Abstract
We describe an on-chip microflow injection (μFI) approach for the determination of aminoglycoside antibiotics using chemiluminescence (CL) detection. The method is based on the inhibition of the Cu(II)-catalyzed CL reaction of luminol and hydrogen peroxide by the aminoglycosides due to the formation of a complex between the antibiotic and Cu(II). The main features of the method include small sample volumes and a fast response. Syringe pumps were used to insert the sample and the reagents into the microfluidic device. CL was collected using a fiber optic bundle connected to a luminescence detector. All instrumental, hydrodynamic and chemical variables involved in the system were optimized using neomycin as the aminoglycoside model. Inhibition is proportional to the concentration of the antibiotics. The dynamic ranges of the calibration graphs obtained for neomycin, streptomycin and amikacin are 0.3–3.3, 0.9–13.7, and 0.8–8.5 μmol L−1, and the detection limits are 0.09, 0.28 and 0.24 μmol L−1, respectively. The precision of the methods, expressed as relative standard deviation, is in the range from 0.8 to 5.0 %. The method was successfully applied to the determination of neomycin in water samples, with recoveries ranging from 80 to 120 %.

Chemical and instrumental systems of the method
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development of analytical methods involving the use of microfluidic devices is an interesting research trend due to the special features of these miniaturized systems, such as low reagent and sample consumptions, simple manipulation, low analysis time and portability. The integration of separation, reaction and detection in microfluidic chips, which is known as micro total analysis system (μ-TAS) or “lab-on-a-chip” system, is a general objective in the development of miniaturized methods, although it still requires the improvement of component integration for its consolidation and easy application to the analysis of real samples. However, several applications of microfluidic systems for food [1] and environmental [2] analysis have been recently described.
The use of microchips for electrophoretical separations (μCE) has been extensively studied in recent years, achieving very fast separations, at the level of seconds, with high efficiencies [3–6]. On the contrary, the development of liquid chromatography on microchips (μLC) is having a slower development, which is ascribed to the need of overcoming some technical limitations for the miniaturization of instrumental components, such as valves and pumps. In spite of these limitations, several μLC separations have been recently described [7].
Another interesting application area of microfluidic devices involves the development of microflow injection (μFI) systems, which allow the miniaturization of automatic analytical methods by integrating the reaction and the analytical measurements in a limited space. Optical detection is usually used in these systems due to its non invasive nature, rapid response and relatively easy coupling [8]. Absorbance and fluorescence detection have been used in some of these μFI systems. However, chemiluminescence (CL) detection is usually preferred owing to its inherent high sensitivity, low background noise and absence of excitation or stray light, as no light source is used [9]. Most μFI-CL methods described involve the use of luminol or a ruthenium(II) complex. For instance, the luminol-ferricyanide CL system has been used for the determination of glucose [10] and uric acid [11] in human serum, and nitrite in food [12], obtaining detection limits of 18 μg mL−1, 0.5 μg mL−1, and 4 ng mL−1, respectively, and a sample throughput between 12 and 30 h−1. The CL reaction of peroxidisulphate with the complex of ruthenium(II) with 1,10-phenanthroline [13, 14] or 2,2′-bipyridyle [15] has been mainly applied to the determination of therapeutic drugs in pharmaceutical formulations, reaching low detection limits, at the level of ng mL−1, and high sample throughputs. These data show the usefulness of μFI-CL systems for sensitive and fast determinations.
The method presented here is the first attempt to develop a new μFI-CL approach, involving a reverse format, for the determination of aminoglycoside (AG) antibiotics in water samples. Figure 1 shows the chemical structures of the three AG antibiotics assayed in this study, neomycin, streptomycin and amikacin. These compounds are highly polar and soluble in water and they inhibit the bacterial protein synthesis by binding irreversibly to the bacterial ribosome. After parenteral administration, they are excreted unchanged by glomerular filtration in the urine within 24 h. In recent years, AG antibiotics are less frequently applied in human medicine because of their severe adverse effects, such as oto- and nephrotoxicity, and also the availability of well-tolerated β-lactam antibiotics. However, they are frequently used in veterinary medicine for the treatment of bacterial infections such as mastitis, or for prophylaxis to prevent infection [16]. Also, streptomycin has been used as pesticide to control bacterial diseases in certain fruits such as apples [17]. The different applications of AG antibiotics have given rise to the fact that they are considered potential pollutants of aqueous environment owing to their stability and high solubility in water [18].
