Abstract
Antimicrobials are used in large quantities in human and veterinary medicine. Their environmental occurrence is of particular concern due to the potential spread and maintenance of bacterial resistance. After intake by the organisms, the unchanged drug and its metabolized forms are excreted and enter wastewater treatment plants where they are mostly incompletely eliminated, and are therefore eventually released into the aquatic environment. The reliable detection of several antimicrobials in different environmental aqueous compartments is the result of great improvements achieved in analytical chemistry. This article provides an overview of the more outstanding analytical methods based on liquid chromatography tandem mass spectrometry, developed and applied to determine antimicrobial residues and metabolites present in surface, waste, and ground waters.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pharmaceuticals have been identified as emerging environmental contaminants even though it must be assumed that they have been present in different aqueous environmental compartments ever since their introduction. Many studies have highlighted the spread of pharmaceutical residues around the world. In surface water alone, around 100 pharmaceutical compounds and metabolites have been detected. Despite these pollutants having been designed to have specific biological effects in humans and animals, they have received comparatively little attention [1].
Among pharmaceuticals, antimicrobials are of particular concern because they can induce bacterial resistance [2] through continuous exposure, which results in untreatable diseases. These effects have been reported to occur in different aqueous compartments, such as waste effluents of pharmaceutical plants and hospitals [3, 4].
Antimicrobial agents, also known as antibacterial or anti-infectives, comprise synthetic and natural compounds. The term antibiotic originally meant only natural substances produced by bacteria or fungi, but today it is used to refer to both synthetic (or semi-synthetic) compounds, such as sulfonamides (SAs) and quinolones, and natural compounds, such as penicillins (PENs) and tetracyclines (TCs). In this review, the term antimicrobial refers to antibacterial antibiotics, since other antimicrobial agents such as antifungal or antiparasitics are beyond the scope of the present work.
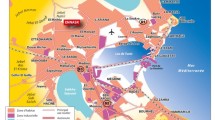
The large quantities of antimicrobials used in human (to treat microbial diseases) and veterinary medicine (to prevent and to treat diseases, and as supplement to promote growth in food animals) have led to their occurrence in the environment. After their medicinal application and excretion via urine and feces, residual human antimicrobials mainly enter municipal sewage treatment plants. The frequent detection in wastewater treatment plant (WWTP) effluents indicates the incomplete removal of many antimicrobials. Most of the veterinary drugs used are antimicrobials, which mainly reach the aquatic environment via animal manure [5], almost in 90 % unchanged form, through direct urination or defecation on the fields or through dispersion on agricultural lands as fertilizer. Figure 1 shows the evolution in manure produced by confined animals in farms of all USA states in ten years (1987–1997).
Distribution of dry manure production in all USA animal farms. Data from the Economic Research Service United States Department of Agriculture, http://www.ers.usda.gov/data/manure
Depending on the mobility of antimicrobials and metabolites in the soil system, they may threaten the surface and ground waters, affecting both terrestrial and aquatic organisms [6]. Overland flow has recently been identified as a pathway through which veterinary antimicrobials, such as sulfonamides and tetracyclines, may be transported to surface waters from arable lands previously treated with animal manure [7]. The same picture is found when the sludge originating in WWTPs is used to fertilize soils. The direct introduction of antimicrobials used in veterinary medicine into surface waters is also possible through fish farming [8]. Antimicrobials are widely used in aquaculture, and the main groups used are TCs and SAs [9].
Production data for antimicrobials and pharmaceuticals in general is not available; however, it is known that large quantities of antimicrobials are used not only with therapeutic purposes but also for growth promotion of farm animals and as additives in soaps, creams, and disinfectants. According to the information reported [10, 11] the use of antimicrobial substances in human prescriptions in comparison with the use in veterinary medicine is balanced in the European Union, whereas in the USA the total amount used in animals is nearly tenfold higher.
Despite the fact that the individual amounts of antimicrobials and their metabolites introduced into the environment are likely low, continuous introduction can lead to cumulative high long-term concentrations, thus promoting the possibility for continual but unnoticed adverse effects, which may accumulate so slowly than changes remain undetected until the effects become irreversible.
For multi-analyte determinations in the analysis of antimicrobials, chromatographic techniques are an excellent tool. Gas chromatography (GC) has a high resolving power owing to the use of capillary columns; however, liquid chromatography (LC) is getting more popular, in part owing to advances achieved through the development of atmospheric pressure ionization (API) interfaces. Therefore, LC has become the technique of choice for the analysis of antimicrobials, which are rather polar, non-volatile, and in some cases thermally labile, without the need for derivatization.
Degradation products and metabolites are often forgotten when analyzing antimicrobials; a notable exception is the macrolide erythromycin, which has been determined as its dehydro-form in acidic samples [12, 13, 39, 57].
