of TCA that can affect the efficiency of method was optimized. For this purpose, various experiments were done using 50, 100, 150, 200, 250, and 300 mg TCA. Based on the results in Fig. 2, the method efficiency enhances up to 150 mg and after that the method efficiency was not improved. It can be concluded that 150 mg is sufficient for precipitation of the proteins and remove their effect on the method efficiency [32]. Thus, next studies were done using 150 mg TCA.
Optimization of TCA amount. Conditions: sample, 5 mL blank milk spiked with 10 ng mL−1 of each analyte; vortexing time, 2 min; sorbent amount, 10 mg; agitation mode in adsorption step, vortexing; adsorption time, 5 min; elution solvent type (concentration, volume), acetic acid (10%, 100 μL); and desorption time, 3 min. The error bars show the minimum and maximum of three repeated determinations
Optimization of vortexing time
In the present work, the mixture of milk and TCA was vortexed to increase the contact area between them and effective precipitating the proteins of milk that can lead to obtain high extraction recovery (ER) and shorten the extraction time. To optimize this factor, various vortexing times were investigated between 0.5 and 4 min. Referring to the data, the ERs of analytes increased up to 2 min and remained constant at longer times. It can be concluded that the vortexing time of 2 min is enough time for complete dispersion and dissolving of TCA in the sample solution [32]. So, 2 min was used as vortexing time in the following steps.
Optimization of sorbent amount
Efficacy of a MDSPE procedure is highly depended to the amount of sorbent since it can alter the number of active adsorption sites. In the current step, the effect of sorbent amount was investigated in the range of 5–25 mg. Regarding to the results in Fig. 3, the ERs of analyte increase up to 20 mg and after that there is no significant difference between the data. It can be concluded that, 20 mg of the sorbent provides sufficient sites for extraction of the analytes and increasing the sorbent amount not improves the method efficiency [28]. So, 20 mg was selected as the optimum sorbent amount to continue the optimization steps.
Optimization of sorbent amount. Extraction conditions: are the same as those used in Fig. 2, except 150 mg TCA and 2 min were utilized as precipitating agent and vortex time
Optimization of agitation mode and time in adsorption step
In SPE based methods like MDSPE, the mixture of sample solution and sorbent was agitated to increase the analytes mass transfer rate from sample onto the sorbent to increase the method efficiency and reduce extraction time. In the present study, sonication and vortexing procedures were used to agitate the mixture of sorbent and sample solution in adsorption step. Referring to the results in Fig. 4a, by vortex agitation higher ERs were obtained which can be related to the effective dispersion of the sorbent in the sample solution [33]. Consequently, vortexing was opted to use in the following studies.
Optimization of agitation mode a and time b in adsorption step Conditions: a as similar as those used in Fig. 3, except 10 mg of sorbent was utilized. b are the same as those used in Fig. 4a, except vortexing was used
The vortexing time used for mixing the sorbent and sample solution is considered as adsorption time and it was assessed in the range of 0.5 to 5 min. Considering the analytical data in Fig. 4b, ERs of the analytes increase by enhancing the adsorption time time until 2 min and then remain constant. At the times ≥ 2 min, the analytes can extract completely using the sorbent. Thus, 2 min was selected to use in the rest of experiments.
Optimization of ionic strength
Regarding to the previously reported papers, adding a salt can increase the ionic strength of solution and influence ERs of the analytes through salting–out and salting–in effects [34]. Considering this point, the concentration of NaCl needed to be optimized. For this purpose, 0.0, 1.0, 2.5, 5.0, and 7.5%, w/v, NaCl were added to the sample solution. Based on the acquired results, up to 5%, w/v, NaCl salting–out effect is predominant (the solubility of analytes is decreased as a result of salt addition and the migration of them onto sorbent surface is enhanced) and ERs increase but at higher concentrations salting–in effect may become predominant. Also occupying the sorbent pores by salt ions can decrease the accessible sites for extraction of the analytes. So, the next studies were performed using 5%, w/v of NaCl.