A high number of methods have been described for the determination of AG antibiotics, mainly using liquid chromatography, capillary electrophoresis and immunoassay, which allow their individual quantification [16, 19, 20]. However, the aim of the study presented here has been the development of a simple and fast method for screening purposes, which has been applied to the analysis of water samples. The method is based on the inhibitory effect of AG antibiotics on the reaction between luminol and hydrogen peroxide catalysed by Cu(II) in basic medium. This inhibition is ascribed to the formation of Cu(II)-AG antibiotic complexes [21].A conventional FI method has been previously described for amikacin determination using the same CL reaction [22], but the detection limit obtained was about twenty-times higher than that obtained for this antibiotic in the μFI method described here.
Experimental
Apparatus and instruments
A glass microreactor model FC_R150.332.2 with dimensions of 12 × 24 mm and an internal volume of 6 μl was assembled to a fluidic chipholder (4515) (Micronit, The Netherlands, www.micronit.com).The CL emission was collected using an optic fibre bundler assembled to a Cary Eclipse Varian spectrofluorimeter (Walnut Creek, CA, USA), which was used as a CL detector. The optic fibre bundler and the microchip were adapted to an X-Y-Z positioner (Oriel Instruments, USA, www.newport.com/oriel/) to allow fine positioning adjustments. The flow was driven through the microfluidic reactor using a KDS220 syringe pump (KD Scientific Inc., MA, USA, www.kdscientific.com) The injections of hydrogen peroxide and Cu(II) mixture and the cleaning solution were carried out with Cheminert VA-CN2 injection valves (Valco, Teknokroma, Barcelona, Spain, www.teknokroma.es). Nylon syringe filters with a pore size of 0.45 μm (Análisis Vínicos, Spain, www.analisisvinicos.com) were used for sample treatment.
Reagents
All chemicals used were of analytical grade. A 2 mmol L−1 luminol (Sigma) stock solution was prepared in 0.5 mol L−1sodium hydrogencarbonate/disodium carbonate (Panreac) buffer solution, adjusted to pH = 10.9 with sodium hydroxide. Copper(II) sulphate pentahydrate (0.01 mol L−1, Panreac), neomycin (200 μg mL−1, Fluka), amikacin (200 μg mL−1,Sigma) and streptomycin (200 μg mL−1, Fluka) solutions were prepared using deionized water, which was purified with a Milli-Q system (Millipore, Bedford, Ma, USA), and stored at 4 °C until use. Hydrogen peroxide solution (10 mmol L−1) was prepared daily by diluting adequately 33 % (w/v) hydrogen peroxide solution (Panreac) in water. The cleaning solution (CS) of the flow-system consisted on 0.4 mol L−1 nitric acid (Merck) solution. Other solutions used were 1 mol L−1sodium chloride (Merck), 1 mol L−1sodium nitrate (Sigma), 1 mol L−1potassium chloride (Merck),1 mol L−1potassium nitrate (Sigma),0.05 mol L−1 disodium hydrogenphosphate (Merck) and 0.05 mol L−1disodium tetraborate (Merck) solutions.
Procedures
Determination of aminoglycoside antibiotics
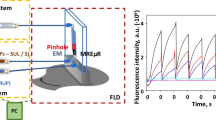
Figure 2 depicts a scheme of the microfluidic approach used, in which a syringe driven system (SDS) propels all solutions from five syringes, at a flow-rate of 1.4 μL s−1. Water was used as carrier (C), in which 5 μL of a mixture from two different syringes containing 10 mmol L−1 hydrogen peroxide and 200 μmol L−1 Cu(II) was inserted as reverse FI mode with an injection valve (IV1). Then the reagent plug merges in the microfluidic chip with a pre-mixed solution containing 0.2 mmol L−1 luminol solution and AG antibiotic standard or sample solution, prepared in the carbonate buffer solution (pH 10.9). A second injection valve (IV2) provides a cleaning cycle between each determination. The reactant mixture goes through the microreactor in which the CL reaction takes place. The signal is recorded at λ = 425 nm with an optic fibre (OF) connected to the luminescence detector. The OF was adapted to a micrometrical system which performs translational movements in a 3-D space extended along the X-Y-Z axes. Each standard or sample solution was assayed three times. The linear calibration graphs were obtained by plotting the analytical signal, which was the difference in the CL intensity obtained in the absence an in the presence of the analyte, versus the antibiotic concentration.