The aim of this review is to provide a number of analytical methods based on liquid chromatography tandem mass spectrometry (LC-tandem MS) developed for the analysis of the antimicrobials mostly present in surface, ground, and wastewaters. These methods are characterized by their extremely high sensitivity and selectivity, which are required by the low concentrations of these substances found in the environment. In previous papers [14, 15], the authors have reviewed the state-of-the-art in the environmental analysis of pharmaceuticals, including antimicrobials, and these reports were devoted mainly to sample preparation, stability, degradation, and matrix effects. In the present review, we focus on the advances achieved in MS detection and on recently reported data on the analysis of metabolites. Finally, some information regarding the removal of antimicrobials in wastewater treatment procedures is also included.
Physico-chemical properties
Often information on the physical and chemical properties of a drug, e.g., the octanol/water partition coefficient (K ow), distribution coefficient (K d), dissociation constants (pK as), vapor pressure or Henry’s law constant (K H), will help to determine whether the compound is most likely to concentrate in the aquatic, terrestrial, or atmospheric environments. Antimicrobials have diverse physico-chemical properties as shown in Table 1. Compounds with high logK ow values may show affinity to sludge or soil, whereas high K d values indicate the tendency for compounds to be adsorbed onto soil materials through the phenomena of adsorption distribution or solid/liquid partition. Both factors contribute to the reduction of their concentrations in the aqueous phase.
Tetracyclines, whose name is derived from the four rings forming their chemical structure, are photodegradable amphoteric substances stable in acids but not in bases, which tend to bind divalent and trivalent metal ions, silanolic groups, and proteins [16, 17] as result of the presence of two ketone groups in their molecular structure. Sulfonamide antimicrobials are generally derivatives of sulfanilamide which have amphoteric properties, but mainly behave as weak acids, due to the N-H bond of the sulfonamidic group, and are able to form salts in strongly acidic or basic media [17]. Aminoglycoside antimicrobials, which are generally composed of two or three aminosugars linked between them by a glycosidic bond, are also susceptible to photodegradation. The high number of amino and hydroxyl moieties are responsible of their strong polar properties. Therefore, these basic compounds are characterized by their high water and poor lipid solubility [17]. Macrolides feature a common nucleus, a lactone macrocycle, to which one or two sugars are attached. This class of antimicrobials are weak bases characterized by their high molecular weight only comparable to polypeptides and polyether antimicrobials (PEs) [17]. The β-lactam antimicrobials (the most used antimicrobials in human medicine) are thermolabile compounds with a limited stability due to the presence of a four-membered (β-lactam) ring in their structure; they are unstable in alcohols and isomerize in acid medium. Quinolones resist acid and basic hydrolysis and between pH 6 and 8 exhibit poor water solubility, but are lipid soluble; however, they are prone to UV-light degradation [17]. Polyether antibiotics are composed of multiple cyclic ethers with a carboxylic acid group at one end and a terminal alcohol group to the other. These compounds are rather lipophilic, and exhibit poor solubility in water, which is even reduced when mono and divalent metal ions are present due to the formation of lipid-soluble cyclic complexes.
Metabolites
After consumption, antimicrobials are metabolized in the organism to different extents and are therefore often excreted only slightly changed (see Fig. 2). In some cases, one metabolite of a certain drug may be the predominant form found in the environment. In general, metabolism takes place in two steps. In the first step, reactive functional groups are introduced into the molecule mainly through oxidation, reduction, or hydrolysis reactions. Finally, the parent drug or its first-step metabolite is covalently bound to polar molecules present in the body, such as sugars, sulfates, and acids. As a consequence, metabolites are more polar than the parent compound, thereby being easily excreted by the organism. It is known that under certain environmental conditions or wastewater treatment procedures, excreted metabolites may be transformed back to the parent compound, for instance through de-glucuronidation. An early study by Berger et al. [18] revealed the back transformation of the glucuronide of amphenicol and N 4-acetylsulfametazine to the active parent compounds during the storage of liquid manure and suggested a possible similar behavior for other N 4-acetylated SAs.
Mean excretion rates (%) for selected antimicrobials and metabolites. Data from Hirsch et al. [22]
The inactive N 4-acetylsulfamethoxazole first-step metabolite of the sulfonamide sulfamethoxazole, which is the most prescribed antimicrobial in human medicine, is known to be excreted in 50 % of the administered dose [19]. In lower rates, 20 % and 13 %, the second-step metabolites of the macrolide clarithromycin, 14-OH-(R)-clarithromycin and 14-OH-(R)-N-demethyl-clarithromycin, respectively, are excreted after undergoing hydroxylation and N-demethylation.
