Abstract
Glassy carbon electrodes (GCE) and carbon paste electrodes (CPE) were modified with imidazole functionalized polyaniline with the aim to develop a sensor for lead (II) in both acidic and basic aqueous solution. The electrodes were characterized by cyclic voltammetry and differential pulse adsorptive stripping voltammetry. The limit of detections obtained with glassy carbon electrode and carbon paste electrode are 20 ng mL-1 and 2 ng mL-1 of lead ion, respectively. An interference study was carried out with Cd(II), As(III), Hg(II) and Co(II) ions. Cd(II) ions interfere significantly (peak overlap) and As(III) has a depressing effect on the lead signal. The influence of pH was investigated indicating that bare and modified GCE and CPE show optimum response at pH 4.0 ± 0.05.

Imidazole functionalized polyaniline modified glassy carbon and carbon paste electrodes were used for lead ion detection by using CV and DPASV techniques. The lower detection limit observed with GCE and CPE are 20 ng mL-1 and 2 ng mL-1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The release of various heavy metals to the environment and human exposure to these toxic metals is increasing day by day. The growth of paint, mining, batteries and PCB industries is the foremost reason for its presence in aqueous medium. Lead is the one of the heavy metals with toxic effects on living organism [1]. Exposure of lead causes toxicity to many organs and tissues including the heart, bones, intestines, kidneys, reproductive and nervous systems [2]. This leads to an ever increasing demand for the development of rapid and sensitive methods for the determination of trace metal contaminants [3]. The development of low cost electrochemical sensor as one of the techniques to determine the trace metal ions holds promise. Thus electrochemical stripping analysis has evoked much interest for monitoring the concentration of various pollutants on a daily basis [4]. The determination of metal ions using stripping voltammetry has many advantages over other methods like liquid phase chromatography, solid phase chromatography, atomic absorption spectroscopy (AAS), X-ray fluorescence etc. The solid electrodes have low adsorptivity, reactivity and sensitivity because of low porosity. Porosity of the solid electrodes can be increased by modifying the surface by various techniques notably by using conducting polymer as a sensing device [5]. Research has been focused on the development of novel lead ion detection sensors of modified solid electrodes with various kinds of functionalized conducting polymers with fast response, long life times, high sensitivity and selectivity [6–8]. Enhancement of physico-chemical properties of solid electrodes which are modified by conducting polymers [9] for interaction with analyte can be achieved by introduction of a specific prosthetic group into a conducting polymer [10]. Polyaniline (PANI) with three oxidation states is relatively stable and has been used for various sensors and devices [11–13]. There are several reports showing the interaction of metal ion with polyaniline backbone [14–16]. Metal ion binding is reported to be enhanced when PANI backbone contains amine or imine nitrogen atoms [17], which act as metal ion acceptors.
The utility of imidazole with N-donor atoms, functionalized to a PANI backbone as a metal ion receptor to form a sensor has gained importance due to its specific property of common metal binding sites in proteins [18]. As the doped IMPANI has higher conductivity compared to the undoped state, binding of metal ion on polymer backbone increases the conductivity [19]. The studies of imidazole containing polyacetylene and polycarbazole as a fluorescence probe for metal ion sensors have been reported [20, 21].
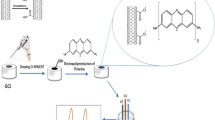
In the present study, the modification of glassy carbon electrode (GCE) and carbon paste electrode (CPE) by conducting polymer imidazole functionalized polyaniline (IMPANI) and the effect of interaction of lead ions on the polymer modified electrodes have been reported. The effect of lead ion binding with modified GCE and CPE was studied by using electroanalytical techniques like cyclic voltammetry (CV), differential pulse anodic stripping voltammetry (DPASV).
Many other techniques have been used for detection of Pb(II) by using various sensing materials in micro to nano molar ratio. Brief reviews of these are tabulated in Table 1. Here, one of the advantages of IMPANI modified sensor (GCE) is that, it can be useful for detection of lead ion in micro molar concentration in basic medium (pH 9).