Scheme of the microfluidic system for aminoglycoside determination with chemiluminescence detection. L+S: luminol (0.2 mmol L−1) and sample mixture; C carrier (water); CS cleaning solution (0.4 mol L−1 HNO3). CLD chemiluminescence detector; PC personal computer; OF optic fibre; SDS syringe driven system; μFChip: microfluidic chip; IV 1 and IV 2 injection valves; xyz-PT xyz positioner; w 1, w 2 and w 3 wastes. [H2O2] = 10 mmol L−1; [Cu(II)] = 200 μmol L−1
Analysis of water samples
Two tap and two river water samples were analysed to determine neomycin, which was used as the analyte model. Each sample (1 mL) was spiked with 0.1 mL of standard neomycin solutions, containing 2.5 or 7.5 nmol, and filtered using a nylon syringe filter with a pore size of 0.45 μm. Then, 500 μL of 2 mmol L−1 luminol solution prepared in carbonate buffer solution (0.5 mmol L−1, pH = 10.9) was added. The solution was diluted to a final volume of 5 mL using deionized water and inserted into the microfluidic system as described above.
Results and discussion
Study of the miniaturized system
A FI system driven by a syringe pump was connected to the microfluidic device to monitor the CL intensity from the reaction between luminol and hydrogen peroxide catalyzed by Cu(II) and its decrease in the presence of neomycin, which was chosen as the AG antibiotic model. As can be seen in Fig. 2, five syringes were used to introduce the reactant solutions, the carrier and a cleaning solution in the microfluidic system. The distribution of the reactants in the syringes and the position of the optic fibre to measure the CL signal from the microfluidic device are two critical experimental variables of the system, owing to the temporal variation of the CL intensity during the development of the reaction.
Several assays were carried out to study the distribution of the reactants in the syringes. A reverse FI mode was selected, in which hydrogen peroxide and Cu(II) solutions were pre-mixed and injected into the flow, which contained the luminol solution in basic medium, as Fig. 2 shows. This reverse mode was chosen to avoid the accumulation of Cu(II) in the internal surface of the microfluidic device, which was observed by a continuous increase of the CL signal when the Cu(II) solution was introduced in the system in a direct mode. The analyte solution was pre-mixed sequentially with each reagent (hydrogen peroxide, Cu(II) and luminol), in order to select the best way to obtain the maximum difference in the CL intensity in the absence and presence of the analyte. The results obtained showed that the CL signal obtained in the presence of the analyte was only slightly lower than that obtained for the blank signal when the analyte was pre-mixed with hydrogen peroxide or Cu(II) solution. However, a relatively high CL inhibition, which was proportional to the analyte concentration, was attained when the analyte was pre-mixed with the luminol solution in basic medium. This behaviour can be ascribed to the fact that this distribution improves the interaction of the analyte with Cu(II), increasing its inhibitory effect. The catalyst/analyte concentration ratio in this instance is lower than in the other assays, in which the analyte was premixed with hydrogen peroxide or with Cu(II) solution. A 0.4 mol L−1 nitric acid solution was used as cleaning solution, which was injected after each standard or sample measurement, to avoid the accumulation of Cu(II) in the microfluidic channel. The assays carried out demonstrated that three injections can be executed without affecting the repeatability of the method.
The position of the optical fibre was fixed by using a micrometrical system in which the fibre was adapted. This system allows the movement of the fibre in a 3-D space extended along the X-Y-Z axes, as can be seen in Fig. 2. In order to enhance the sensitivity of the method, a 40 × magnification lens was coupled between the optic fibre and the microfluidic device.
Optimization of variables
The variables affecting the system were optimized following the univariate method. The analytical signal used was the net CL signal, which is the difference in the CL intensity emitted by the luminol reaction in the absence and in the presence of neomycin, as indicated above. Each analytical result was the average of at least three measurements. Table 1 summarizes the variables studied, the range assayed for each variable and the optimal values chosen.