Little is known about the occurrence of antimicrobial metabolites in the environment. This may mainly be attributed to their relatively high polarity and to the lack of reference substances, which both hinder their analytical determination. In order to better assess the occurrence of antimicrobials in the environment Gobel et al. [20] have pointed out the need to consider the metabolites of dosed drugs. In their work, the elimination of N 4-acetylsulfamethoxazole and dehydro-erythromycin were studied among other residues, and for the acetylated form a tentative fragmentation process was presented. In an earlier study, Hilton and Thomas [21] included N 4-acetylsulfamethoxazole among the pharmaceuticals investigated in effluent and surface water. Findings indicated that while sulfamethoxazole could not be quantified in any sample (<50 ng L−1), N 4-acetylsulfamethoxazole was present in all samples and at quite high concentrations (<50–2,200 ng L−1). Regarding erythromycin, it is never detected in its original form but as a degradation product with the loss of one molecule of water. This dehydration process is known to occur in acidic aqueous solution (pH<7). In order to elucidate which form is mainly present in environmental waters Hirsh et al. [22] carried out a simple experiment consisting of extracting spiked samples with erythromycin at different pH values. The findings indicated that only the degradation product could be detected; thus, the dehydration process takes place in the natural aquatic environment, contrary to earlier explanations pointing out the formation of the degradation product during the ionization process when analyzing by mass-spectrometry-based methods. In this work, in addition to erythromycin other antimicrobials were investigated in wastewater treatment plant effluents and surface waters. The highest concentration in the effluents was reached by dehydro-erythromycin with a mean value of 2,500 ng L−1 (maximum of 6,000 ng L−1).
The study of degradation products and epimers of TCs in the environment has scarcely been addressed. This can be likely due to the low proportion relative to the parent TC in which they are formed, especially for degradation products. These products are known to be formed through hydrolysis and photolysis reactions yielding the epi-tetracyclines, anhydro-tetracyclines, and iso-tetracyclines. Nevertheless, their consideration is important because of degradation products are known to be more soluble in water phases than the parent compounds, which increases their mobility potential. In recent work, Halling-Sorensen et al. [23] evaluated the occurrence of oxytetracycline (OTC) in soil interstitial water as regards the parent and eight degradation products, namely, 4-epi-OTC, α- and β-Apo-OTC, 4-epi-N- and N-desmethyl-OTC, 4-epi-N- and N-didesmethyl-OTC, and 2-acetyl-2-decarboxamido-OTC. Results indicated that OTC and 4-epi-OTC were the only compounds found to be at significant concentrations in soil interstitial water, whereas all other degradation products were present at <2 % relative to OTC.
Removal in WWTPs
A variety of antimicrobial residues have been found in WWTP effluents in concentrations up to the low μg L−1 range. Mean values for receiving surface waters are generally around one order of magnitude lower than the median values for the WWTP effluents. Elimination rates differ considerably depending on the compound, on the environmental conditions, and on the process conditions with adsorption and degradation being the main processes taking place during the wastewater treatment for the removal of contaminants. In a recent paper, Castiglioni et al. [24] classified a number of pharmaceuticals, including a few antimicrobials, depending on the seasonal variation in their removal rates; the following three groups were defined: those most removed in summer, those with no seasonal influence on their elimination, and those not removed either in winter or in summer.
Hydraulic retention times are generally shorter than the degradation half-lives of most antimicrobials entering WWTPs, and as a result polar compounds are discharged into the effluents before complete degradation can occur. According to several authors [25–30], elimination rates of antimicrobials during the sewage treatment are between 50 and 99 %. In general, more polar antimicrobials are not effectively eliminated during the process, whereas rather lipophilic compounds are more easily removed from the waste water by adsorption on the sewage sludge through hydrophobic interactions [31, 39]. β-Lactams are expected to be easily eliminated in WWTPs due to the lability of the β-lactam ring towards chemical and microbial degradation; however, concentrations up to 330 ng L−1 of clarithromycin were detected in WWTP effluents in Sweden [26]. TCs and quinolones are effectively removed by retention in sludge through precipitation as metal complexes and by adsorption through hydrophobic interactions, respectively [27–29]. Low removals (around 60 %), however, have been reported for SAs and trimetoprim [30, 32].