Optimization of elution solvent type, concentration, and volume
To acquire an efficacious desorption of the adsorbed analytes from the surface of the sorbent, choosing a suitable solvent is critical. To find the best desorption solvent, three solvents solutions including ammonia, acetic acid, and formic acid at the concentration of 10%, v/v of each were tested. The results obtained in Fig. 5, revealed that the best ERs for analytes were obtained when ammonia solution was used as the eluent. It can be realted to the fact that, ammonia solution can desorb all analytes via formation of complex with the analytes compared to the DES. Therefore, this solvent was selected to use in the following experiments.
Optimization of elution solvent type. Extraction conditions: as similar as those used in Fig. 4, except the experiments were done using 2 min as adsorption time and 5%, w/v, NaCl
In the following, the concentration of ammonia solution was also evaluated in the range of 5–25%, v/v, to acquire the quantitative recoveries. Based on the outcomes in Fig. 6, 10%, v/v, of ammonia solution is sufficient for the effective desorption the studied heavy metals from the surface of magnetic sorbent and at higher concentrations of ammonia solution, no significant improvement was seen in the obtained results. Thus, the following experiments were done using 10%, v/v, ammonia solution.
Optimization of ammonia solution concentration. Extraction conditions: are the same as those used in Fig. 5, except ammonia was used as elution solvent
In the present work, the volume of elution solvent is another parameter that can affect the efficiency of desorption step, the enrichment factors (EFs), and consequently limits of detection (LODs). In the suggested MDSPE method, the volume of eluent should be chosen as low as possible to acquire low LODs which is important factor to sensitive analysis of the analytes. For optimizing this parameter, various volumes (50, 100, 150, 200, and 250 μL) of ammonia solution (10%, v/v) was used in the extraction procedure. Based on the obtained data (Fig. 7), 100 μL is sufficient for the effective elution of the analytes and was selected for the further studies. It can be attributed to the fact that 100 μL of ammonia solution is sufficient volume for desorption of whole analytes.
Extraction conditions: are the same as those used in Fig. 6, except the ammonia solution concertation was 10% v/v
Optimization of desorption time
In MDSPE methods, the time spent for desorption of the adsorbed analytes from the sorbents surface is defined as desorption time. This time can affect the elution of analytes and consequently ERs. In the present work, vortexing was utilized to agitate the mixture of sorbent particles and elution solvent. This time was varied in the range of 0.5 to 4 min. Based on the results (Fig. 8), 2 min is sufficient for the efficient elution of the analytes and was selected as the optimum time for the next studies.
Optimization of desorption time. Extraction conditions: are the same as those used in Fig. 7, except 100 µL ammonia solution was used as the elution solvent
Investigation of coexisting ions
Like other matrices, milk samples also may have various cations and anions that can interfere in quantification of the studied heavy metals. The interference effect can be seen when concentrations of the added ions or species cause significant changes in the analytical signals (higher than ± 5%). For investigating coexisting effect, 5 mL of blank milk sample spiked with the studied analytes at the concentration of 10 ng mL−1 and various concentrations of coexisting ions were analyzed using the offered method. The tolerable concentration ratios of the studied coexisting ions to the selected analytes are summarized in Table 1. The outcomes reveal that the evaluated ions do not interfere with the analytes determination at high concentrations.
Reusability
To evaluate the sorbent reusability in extraction of the analytes from milk samples, several experiments were done by 20 mg of the sorbent in the repeated applications. The obtained results depicted that there was no memory effect and the sorbent can be used at least for 3 times (relative standard deviations, RSD ≤ 7.3%) without obvious alteration in the method efficiency.
Analytical data
In the present step, some figure of merits of the offered method including limit of detection (LOD), limit of quantification (LOQ), linear range (LR), absolute recovery (AR), EF, correlation coefficient (r), and relative standard deviation (RSD) were evaluated to investigate the success of it. The results are listed in Table 2. The LODs and LOQs calculated based on signal to noise ratios of 3 and 10 ranged from 0.42–0.63 and 1.4–2.1 ng mL−1, respectively. The acquired ERs were in the range of 66–82%. The calibration curves for all analytes were obtained by performing the introduced method on seven spiked milk samples at different concentrations including 3, 5, 10, 25, 50, 75, and 100 ng mL−1 (each analyte). Linearity of the suggested method demonstrated by LRs was broad in the range of 2.0–100 ng mL−1 with r ≥ 0.995. In addition, to assess repeatability of the work, intra– and inter–day precisions were explored at concentration of 10 ng mL−1 of each analyte. They were less than 3.2 and 3.9% for intra– (n = 6) and inter–day (n = 4) precisions, respectively. The ARs and EFs were in the ranges of 66–82% and 33–41, respectively.