The emission wavelength chosen was 425 nm, which corresponds to the emission of the aminophthalate ion formed by the oxidation of luminol. The study of the influence of the gate time in the net CL signal showed that it increased up to a value of 800 ms, remaining constant at higher values. The highest emission slit of the instrument, 20 nm, was chosen to obtain the maximum CL signal, bearing in mind that it is not interfered by potential stray radiation as it occurs in fluorimetry. The energy of the detector was studied in a range between 600 and 1000 V, obtaining an exponential increase in the CL signal as the energy was increased. A value of 800 V was chosen because higher values caused the saturation of the detector and the CL signal cannot be measured.
Regarding the hydrodynamic variables, the flow rate is crucial for reaching the maximum net CL signal. The influence of this variable was studied in the range of 0.1–2.1 μL s−1, obtaining an increase of the net CL signal by raising the flow-rate until an optimum value of 1.4 μL s−1. The CL signals obtained in the absence and in the presence of the analyte remained practically constant at higher flow-rate values. The study of the influence of the injection volume of the hydrogen peroxide and Cu(II) mixture solution was studied in an interval between 1 and 5 μL, obtaining increasing CL values as the injection volume increased. This last value was selected as optimal bearing in mind that the internal volume of the microfluidic device is 6 μL and, also, because a band broadening was obtained when higher values were assayed. The position of the optic fibre in the microfluidic system is a critical variable to obtain the maximum CL signal, as indicated above. This study was carried out by placing the microfluidic device and the fibre in a system that can be moved in the x-y-z directions (Fig. 2). The optimal CL signal was obtained when the fibre was placed 7.5 mm in the x axis, 0 mm in the y axis and 7.5 mm in the z axis.
Regarding the optimization of the chemical variables, Fig. 3a shows the influence of Cu(II) concentration on the system, which was studied in the range 10–300 μmol L−1. A 200 μmol L−1 concentration was selected for the development of the method. The influence of hydrogen peroxide concentration was assayed in the range 2.5–35 mmol L−1, obtaining that the net CL signal remained constant and maximum from a 10 mmol L−1concentration. The behaviour of the luminol concentration on the system is shown in Fig. 3b, in which can be seen that the best CL signal was obtained for a 0.2 mmol L−1 luminol concentration. Figure 3c shows the influence of the pH on the system, in which can be seen that the CL signal was independent of this variable in the pH range of 10.3–11.2, choosing a value of 10.9 for the development of the method. A borate buffer solution was assayed to fit this pH, but it decreased the CL signal. Better results were obtained using phosphate and carbonate buffer solutions, selecting the last one for the development of the method. The influence of the concentration of this buffer solution was studied in an interval 10–100 mmol L−1, achieving the best CL signal for a value of 50 mmol L−1. Several salts were assayed in order to evaluate the influence of the ionic strength. The addition of sodium or potassium nitrate, in a concentration interval from 0 to 500 mmol L−1 did not modify practically the CL signal, but a slight decrease was obtained in the presence of sodium or potassium chloride.
Analytical features of the method
Table 2 shows the features of the calibration graphs obtained for neomycin, streptomycin and amikacin under the optimum experimental variable values. The limits of detection (LOD), calculated according to IUPAC recommendation [23], are also included, in which can be seen that neomycin shows the lower LOD value because this antibiotic, which is a four-ring AG antibiotic, binds Cu(II) with higher affinity than the three-ring AG antibiotics, such as streptomycin and amikacin [21]. These compounds coordinate Cu(II) ions mostly by their terminal amino sugar donors, while neomycin binds Cu(II) by its deoxystreptamine residue, improving the stability of the complex.
Precision data, expressed as the percentage of relative standard deviation, are also shown in Table 2. These values were obtained for two concentration levels of each analyte (n = 10), the lowest concentration of each calibration graph, which corresponds to the quantification limit, and an analyte concentration corresponding to the centre of the calibration graph. The values obtained ranged between 0.8 and 5.0 %. The estimated sampling frequency under the working conditions was about 15 h−1, including three measurements and the corresponding cleaning steps, which is a relatively low value owing to the reverse mode used, although similar sample throughputs have been described in other μFI methods [10, 11].