Regarding the occurrence of antimicrobials during wastewater treatment procedures, in general, studies have addressed final effluents [33, 34, 57]. Raw influents have scarcely been considered [20, 32, 35]; nevertheless, for a complete assessment of the suitability of current treatments, the study has to be extended to primary, secondary, and tertiary effluents (whenever these exist). Golet et al. have followed such an integrated approach [20] in the study of selected macrolides, sulfonamides, and the metabolites N 4-acetylsulfamethoxazole and dehydro-erythromycin along the wastewater treatment process in two municipal WWTPs in Switzerland. Results indicated that the compounds investigated are not efficiently eliminated, and therefore they reach surface waters. Tertiary treatment seems to not efficiently decrease the concentration of contaminants present in the secondary effluent; on the contrary, in quite a number of antimicrobials their concentrations increased in the tertiary effluent, for instance sulfapyridine and the acetylated metabolite. As regards N 4-acetylsulfamethoxazole, it is eliminated exclusively in the primary treatment (mechanical process) with concentrations of 518 and 943 ng L−1 in primary effluents, 86 ng L−1 and <LOQ in secondary effluents and 82 and 71 ng L−1 in the final tertiary effluent. The high removal rate of N 4-acetylsulfamethoxazole has to be considered when evaluating the extent of elimination of sulfamethoxazole along wastewater treatment, since the removal of the parent form is quite poor and this would lead to an underestimation of its attenuation. A similar study was conducted in one WWTP in Spain [32]; however, the process comprises a pre-treatment and primary and secondary treatments. The overall removal efficiency reported for sulfamethoxazole was around 60 %, in agreement with those values found in the literature; however, N 4-acetyl metabolite was not considered.
There is a lack of information regarding production data for antimicrobials and for pharmaceuticals in general. Little data on prescribed dosages in human medicine is available. Thus, the evaluation of overall environmental loads of such substances is intricate. Despite that, Lindberg et al. [35] recently performed a survey on the occurrence of antimicrobials in five WWTPs in Sweden in order to assess the amount of antimicrobials discharged into the environment. The total amounts found (considering final effluent and sludge) normalized to the number of population in the area where the plant is located were compared with theoretical predictions based on consumption data. The authors concluded that quite reliable values of antibiotic loads could be estimated from consumption data without additional measurements.
Analytical determination
Sample preparation
Commonly used methods for analysis usually include extraction for enrichment and clean-up of aqueous samples. Prior to analysis filtration is often carried out on 0.45- or 0.2-μm glass-fiber filters. When analyzing aminoglycosides, however, filtration should be avoided because significant loss occurs due to their extremely high sorption ability [36]. Solution pH is critical, because it determines the chemical form of analytes in the samples, their stability, and the interaction between analyte and sorbent when further solid-phase extraction (SPE) is carried out. Most antimicrobials are acidic substances; thus the acidification of water samples in order to obtain their neutral or acidic forms allows the retention of the negatively charged organic matter usually present in natural samples, in anionic exchange SPE cartridges, which improves the further retention of target compounds. Nevertheless, in some cases, acidic pH values can promote the degradation of the compound, as would be the case of penicillin G, and therefore the acidification of the sample is performed just before extraction [37]. Similarly, when extracting PEs, the pH value of the sample has to be adjusted to neutral (around 7.5) just before the extraction since they are acid- and/or base-labile [38].
Because TCs, SAs, and PEs form complexes with metal ions, special precautions have to be taken, for example, to heat all glassware at 450 °C for 1 h followed by rinsing with a strong chelating agent, such as Na2EDTA [38, 39]. Temperature control is needed when analyzing TCs and macrolides since temperatures higher than room temperature cause their transformation into epi- or anhydrous forms [40], and higher than 100 °C promotes the degradation of macrolides [41]. The use of glassware has also to be avoided when analyzing strongly polar compounds, such as aminoglycoside, in order to prevent losses by adsorption [36] and for penicillins in order to avoid the formation of epimers that are catalyzed by both heavy metal ions and basic media [14].
Additional problems in determining TCs are the formation of keto–enol tautomers in alkaline aqueous solutions [42] and the formation of 4-epimer isomers in acidic aqueous solutions [43]. Although these reversible phenomena have been known to occur for a long time, very few recent papers have focused on detecting TCs and their degradation products and epimers in environmental analysis [23, 44, 45, 61]. The strong affinity of PEs by mono and divalent metal ions strongly suggests the conversion of such compounds into their single sodium adduct species in the water sample before analysis in order to perform sensitive, specific, and reproducible determinations. To fulfil such requirements, addition of sodium chloride to the water samples before extraction may be employed [38].
A literature survey reveals that different extraction techniques are employed to extract antimicrobials, including lyophilization (Lyo), liquid–liquid extraction (LLE), and SPE, but mainly enrichment and clean-up are usually carried out on SPE cartridges of a number of sorbent materials: Lichrolut EN, Lichrolut C18, Oasis MCX, Oasis HLB, and Widepore CBX. Oasis HLB cartridges are more often employed owing to their better recovery of both polar and non-polar compounds. The use of Oasis MCX-HLB in tandem is an excellent approach to ensure the elimination of the interfering organic substances, since the presence of organic matter may result in the reduction of extraction efficiencies, therefore hindering detection. Polar solvents such as acetone, MeOH, acetonitrile (ACN), or ethyl acetate are employed for elution (see Table 2).