Experimental
Apparatus and reagents
The electrochemical studies were performed with a CHI 660D electrochemical workstation (CH Instruments, USA). The modified GCE and modified CPE were used as working electrodes. Atomic absorption spectroscopic studies were carried out by using Series AA spectrometer from Thermo Electron Corporation, USA. Reagent grade 2-aminophenol was purchased from Sigma-Aldrich (http://www.sigmaaldrich.com). Analytical reagent grade acetic anhydride, epichlorohydrin, imidazole, ammonium persulfate, lead chloride, sodium hydroxide, hydrochloric acid, graphite powder and potassium carbonate were purchased from s d Fine Chem Ltd. (http://www.sdfine.com), were used without further purification. The buffer tablets of acidic, neutral and basic (pH 4.0 ± 0.05, 7.0 ± 0.05, and 9.2 ± 0.05) were purchased from Merck Chemicals (http://www.merck-chemicals.in) and were used without further purification. Aniline was purified by double distillation under reduced pressure and stored at low temperature. Solvents used were of reagent grade except the tetrahydrofuran which was distilled twice over pressed sodium and preserved under inert conditions.
Polymerization of poly (2-(1-imidazoylium-2-hydroxypropoxy) aniline hydrochloride
Imidazole functionalized polyaniline has been synthesized by a sequence of reactions as shown in Scheme 1. In the first step, by using acetic anhydride, ortho amino group of o-hydroxy aniline was protected and o-hydroxy acetanilide was obtained (1). The resultant o-hydroxy acetanilide was mixed with epichlorohydrin in dry THF in the presence of a weak base, K2CO3 at 70 °C to produce 2-(2-oxiranylpropoxy) acetanilide (2). In the next step the basic imidazole group was incorporated to 2-(2-oxiranylpropoxy) acetanilide, and by ring opening reaction takes place to form 2-(1-imidazoyl-2-hydroxy propyloxy) acetanilide (3). In the next step, amino group was deprotected by refluxing 2-(1-imidazoyl-2-hydroxy propyloxy) acetanilide in 1:2 ratio of concentrated HCl and absolute ethanol for 24 h. This yielded 2-(1-imidazoylium-2-hydroxy propyloxy) aniline hydrochloride (4) monomer. The obtained monomer was directly polymerized on GCE by electrochemical polymerization methods. For modification of CPE, the monomer was further polymerized by chemical synthesis method. The obtained monomer 2-(1-imidazoylium-2-hydroxy propyloxy) aniline hydrochloride was used for the oxidative polymerization using ammonium persulfate solution at room temperature to obtain the final product IMPANI. The synthesized polymer was mixed with CPE in proper ratio to modify the CPE. The interaction of lead ion with modified electrodes which contains IMPANI has been shown in the Scheme 2.
Modification of GCE by IMPANI
The electrochemical studies have been carried out in a one compartment cell with 3 electrodes system. Platinum wire and saturated calomel electrode were used as counter and reference electrodes respectively. The polished surface of GCE used as a working electrode, was dried under argon to remove residual moisture without damaging the active part of the electrode. IMPANI monomer was polymerized on GCE by CV technique (Fig. 1). IMPANI monomer of 0.01 M (0.03 g) and 10 mL, 1M HCl were mixed in the reaction cell. Supporting electrolyte KCl (0.1 M) was added to the mixture. The potential was swept from 1.4 V to -1.4 V range for 20 cycles. A black powder of IMPANI polymer was observed to grow on the surface of GCE. After the polymerization, the surface was washed with a copious amount of deionized water to remove any residual monomer. The modified GCE was dried using a vacuum desiccator for 1 h.
Preparation and modification of carbon paste electrode
The fine graphite powder (average particle size ~50 μm, density 0.2–0.3 gm L-1, and >99% assay) and paraffin oil binder were mixed in the ratio of 4:1 to obtain a homogeneous paste. The obtained paste was filled tightly in a glass tube of 3 mm diameter to form an electrode. This carbon paste electrode was connected to the external circuit by attaching a copper wire. Furthermore, before conducting the experiment, each time the electrode surface was cleaned mechanically by smoothening some paste off and then polishing on a piece of smooth emery paper. Modification of CPE was carried out using IMPANI. Various percentages of IMPANI and graphite mixture were evaluated for maximum response and 77:3:20 percentage ratio of graphite: IMPANI: paraffin oil was found to be the optimum composition for making the electrode. Further experiments throughout this work were carried out with this optimum composition of modified electrode.
Buffer solutions of pH 4.0 ± 0.05 (acidic), pH 7.0 ± 0.05 (neutral) and pH 9.2 ± 0.05 (basic) were prepared using standard buffer tablets. 1×10−3 M, to 1 × 10−9 M PbCl2 solutions were prepared using these buffers. From the stock solution 10 mL of the analyzing samples were used each time.