A comparison of the figures of merit of this work with those of other methods for the determination of aminoglycoside antibiotics involving CL detection [22, 24–30] is shown in Table 3. As can be seen, the luminol-hydrogen peroxide-Cu(II) system has been described for the CL detection of amikacin alone [24] and AG mixtures [25] by liquid chromatography, reaching very low LOD values in this last method, which includes a previous preconcentration step. However, a relatively high LOD for amikacin was obtained by using the same CL system in a conventional FI method [22]. Conventional FI methods using other luminol systems have been also described for several individual AG antibiotics [26–28], but the method described here is the first one which uses a microfluidic system for AG determination using CL detection.
Applications
The method was applied to the analysis of two tap and two river water samples using the procedure above described. Aliquots of the samples were first analysed to check the potential presence of AG antibiotics but no analytes were detected. A recovery study was carried out by adding two different neomycin amounts to each sample, so that the analyte final concentrations were 0.5 and 1.5 μmol L−1. Table 4 shows the recovery values obtained, which ranged between 80.0 and 120.0 %.
Conclusions
The method describes for the first time the miniaturized determination of AG antibiotics by developing a FI system in a microfluidic device. The results obtained confirm the usefulness of this approach for quantitative purposes, using the inhibition caused by these compounds on the CL reaction between luminol and hydrogen peroxide catalyzed by Cu(II). The use of microfluidics provides several advantages, such as simplicity and low consume of sample and reagents. Also, the method allows the fast automatic determination of AG antibiotics and can readily be adapted to the miniaturized determination of other analytes, owing to the versatility of the system. Thus, for instance, the use of microfluidic systems is a trend in the development of new bioassays [31].
References
Atalay YT, Vermeir S, Witters D, Vergauwe N, Verbruggen B, Verboven P, Nicolaï BM, Lammertyn J (2011) Microfluidic analytical systems for food analysis. Trends Food Scien Technol 22:386–404
Jokerst JC, Emory JM, Henry CS (2012) Advances in microfluidics for environmental analysis. Analyst 137:24–34
Ríos A, Escarpa A, González MC, Crevillén AG (2006) Challenges of analytical microsystems. Trends Anal Chem 25:467–479
Escarpa A, González MC, López-Gil MA, Crevillén AG, Hervás M, García M (2008) Microchips for CE: breakthroughs in real-world food analysis. Electrophoresis 29:4852–4861
Ohno KI, Tachikawa K, Manz A (2008) Microfluidics: applications for analytical purposes in chemistry and biochemistry. Electrophoresis 29:4443–4453
Kenyon SM, Meighan MM, Hayes MA (2011) Recent developments in electrophoretic separations on microfluidic devices. Electrophoresis 32:482–493
Kutter JP (2012) Liquid phase chromatography on microchips. J Chromatogr 1221:72–82
Kuswandi B, Nuriman, Huskens J, Verboom W (2007) Optical sensing systems for microfluidic devices: A review. Anal Chim Acta 601:141–155
Zhang Z, He D, Liu W, Lv Y (2005) Chemiluminescence micro-flow-injection analysis on a chip. Luminescence 20:377–381
Lv Y, Zhang Z, Chen F (2003) Chemiluminescence microfluidic system sensor on a chip for determination of glucose in human serum with immobilized reagents. Talanta 59:571–576
He D, Zhang Z, Huang Y, Hu Y, Zhou H, Chen D (2005) Chemiluminescence microflow injection analysis system on a chip for the determination of uric acid without enzyme. Luminescence 20:271–275
He D, Zhang Z, Huang Y, Hu Y (2007) Chemiluminescence microflow injector analysis system on a chip for the determination of nitrite in food. Food Chem 101:667–672
Al Lawati HAJ, Suliman FEO, Al Kindy SMZ, Al-Lawati AM, Varma GB, Nour IEM (2010) Enhancement of on chip chemiluminescence signal intensity of tris(1,10-phenantroline)-ruthenium(II) peroxydisulphate system for analysis of chlorpheniramine maleate in pharmaceutical formulations. Talanta 82:1999–2002