In order to avoid undesirable matrix effects (ion suppression or signal enhancement) due to interfering matrix-related components control experiments should be always carried out with matrix-free blanks.
High enrichment factors are required to achieve the low limits of detection necessary for the environmental analysis of antimicrobials. In response to such demands, Hartig et al. [46] reported a robust SPE procedure for the extraction and purification of sulfonamide drugs in surface waters and waste water effluents in which 1-L acidified samples (pH 2.5) were extracted using Lichrolut EN SPE cartridges regardless of their origin. A concentration factor as high as 670 was employed, achieving recovery rates between 50 and 90 %.
Analytical procedure
Chromatographic separation
The chromatographic column preferably used has an end-capped C18 phase; however, aminoglycosides are not retained in alkyl-bonded silica columns. Therefore, the common approach in this case is to use ion-pair chromatography, where ion-pair agents compatible with MS detection (high volatility) must be used, such as heptafluorobutyric acid. In order to reduce ion suppression it is strongly recommended to avoid the use of trifluoroacetic acid.
The influence of the LC mobile phase on the ionization process has been the aim of several studies [47, 48].Typical mobile phases comprise water/ACN or water/MeOH mixtures where volatile organic modifiers such as acetic/acetate buffer, formic acid, or acetic acid are added to improve ionization efficiencies and control pH (see Table 2). The presence of non-volatile compounds in the mobile phase causes clogging in the orifice plate and significant drop in signal intensity. The methanolysis observed for penicillins when prepared in MeOH is not observed during LC separation using methanolic mobile phases: in fact, an improvement of response is observed in ESI+ mode.
Tandem MS detection
Instrumentation
Mass spectrometry as a technique for chromatographic detection provides high sensitivity and specificity, especially in multiple reaction monitoring (MRM) mode, and allows compound confirmation and provides structural information. In general, LC-MS can be used for quantification purposes only when the analyte is present in simple matrices, such as tap water and bottled water, whereas LC-tandem MS is required to quantify with confirmation of identity of residues in complex matrices such as wastewaters.
The analyzers used most as LC detectors are the quadrupole (Q), ion trap (IT), and time of flight (TOF) either alone or combined to give tandem mass spectrometers as the triple quadrupole (TQP) and hybrid instruments, such as the quadrupole/time of flight (Q-TOF), ion trap/time of flight (IT-TOF), and the quadrupole/linear ion trap (Q-LIT). IT instruments are able to perform many stages of MS (MSn), thereby achieving an extremely high sensitivity since they can record a complete mass spectrum of each pulse of ions introduced into the trapping volume [49]. Sensitivity achieved by TOF and TQP instruments is quite similar but for increased specificity TOF is recommended; however, the significantly lower effective linear dynamic range compared to that provided by TQP instruments limits their use in quantitative determinations. The recently introduced Q-TOF instruments are of great interest in confirming proposed analyte identities owing to the accurate masses provided for both precursor and product ions and the possibility of recording a full-scan product-ion spectrum [18, 50]; however, the main drawback of such instruments is their lower sensitivity compared to TQP instruments working in MRM mode. An attempt to improve sensitivity in Q-TOF detection of selected penicillins and quinolones in surface and groundwater was carried out by Pozo et al. [37] by increasing the volume of sample extracted (1 L); however, the LOD was still higher than that obtained by TQP detection. These findings are in agreement with the results reported by Stolker et al. [51] who compared the performances of TQP and Q-TOF detections for the screening and confirmation of selected antimicrobials belonging to different classes, namely chloramphenicol, erythromycin, and sulfamethoxazole, in surface, ground, and drinking water. The method allowed screening and confirmation of a large number of trace pharmaceuticals in the range 1–100 ng L−1 in one run. Comparing the performances of triple quadrupole and Q-TOF, authors concluded that fully satisfactory results were obtained with both techniques; however, Q-TOF has the advantage of the enhanced selectivity owing to information provided by the accurate mass measurements of product ions. Method characteristics such as linear dynamic range and repeatability were found to be similar for both techniques, but LODs of TQP were somewhat lower.
The IT-TOF analyzer is a variation of the Q-TOF obtained by substituting the quadrupole by an IT and therefore combines the extremely high IT sensitivity with the excellent TOF resolution [49]. The new Q-LIT mass spectrometer is also a powerful tool providing the specificity and robustness of the TQP instruments with the full-scan tandem MS sensitivity of IT analyzers, thereby increasing the instrumental dynamic range [52, 53]. Its capability to decouple some of the ion processing steps in the production of a product ion mass spectrum overcomes most of the limitations, such as the low-mass cut off, of conventional ion trap MS instruments, and thus it may be suitable for the analysis of small molecules. An additional advantage is the selective detection shown for multiply charged ions over singly charged ions. To the best of the author’s knowledge no application in environmental analysis of antimicrobial residues has been reported so far.