Results and discussion
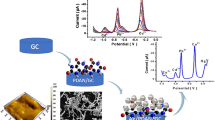
The modified GCE and CPE electrode were evaluated for determination of lead metal ions in chloride form. Various techniques like CV, DPASV were used to evaluate the sensitivity of lead ions. GCE was used to polymerize the IMPANI and the metal ion sensing capability of the modified electrode surface was evaluated. Initially lead chloride solution of 1 × 10−3 M prepared in buffer solution of pH 4 was taken in a one compartment reaction cell. CV was performed in the potential range of 1.4 V to -1.4 V (Fig. 2). A sharp metal ion adsorption peak was observed in the potential region of -0.5–0.7 V. Furthermore, the sharp peak was observed again with lower intensity when PbCl2 solution of 1 × 10−4 M was used, indicating the lead adsorption by the modified GCE. Several experiments were carried out by decreasing the concentrations of lead to determine the lower limit of detection (LLOD) of modified GCE. Even though response to the concentration of 1 × 10−7 M was not observed in CV technique, DPASV showed response (Fig. 3). Response to low concentration of 10-8 M was observed in stripping technique as it is a more sensitive technique compared to potentiodynamic techniques [31]. DPASV parameters were tuned as potential range was adjusted to 0.0 V to -1.4 V. The optimization of parameters for DPASV was carried out by trial and error method (see ESM). Maintaining above parameters the observed response for lead ion at low concentrations of 1 × 10−8 PbCl2 (equal to 20 ng mL-1 of lead ion) was realized for modified GCE.
CPE was also used to evaluate the response of IMPANI to lead ion. Modified CPE shows more sensitivity towards lead ion as compared to modified GCE. Using modified CPE as working electrode two electrochemical techniques namely CV (Fig. 4) and DPASV (Fig. 5) were employed for lead ion detection. Pulse technique was employed to detect the lead at lower concentration of 1 × 10−9 PbCl2 (equal to 2 ng mL-1 of lead ion). DPASV parameters were tuned as potential range was adjusted to 0.0 V to -1.4 V. Compared to unmodified CPE, IMPANI modified CPE is more sensitive towards lead ion. This is due to the enhancement in porosity of modified electrode over unmodified electrode. Moreover, IMPANI will readily form complex with M2+ ions. Using GCE sensitivity of IMPANI to lead ion was observed in both acidic as well as basic medium. However, modified CPE was sensitive only in acidic medium, i.e., at pH 4.0 ± 0.05 solution.
Interference study
The selectivity of fabricated electrode was studied by examining the effect of some heavy metal ions like Hg(II), Cd(II), Co(II) and As(III) on the response for Pb(II). Stripping response on IMPANI modified electrodes were observed only with As(III) and Cd(II) at above 1 × 10−5 M and 1 × 10−6 M respectively (Fig. 6). In As(III) stripping voltammetry, stripping peak was observed at around +0.3 V which is far away from Pb(II) peak (at -0.7 V) and hence does not interfere. However, suppression of the stripping response of Pb(II) in the presence of As(III) was observed (Fig. 3S, Fig. 4S, Fig. 5S and Fig. 6S in ESM). In case of Cd(II) stripping response is at -0.8 V. Significant increase in the stripping response of lead peak from 10-5 M CdCl2 to 10-4 M CdCl2 mixture was observed. This indicates that Pb(II) mixed with Cd(II) can be analyzed successfully if the Cd(II) concentration is below 10-4 M.
The pH study was carried out for fabricated sensors to optimize the pH at which sensor shows maximum sensitivity. DPASV analyses were carried out with pH 4, pH 7 and pH 9 for both modified GCE (Fig. 7S in ESM) and CPE (Fig 8S in ESM). It was observed that, IMPANI sensors were not able to detect lead ion at pH 7. In basic pH, even though modified GCE sensor senses lead, sensitivity is lower compared to acidic pH. Hence IMPANI modified electrodes are suitable lead ion sensors at acidic pH rather than basic or neutral medium.
The variation of peak current and voltage for various concentrations of metal ions, Pb(II), Cd(II) and As(III) with IMPANI modified GCE and CPE sensors are tabulated (See ESM Table 1S). Calibration curve for both GCE and CPE of Pb(II) detection are as shown in Fig 9S and 10S in ESM.
Detection of lead at basic pH
It is important to determine the lead content in both acidic and basic medium [32]. Hence lead sensing was repeated at pH 9.2 ± 0.05 solutions with the modified GCE. To prevent lead precipitation at pH 9 solution at lower concentrations (1 × 10−3 M) was prepared by slow addition of buffer solution to the stock solution of lead. CV of this solution at pH 9 (Fig. 1S in ESM) does not shown potential shift in peak position from pH 4. This indicates stripping of lead from IMPANI complex occurs at the same oxidation potential in both acidic and basic media. As before CV analysis was carried out for various concentration of PbCl2 at pH 9.2 ± 0.05. Furthermore DPASV (Fig. 2S in ESM) technique was used to detect the LLOD which was observed to be 1 × 10−7 M (828 ng mL-1) of Pb(II).