Al Lawati HAJ, Al Dahmani ZM, Suliman FEO, Al Kindy SMZ, Al-Lawati AM (2011) Analysis of fexofenadine in pharmaceutical formulations using tris(1,10-phenantroline)-ruthenium(II) peroxydisulphate chemiluminescence system in a multichip device. Luminescence 26:762–767
Al Lawati HAJ, Al Gharibi E, Al Kindy SMZ, Suliman FEO, Al-Lawati AM (2011) High throughput method for the analysis of cetrizine hydrochloride in pharmaceutical formulations and in biological fluids using a tris(2,2′-bypyridyl)ruthenium(II)-peroxydisulphate chemiluminescence system in a two-chip device. Talanta 85:906–912
McGlinchey TA, Rafter PA, Regan F, McMahon GP (2008) A review of analytical methods for the determination of aminoglycoside antibiotics and macrolide residues in food matrices. Anal Chim Acta 624:1–15
Mayerhofer G, Schwaiger-Nemirova I, Kuhn T, Gisch L, Allerberger F (2009) Detecting streptomycin in apples from orchards treated for fire blight. J Antimicr Chemother 63:1076–1077
Diaz-Cruz MS, Barceló D (2006) Determination of antimicrobial residues and metabolites in the aquatic environment by liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 386:973–985
Stead DA (2000) Current methodologies for the analysis of aminoglycosides. J Chromatogr B 747:69–93
García-Ruiz C, Marina ML (2006) Recent advances in the analysis of antibiotics by capillary electrophoresis. Electrophoresis 27:266–282
Jeżowska-Bojcuk M, Szczepanik W, Mangani S, Gagelli E, Gagelli N, Valensin G (2005) Identification of copper(II) binding sites in the aminoglycosidic antibiotic neomycin B. Eur J Inorg Chem 15:3063–3071
Ramos-Fernández JM, Bosque-Sendra JM, García-Campaña AM, Alés-Barrero F (2005) Chemiluminescence determination ok amikacin based on the inhibition of the luminol reaction catalyzed by copper. J Pharm Biomed Anal 36:969–974
Long GL, Winefordner JD (1983) Limit of detection. A closer look at the IUPAC definition. Anal Chem 55:712A–724A
Serrano JM, Silva M (2006) Determination of amikacin in body fluid high-performance liquid–chromatography with chemiluminescence detection. J Chromatogr B 843:20–24
Serrano JM, Silva M (2006) Rapid and sensitive determination of aminoglycoside antibiotics in water samples using a strong cation-exchange chromatography non-derivatisation method with chemiluminescence detection. J Chromatogr A 1117:176–183
Yang C, Zhang Z, Wang J (2010) New luminol chemiluminescence reaction using diperiodatoargentate as oxidate for the determination of amikacin sulfate. Luminescence 25:36–42
Wan G-H, Cui H, Zheng H-S, Pang Y-Q, Liu L-J, Yu X-F (2006) Flow-injection determination of streptomycin residues in milk using the luminol-periodate-Mn2+ chemiluminescence system. Luminescence 21:36–42
Santos LHMLM, Araújo AN, Reis B, Montenegro MCBSM (2010) Development of a multicommutated flow system with chemiluminometric detection for quantification of gentamicin in pharmaceuticals. J. Aut. Met. Manag. Chem, Article ID, 913207
Fernández-Ramos JM, García-Campaña AM, Alés-barrero F, Bosque-Sendra JM (2006) Determination of gentamicin in pharmaceutical formulations using peroxyoxalate chemiluminescent detection in flow-injection analysis. Talanta 69:763–768
Li B, Zhang Z, Wu M (2000) Flow injection chemiluminescence determination of gentamycin using on-line electrogenerated Co(III) as the oxidant. Anal Lett 33:1577–1590
Hartwell SK, Grudpan K (2010) Flow based immune/bioassay and trends in micro-immuno/biosensors. Microchim Acta 169:201–220
Acknowledgments
Authors gratefully acknowledge financial support from the Spanish MICINN (Ministerio de Ciencia e Innovación) (Grant No. CTQ2009-08621/BQU), the Junta de Andalucía (Grant No. PO9-FQM-493) and the FEDER-FSE program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sierra-Rodero, M., Fernández-Romero, J.M. & Gómez-Hens, A. Determination of aminoglycoside antibiotics using an on-chip microfluidic device with chemiluminescence detection. Microchim Acta 179, 185–192 (2012). https://doi.org/10.1007/s00604-012-0878-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-012-0878-2