Application
Some conventional interface systems used years ago, e.g., thermospray and particle beam, do not fit to the requirements for environmental analysis due to their poor sensitivity and robustness; however, two mild ionization interfaces, electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI), satisfy the requirements. Recently a new API interface has been developed, the so-called atmospheric pressure photo-ionization (APPI) [54, 55]. APPI is a modification of the APCI source in which the corona is replaced by a gas-discharge lamp, emitting radiation in the UV region able to selectively ionize the analytes in the presence of the LC mobile phase. To the best of the knowledge of the authors, this new interface has only been applied for detecting chloramphenicol in fish meat [56].
The electrospray source appears to be the most frequently employed mode of ionization in antimicrobial residue determination, since it is particularly suitable for both polar and non-polar analytes and for thermally labile substances, although it is known to be more prone to signal suppression than APCI.
Positive ionization is often preferred when both positive and negative ionization are possible, such as for the β-lactams and penicillins. Therefore, ESI+ is employed with only a few exceptions, such as cephalosporins, novobiocin, and chloramphenicol, which ionize better in ESI− mode [57, 58].
The first step in the tandem MS detection is the selection of the precursor ion. In residue analysis it is preferable that the ionization spectrum consists of a molecular or quasi-molecular ion due to a singly charged droplet of the analyte and negligible fragmentation. The protonated molecular ions, [M+H]+, are generally considered as the best precursor ions; however, for a better sensitivity, selectivity, and reproducibility the [M+NH4]+ and [M+Na]+ ions are selected for penicillins and PEs, respectively [38, 46, 57]. In a recent study by Pozo et al. [37], addition of formic acid was carried out to avoid the formation of the sodium adduct of penicillins, which seemed to present a deficient fragmentation in the collision cell. The formation of ammonium adducts has also been reported to occur during the ionization process, when analyzing sulfonamides using ammonium additives in the mobile phase, which significantly worsen sensitivity. However, the formation of adducts may not always constitute a drawback, since when internal standards are used they will show the same specific formation of adducts in a given system, which may even facilitate their detection. The formation of the stable single sodium adduct species of PEs is a somewhat difficult process, since other alkali metal ions present as impurities during the analysis can also form adducts driven by the high affinity of PEs for alkali metal ions. In order to avoid that, an excess of sodium cations has to be guaranteed, for example, simply by adding sodium chloride to the water samples prior to extraction and adding formic acid to the mobile phase in order to prevent deprotonation of the terminal carboxyl groups and subsequent formation of adducts with more than one metal ion [38]. In the same way, the precursor ion [M+Na]+ is also preferred to [M+H]+ for novobiocin (C31H36N2O11, a coumarin antibiotic) for increased sensitivity. In the course of its fragmentation process, the noviose moiety is lost yielding the sodiated complementary fragments [C9H15NO5 + Na]+ and [C22H21NO6 + Na]+ at m/z 240 and 410, respectively. Nevertheless, Miao et al. [58] recently reported a more intense signal an a higher number of fragment ions operating in negative ionization mode, with [M−H]− as the precursor ion, and proposed a fragmentation pattern of [M−H]− for novobiocin.
The application of multi-residue methods allows a large amount of data to be obtained after a single sample preparation step and in a single LC-tandem MS run. In addition, under specific conditions, compounds belonging to the same antimicrobial class usually form common fragments in the fragmentation process. These fragments may be considered as class-specific fragment ions, which contribute to a best performance (see Fig. 3 and Table 3). This would be the case with the four components of gentamicin, which form a common fragment detected at m/z 322 as a consequence of the cleavage of the purpurosamine group. β-Lactam rings also undergo cleavage during fragmentation providing β-lactams with class-specific fragment ions at m/z 160 and 114. Common neutral losses for the same class of antimicrobials also aids their detection, e.g., loss of the sugars desosamine and cladinose in macrolides, loss of H2O, CO2, and the piperazine substituent in quinolones, loss of the sulfanilamide moiety in SAs (m/z 156), and of NH3 and H2O for TCs. In a very interesting study, Kamel et al. [59] carried out the mass spectral characterization of selected TCs by electrospray ionization and multiple stage mass spectrometry using TQP and IT instruments. Compositions of product ions and mechanism of decomposition could be determined by comparison of spectra of deuterated and non-deuterated species.
Regarding metabolites and degradation products, according to Halling-Sorensen et al. [23] OTC and its degradation products (4-epi, apo, and demethyl derivatives) follow similar fragmentation processes, involving neutral losses of H2O and NH3 from the precursor ion [M+H]+.