AAS studies were carried out for PbCl2 at lower concentrations for determining the lead ion concentration before and after IMPANI complex formation. Samples collected before and after stripping studies during DPASV technique were analyzed by AAS. The lead ion concentration from AAS results, before and after stripping experiments was to be found in the same range. This indicates that the IMPANI complex formation with lead is a reversible process and hence the sensor can be used more than once.
Conclusions
Functionalized conducting polymer, IMPANI was used to modify GCE and CPE and evaluated for lead ion detection in aqueous solution at both acidic and basic medium. The modified electrodes showed excellent selectivity towards lead ion, arising from the complexation of divalent lead ions with IMPANI. The modified electrodes showed reversibility towards lead ion. Detection of lead ion by modified electrodes was evaluated by CV and DPASV techniques. The LLOD obtained with glassy carbon and carbon paste electrodes at pH 4 are 20 ng mL-1 and 2 ng mL-1 of lead ion. Even modified GCE can be used for detection of lead in basic medium (pH 9). The modified CPE showed higher sensitivity than GCE in acidic medium. These sensors are easy to fabricate and convenient to use in field. The IMPANI modified GCE and IMPANI modified CPE sensors could be used for lead detection in the range of 1 × 10−3 to 1 × 10−8 M and 1 × 10−3 to 1 × 10−9 M respectively.
References
Xu H, Zeng L, Xing S, Xian Y, Shi G, Jin L (2008) Ultrasensitive voltammetric detection of trace lead (II) and cadmium (II) using MWCNTs-nafion/bismuth composite electrodes. Electroanalysis 20:2655–2662
Coon T, Miller M, Shirazi F, Sullivan J (2006) Lead toxicity in a 14-year-old female with retained bullet fragments. Pediatrics 117:227–230
Wang J, Lu J, Luo D, Wang J, Jiang M, Tian B (1997) Renewable-reagent electrochemical sensor for monitoring trace metal contaminants. Anal Chem 69:2640–2645
Esteban M, Casassas E (1994) Stripping electroanalytical environmental analysis. Tren Anal Chem 13:110–117
Manisankar P, Vedhi C, Selvanathan G, Arumugam P (2008) Differential pulse stripping voltammetric determination of heavy metals simultaneously using new polymer modified glassy carbon electrodes. Microchim Acta 163:289–295
Zott H, Heusinger H (1975) Electron spin resonance investigations of radicals and trapped Electrons in γ-irradiated 3,4-polyisoprene and 1,2-polybutadiene. Macromolecules 8:182–185
Zon A, Ion I, Popescu A, Ungureanu M, Moutet JC, Aman ES (1997) A ferrocene crown ether-functionalized polypyrrole film electrode for the electrochemical recognition of barium and calcium cations. Adv Mater 9:711–713
He X, Su Z, Xie Q, Chen C, Fu Y, Chen L, Liu Y, Ma M, Deng L, Qin D, Yao S, Luo Y (2011) Differential pulse anodic stripping voltammetric determination of Cd and Pb at a bismuth glassy carbon electrode modified with nafion, poly(2,5-dimercapto-1,3,4-thiadiazole) and multiwalled carbon nanotubes. Microchim Acta 173:95–102
Ranjith K, Swathi SK, Kumar P, Ramamurthy PC (2011) Pulsed laser deposition film of a donor–acceptor–donor polymer as possible active layer in devices. J Mater Sci 46:2259–2266
McQuade DT, Pullen AE, Swager TM (2000) Conjugated polymer-based chemical sensors. Chem Rev 100:2537–2574
Mangombo ZA, Baker P, Iwuoha E, Key D (2010) Tyrosinase biosensor based on a boron-doped diamond electrode modified with a polyaniline-poly(vinyl sulfonate) composite film. Microchim Acta 170:267–273
Ramamurthy PC, Tiwary AK, Hardaker SS, Gregory RV (2002) Thermal characterization of high molecular weight leucoemeraldine base polyaniline. Poly Preprints 43:1242–1243
Lodha A, Kilbey SM, Ramamurthy PC, Gregory RV (2001) Effect of annealing on electrical conductivity and morphology of polyaniline films. J Appl Poly Sci 82:3602–3610
Tang L, Wu T, Kan J (2009) Synthesis and properties of polyaniline–cobalt coordination polymer. Synth Met 159:1644–1648