Analysis of milk samples
In this section, in order to investigate efficiency of the developed MDSPE process, the validated method was further assessed with analyzing ten milk samples. The samples were analyzed under optimal conditions. The data showed that Cu2+ and Zn2+ were found in all samples in the range of 246–475 and 179–269 ng mL−1, respectively. In the following, to study the matrix effect, five milk samples (out of ten) were randomly selected and the added–found method was applied on them. To acquire this aim, the milk samples along with a blank milk sample were spiked with studied metals at concentrations of 10, 25, and 50 ng mL−1 and after that were extracted and analyzed using the developed analytical method. The obtained mean relative recoveries (Table 3) reveal that the samples matrix effect is not significant in this study. All experiments were performed in triplicates and standard deviation of these results were calculated.
Comparison of the present work with previous ones
Some characters of the suggested approach including LOD, RSD, LR, AR, RR, and LOQ were compared with the previously reported works used in determination of the studied heavy metals. The outcomes are listed in Table 4. The results revealed that LODs and LOQ of analytes using the suggested method were lower than the other methods. The RSDs obtained with present work are comparable or better than previously reported ones. LRs of the current approach are wider than or comparable with those of other ones. Considering these points, the suggested method efficiently can be used in the determination of heavy metals in milk samples. The RR values of the method were comparable with other method while the introduced method has AR values less than the other method [37].
Conclusions
In the current work, a magnetic sorbent was prepared by coating CoFe2O4 with ChCl: p–aminophenol DES and utilized as sorbent in MDSPE of heavy metals from milk samples prior to their determination with ICP–OES. The suggested method is environmentally friendly and economical due to remove the conventional toxic and expensive complexing agents. The offered approach provides satisfactory results including low LODs (0.42–0.63 ng mL−1) and LOQs (1.4–2.1 ng mL−1), high ERs (66–82%), and wide LRs (1.8–100 ng mL−1). In addition, having no serious matrix effect assisted the method to be applied on different milk samples marketed in Tabriz, Iran to guarantee their safety.
Abbreviations
- ChCl:
-
Choline chloride
- DES:
-
Deep eutectic solvent
- ER:
-
Extraction recovery
- ICP–OES:
-
Inductively coupled plasma–optical emission spectrometry
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- LR:
-
Linear range
- MDSPE:
-
Magnetic dispersive solid phase extraction
- RSD:
-
Relative standard deviation
- SPE:
-
Solid phase extraction
References
L. Iannotti, E. Muehlhoff, D. Mcmahon, J. Dev. Eff. 5, 82 (2013)
Z. Suturović, S. Kravić, S. Milanović, A. Đurović, T. Brezo, Food Chem. 155, 120 (2014)
Ö.G. Manav, Ş Dinç-Zor, G. Alpdoğan, Microchem. J. 144, 124 (2019)
V. Samanidou, S. Nisyriou, J. Sep. Sci. 31, 2068 (2008)
L. Makedonski, K. Peycheva, M. Stancheva, Food Control 72, 313 (2017)
T. Okhravi, S.M. Sorouraddin, M.A. Farajzadeh, A. Mohebbi, Anal. Bioanal. Chem. 412, 1675 (2020)