Conclusions
The requirement for quantitative data at environmentally relevant concentrations (ng L−1 range) reinforces the need for powerful analytical techniques, which ensure low detection limits and are certain to confirm analyte identities. LC-tandem MS fulfils these criteria. Hybrid Q-TOF instruments allow an ultimate identity confirmation of unknowns by the accurate mass measurement of product ion spectra. Despite that, its application in environmental analysis is still limited due to its lower sensitivity compared to TQP analyzers. Therefore, in the analysis of antimicrobials at environmentally relevant concentrations, typically in the low ng L−1 level, the technique of choice so far is LC-tandem MS with TQP instruments for both detection and confirmation purposes. For further improvement in antimicrobial environmental analysis the use of the recently introduced hybrid Q-LIT mass analyzer might be a very interesting tool.
Despite the increasing number of studies devoted to the analysis of metabolites and degradation products, they are only available for some antimicrobial compounds, such as TCs. This may be due to their usually high polarity and to the lack of reference substances, which both make their analytical determination difficult.
Most efforts in environmental analysis have to be focused on the minimization of matrix effects. Suppression or enhancement of the analyte signal is a complex effect, whose extent seems to be dependent on several experimental and instrumental conditions. Strategies to diminish those unwelcome effects together with improved calibration approaches are required to obtain reliable data on the occurrence and fate of antimicrobial residues in the environment. To attain these challenges, enhanced extraction and purification procedures need to be developed, and more labeled internal standards should be commercially available.
References
Halling-Sorensen B, Nors Nielsen S, Lanzky PF, Ingerslev F, Holten Lützhoft HC, Jorgensen SE (1998) Chemosphere 36:357–393
Chee-Sanford JC, Aminov RI, Krapac IJ, Garrigues-Jeanjean N, Mackie RI (2001) Appl Environ Microbiol 67:1494–1502
Guardabassi L, Petersen A, Olsen JE, Dalsgaard A (1998) Appl Environ Microbiol 64:3499–3508
Goñi-Urriza M, Capdepuy M, Arpin C, Raymond N, Caumette P, Quentin P (2000) Appl Environ Microbiol 66:125–132
Haller MY, Müller SR, McArdell CS, Alder AC, Suter MJF (2002) J Chromatogr A 952:11–120
Wollenberger L, Halling-Sorensen B, Kusk KO (2000) Chemosphere 40:723–730
Kay P, Blackwell PA, Boxall ABA (2005) Chemosphere 59:951–959
Capone DG, Weston DP, Miller J, Shoemaker C (1996) 145:55–75
Baticados MCL, Paclibare JO (1992) The use of chemoterapheutic agents in aquaculture in the Philippines. In: Shariff M, Subasinghe RP, Arthur JP (eds) Diseases in Asian aquaculture I, Manila, Philippines. Fish Health Section, Asian Fishery Society, pp 531–546
Kümerer K (2001) Chemosphere 45:957–969
Mellon M, Benbrook C, Benbrook KL (2001) - http://www.ucsusa.org/publications
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Environ Sci Technol 36:1202–1211
Yang S, Carlson KH (2004) J Chromatogr A 1038:141–155
Díaz-Cruz MS, Barceló D (2005) Trends Anal Chem 24:645–657
Petrovic M, Hernando MD, Díaz-Cruz MS, Barceló D (2005) J Chromatogr A 1067:1–14
Oka H, Matsumoto H (2000) J Chromatogr A 882:109–133
Thiele-Bruhn S (2003) J Plant Nutr Soil Sci 166:145–167
Berger K, Petersen B, Büning-Pfaue H (1986) Arch Lebensmittelhyg 37:85–108
Vree TB, Hekster YA (1985) Pharmacokinetics of sulfonamides revisited, vol 34. Carger, Basel, New York
Gobel A, McArdell CS, Suter MJS, Giger W (2004) Anal Chem 76:4756–4764
Hilton MJ, Thomas KV (2003) J Chromatogr A 1015:129–141
Hirsch R, Ternes TA, Haberer K, Kratz KL (1999) Sci Tot Environ 225:109–118