Wang A, Feng J, Li Y, Xi J, Dong W (2010) In-situ decorated gold nanoparticles on polyaniline with enhanced electrocatalysis towards dopamine. Microchim Acta 171:431–436
Dimitriev OP (2004) Doping of polyaniline by transition-metal salts. Macromolecules 37:3388–3395
Nikolaidis MG, Sejdic JT, Cooney RP, Bowmaker GA (2006) Spectroscopic studies of interactions of polyaniline with some Cu(II) compounds. Curr Appl Phys 6:457–461
Joszai V, Nagy Z, Osz K, Sanna D, Natale GD, Mendola DL, Pappalardo G, Rizzarelli E, Sovago I (2006) Transition metal complexes of terminally protected peptides containing histidyl residues. J Inorgan Biochem 100:1399–1409
Zeng Q, Cai P, Li Z, Qin J, Tang BZ (2008) An imidazole-functionalized polyacetylene: convenient synthesis and selective chemosensor for metal ions and cyanide. Chem Comm 9:1094–1096
Li Z, Lou X, Yu H, Li Z, Qin J (2008) An imidazole-functionalized polyfluorene derivative as sensitive fluorescent probe for metal ions and cyanide. Macromolecules 41:7433–7439
Joseph A, Ramamurthy PC, Subramanian S (2012) Imidazole functionalized polyaniline: synthesis, characterization and Cu (II) coordination studies. J Appl Poly Sci 123:526–534
Wang JJ, Wang XJ, Geng Y, Tung CH, Wu LZ (2009) Colorimetric and electrochemical Pb2+ detection by imine-bridged tetrathiafulvalene-pyridine derivatives. Sci China Ser B-Chem 52:765–770
Wang J, Tian B (1993) Mercury-free disposable lead sensors based on potentiometric stripping analysis of gold-coated screen-printed electrodes. Anal Chem 65:1529–1532
Noh MF, Tothill IE (2006) Development and characterization of disposable gold electrodes and their use for lead(II) analysis. Anal Bioanal Chem 386:2095–2106
Song W, Zhang L, Shi L, Li DW, Li Y, Long YT (2010) Simultaneous determination of cadmium(II), lead(II) and copper(II) by using a screen-printed electrode modified with mercury nano-droplets. Microchim Acta 169:321–326
Yang G, Qu X, Shen M, Wang C, Qu Q, Hu X (2008) Electrochemical behavior of lead(II) at poly(phenol red) modified glassy carbon electrode and its trace determination by differential pulse anodic stripping voltammetry. Microchim Acta 160:275–281
Xiao-bo J, Yun-hua W, Jun-jie F, Sheng-shui H (2004) Electrochemical determination of trace amounts of lead (II) and cadmium (II) at a calix[6]arene modified carbon paste electrode. Wuhan Uni J of Nat Sci 6:943–948
Li XG, Feng H, Huang MR, Gu GL, Moloney MG (2012) Ultrasensitive Pb(II) potentiometric sensor based on copolyaniline nanoparticle in plasticizer free membrane with long life time. Anal Chem 84:134–140
Rahman MA, Won MS, Shim YB (2003) Characterization of EDTA bonded conducting polymer modified electrode: Its application for the simultaneous determination of heavy metal ions. Anal Chem 75:1123–1129
Ardakani MM, Kashani MK, Niasari MS, Ensafi AA (2005) lead ion selective electrode prepared by sol-gel and PVC membrane techniques. 107:438–445
Takeuchi RM, Santos AL, Medeiros MJ, Stradiotto NR (2009) Copper determination in ethanol fuel samples by anodic stripping voltammetry at a gold microelectrode. Microchim Acta 164:101–106
Cigala RM, Crea F, Stefano CD, Lando G, Milea D, Sammartano S (2010) Electrochemical study on the stability of phytate complexes with Cu2+, Pb2+, Zn2+ and Ni2+: A comparison of different techniques. J Chem Engg Data 55:4757–4767
Acknowledgements
Authors gratefully acknowledge the financial support from DST No SR/S3/ME/025/2008, and technical support from Advanced Characterization Centre, IISc.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 2370 kb)
Rights and permissions
About this article
Cite this article
Kumar, P., Joseph, A., Ramamurthy, P.C. et al. Lead ion sensor with electrodes modified by imidazole-functionalized polyaniline. Microchim Acta 177, 317–323 (2012). https://doi.org/10.1007/s00604-012-0787-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-012-0787-4