F. de S. Dias, S.C. de S.R. Neto, L. de N. Pires, V.A. Lemos, (2020) Anal. Methods 12(6), 865-871
M. Tuzen, Food Chem. 80, 119 (2003)
J. Chen, S. Xiao, X. Wu, K. Fang, W. Liu, Talanta 67, 992 (2005)
H. Karami, M.F. Mousavi, Y. Yamini, M. Shamsipur, Anal. Chim. Acta 509, 89 (2004)
A.A. Fallah, S.S. Saei-Dehkordi, A. Nematollahi, T. Jafari, Microchem. J. 98, 275 (2011)
V. Chand, S. Prasad, Microchem. J. 111, 53 (2013)
A. Papadopoulos, N. Assimomytis, A. Varvaresou. Cosmetics 9, 21 (2022)
T. Daşbaşi, H. Muğlu, C. Soykan, A. Ülgen, J. Macromol. Sci. A 55, 288 (2018)
M.R. Afshar Mogaddam, M.A. Farajzadeh, S. Azadmard Damirchi, M. Nemati, J. Chromatogr. A 1630, 461523. https://doi.org/10.1016/j.chroma.2020.461523
A. Mohebbi, M.A. Farajzadeh, J. Chromatogr. A 1625, 461340 (2020)
A. Posyniak, J. Zmudzki, K. Mitrowska, J. Chromatogr. A 1087, 259 (2005)
E. Yavuz, Ş Tokalıoğlu, Ş Patat, Food Chem. 263, 232 (2018)
P. Montoro-Leal, J.C. García-Mesa, M.T. Siles Cordero, M.M. López Guerrero, E. Vereda Alonso, Microchem. J. 155, 104796 (2020)
A. Mohebbi, M.A. Farajzadeh, M.R. Afshar Mogaddam, M. Nemati, Food Anal. Methods 14, 1216 (2021)
Y. Sun, J. Tian, L. Wang, H. Yan, F. Qiao, X. Qiao, J. Chromatogr. A 1422, 53 (2015)
W. Li, J. Zhang, W. Zhu, P. Qin, Q. Zhou, M. Lu, X. Zhang, W. Zhao, S. Zhang, Z. Cai, Talanta 208, 120440 (2020)
M. Mehrabian, E. Noroozian, Sh. Maghsoudi, Microchem. J. 165, 106104 (2021)
C. Chen, J. Hu, D. Shao, J. Li, X. Wang, J. Hazard. Mater. 164, 923 (2009)
S. Su, B. Chen, M. He, B. Hu, Z. Xiao, Talanta 119, 458 (2014)
M. Zhang, W. Guo, Food Chem. 406, 135034 (2023)
H.R. Noormohamadi, M.R. Fathi, M. Ghaedi, J. Colloid Interface Sci. 531, 343 (2018)
MR Afshar Mogaddam, MA Farajzadeh, S Azadmard Damirchi, M Nemati, (2020) J. Chromatogr. A 1630, 461523
S.M. Sorouraddin, M.A. Farajzadeh, T. Okhravi, Sep. Sci. Technol. 55, 2955 (2020)
H. Abdolmohammad-Zadeh, E. Rahimpour, Anal. Chim. Acta 881, 54 (2015)
P.A. Mohammadzadeh Baghaei, M.R. Afshar Mogaddam, M.A. Farajzadeh, A. Mohebbi, S. M. Sorouraddin, J. Food Compost. Anal. 117, 105125 (123)
MA Farajzadeh, MR Afshar Mogaddam, (2016) J. Sep. Sci. 39, 1160
M. Rajabi, S. Arghavani-Beydokhti, B. Barfi, A. Asghar, Anal. Chim. Acta 957, 1 (2017)
M. Nemati, M.R. Afshar Mogaddam, M.A. Farajzadeh, M. Tuzen, J. Khandaghi, (2021) J. Chromatogr. A 1660, 462653
S. Arghavani-Beydokhti, M. Rajabi, A. Asghari, Appl. Organometal Chem. 32, e4279 (2018)
T. Daşbaşı, Ş Saçmacı, N. Çankaya, C. Soykan, Food Chem. 211, 68 (2016)
M. Ghaedi, K. Niknam, A. Shokrollahi, E. Niknam, H.R. Rajabi, M. Soylak, J. Hazard. Mater. 155, 121 (2008)
Acknowledgements
The authors thank the Research Council of the Islamic Azad University, Tabriz Branch for financial support and Tabriz University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There was no any conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rouhi, M., Abolhasani, J., Afshar Mogaddam, M.R. et al. Deep eutectic solvent modified cobalt ferrite nanoparticles in dispersive solid phase extraction of some heavy metals from milk samples prior to ICP–OES. J IRAN CHEM SOC 20, 2481–2490 (2023). https://doi.org/10.1007/s13738-023-02819-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02819-5