Halling-Sorensen B, Sengelov G, Tjornelund J (2002) Arch Environ Contam Toxicol 42:263–271
Castiglioni S, Bagnati R, Fanelli R, Pomati F, Calamari D, Zuccato E (2006) Environ Sci Technol 40:357–363
Ternes T, Bonerz M, Schmidt T (2001) J Chromatogr A 938:175–185
Giger W, Alder AC, Golet EM, Kohler HPE, McArdell CS, Molnar E, Siegrist H, Suter MJF (2003) Environ Anal CHIMIA 57:485–491
Daughton CG, Ternes TA (1999) Environ Health Perspect 107:907–938
Yang S, Cha JM, Carlson KH (2005) J Chromatogr A 1097:40–53
Golet EM, Xifra I, Siegrist H, Alder AC, Giger W (2003) Environ Sci Technol 37:3243–3249
Bendz D, Paxéus NA, Ginn TR, Loge FJ (2005) J Hazard Mat 122:195–204
Tolls J (2001) Environ Sci Technol 35:3397–3406
Carballa M, Omil F, Lema JM, Llompart M, García-Jares C, Rodríguez I, Gómez M, Ternes TA (2004) Water Res 38:2918–2926
Miao XS, Bishay F, Chen M, Metcalfe CD (2004) Environ Sci Technol 38:3533–3541
Wiegel S, Aulinger A, Brockmeyer R, Harás H, Löffler J, Reincke H, Schmidt R, Stachel B, von Tümpling W, Wanke A (2004) Chemosphere 57:107–126
Lindberg RH, Wennberg P, Johansson MI, Tysklind M, Andersson BAV (2005) Environ Sci Technol 39:3421–3429
Löffler D, Ternes TA (2003) 1000:583–588
Pozo, OJ, Guerrero C, Sancho JV, Ibáñez M, Pitarch E, Hogendoorn E, Hernández F (2006) J Chromatogr A 1103:83–93
Cha JM, Yang S, Carlson KH (2005) J Chromatogr A 1065:187–198
Ternes T (2001) Trends Anal Chem 20:419–434
Yang S, Cha JM, Carlson KH (2004) Rapid Commun Mass Spectrom 18:2131–2145
Jacobsen MA, Halling-Sorensen B, Ingerslev SH, Hansen J (2004) J Chromatogr A 1038:157–170
Naidong W, Roets E, Busson R, Hoogmartens J (1990) J Pharm Biomed Anal 8:881–889
Bryan PD, Hawkins KR, Stewart JT, Capomacchia AC (1992) Biomed Chromatogr 6:305–310
Hamscher G, Sczesny S, Hoper H, Nau H (2002) Anal Chem 74:1509–1518
Soeborg T, Ingerslev F, Halling-Sorensen B (2004) Chemosphere 57:1551–1524
Hartig C, Store T, Jekel M (1999) J Chromatogr A 854:163–173
Zhou S, Hamburger M (1995) Rapid Commun Mass Spectrom 9:1516–1521
Zhou S, Hamburger M (1996) J Chromatogr A 755:189–204
Hager JW (2004) Anal Bioanal Chem 378:845–850
Ibáñez M, Sancho JV, Pozo OJ, Niessen W, Hernández F (2005) Rapid Commun Mass Spectrom 19:169–178
Stolker AAM, Niesing W, Fuchs W, Vreeken RJ, Niessen WMA, Brinkman UATH (2004) Anal Bioanal Chem 378:1754–1761
Hager JW, Le Blanc JCY (2003) J Chromatogr A 1020:3–9
Hager JW (2002) Rapid Commun Mass Spectrom 16:512–526
Raffaelli A, Saba A (2003) Mass Spectrom Rev 22:318–331
Hanold KA, Fisher SM, Cormia PH, Miller E, Syage JA (2004) Anal Chem 76:2842–2851
Takino M, Daishima S, Nakahara (2003) J Chromatogr A 1011:67–75
Hirsch R, Ternes TA, Haberer K, Mehlich A, Ballwanz F, Kratz KL (1998) J Chromatogr A 815:213–223
Miao XS, Metcalfe CD (2003) J Mass Spectrom 38:27–34
Kamel AM, Fonda HG, Brown PR, Munson B (2002) J Am Soc Mass Spectrom 13:543–557
Zhu J, Show DD, Cassada DA, Monson SJ, Spalding RF (2001) J Chromatogr A 928:177–186
Halling-Sorensen B, Lykkeberg A, Ingerslev F, Blackwell P, Tjornelund J (2003) Chemosphere 50:1331–1342
Sacher F, Lange FT, Brauch HJ, Blankenhorn I (2001) J Chromatogr A 938:199–210
Acknowledgements
This work has been funded by the EU Framework VI Programme (Project NOMIRACLE, Co.No: 003956-2), and by the Spanish Ministry of Science and Technology (Project EVITA, CTM2004-06265-CO3-01/TECNO). M.S. Díaz-Cruz acknowledges her Ramon y Cajal contract from the Spanish Ministry of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Díaz-Cruz, M.S., Barceló, D. Determination of antimicrobial residues and metabolites in the aquatic environment by liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 386, 973–985 (2006). https://doi.org/10.1007/s00216-006-0444-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-006-0444-z