Abstract
We report a multi-residue pretreatment technique, termed temperature-assisted ionic liquid dispersive liquid-liquid microextraction, and demonstrate its application to simultaneous extraction of polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs). An ionic liquid was used as the extraction solvent and dispersed into the liquid sample (water, urine) with the help of methanol and at elevated temperature. Parameters such as extraction solvent and its volume, disperser solvent and its volume, extraction time, centrifugation time, salt addition, extraction temperature and sample pH were optimized. Under the optimized conditions, an up to 278-fold enrichment factor and an >83.4% extraction recovery were obtained. A linear relationship is obtained in the range of 0.5–500 ng mL−1. The limits of detection (at S/N = 3) and relative standard deviations (for n = 5) range from 0.1 to 0.4 ng mL−1 and from 1.0% to 5.6%, respectively. The recoveries for water and urine samples additionally spiked with PCBs and PBDEs are between 81.0 and 127.1% and 84.0 and 110.3%, respectively. The method was successfully applied to the determination of PCBs and PBDEs in real river water and in human urine samples.

Under optimized conditions (temperature, 40 °C; water or urine sample, 5.0 mL; spiking level, 10.0 ng mL−1; disperser solvent (methanol), 1.0 mL; extraction solvent ([C8MIM][PF6]), 40.0 μL; centrifugation time, 8 min; pH, 1.0), the enrichment factors of PCBs and PBDEs by temperature-assisted ionic liquid dispersive liquid-liquid microextraction are in the range from 278 to 343, and acceptable extraction recoveries (>83.4%) are obtained. The limits of detection (at S/N=3) and relative standard deviations (n = 5) for all analytes were 0.1–0.4 ng mL−1 and 1.0–5.6%, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) are typical persistent organic pollutants, which are ubiquitous in environment and have a wide range of industrial applications such as dielectric fluid, hydraulic liquid and fire retardant. Owing to their high toxicity, lipophilic property and widespread dispersal in the global environment, PCBs and PBDEs pose real threat to human health and ecological systems. More than forty years have passed since the accidental poisoning with PCBs in Yusho, Japan in 1968. High concentration of PCBs was still detected in the serum of the Yusho victims and the mean urinary concentrations of nitrite and nitrotyrosine were significantly higher than those in the controls [1]. In recent years, PCBs and PBDEs were reported to be still detected in human serum, breast milk and adipose tissues [2–5].
Environmental pollutants are incorporated into the organism by different routes, and can be stored and distributed in different tissues, which lead to an internal concentration that can induce different alteration, adverse effect and/or disease. Thus, it is necessary to develop new techniques for the residual analysis of pollutants at trace levels in organism samples such as saliva, urine, nails, hair, and semen or breast milk [6]. To achieve effective extraction of target analytes from these matrices, several sample preparation methods have been developed including liquid-liquid extraction, microwave-assisted extraction, matrix solid-phase dispersion, hollow fiber-liquid phase microextraction, dispersive liquid–liquid microextraction (DLLME) etc. [7–11]. Among them, DLLME has attracted much interest from scientists working in separation science. Since its introduction in 2006, DLLME has been proved to be an efficient, quick and sensitive extraction method for organic or inorganic analytes [11]. It was applied to the extraction of PCBs and PBDEs from different matrices, during which chlorinated solvents were preferred as extraction solvents [12–18]. Even though these conventional solvents have demonstrated good recovery values, they are volatile, toxic and flammable solvents.
To overcome the weaknesses of the previous procedure, ionic liquids (ILs) have been recently suggested as extraction solvents in DLLME procedures [19–21]. They are very simply molten salts, consisting of cations and anions, and possess high thermal stability, negligible vapor pressure, tunable viscosity and miscibility with water and organic solvents, making them attractive alternatives to environmentally unfriendly solvents [22].
The objective of this paper was to develop temperature-assisted ionic liquid dispersive liquid-liquid microextraction (TA-IL-DLLME) method for extraction of PCBs and PBDEs. Obviously, this method is based on the temperature change making IL complete dispersion in the aqueous phase and increase the chance of partitioning between the two phases, and the IL was concentrated into one drop by cooling and centrifugation. The performance of this method was evaluated using ILs as solvents for the extraction of PCBs and PBDEs from water and urine samples. All the variables, affecting TA-IL-DLLME procedure, were investigated and optimized in detail. Finally, the developed method was applied to the determination of the target analytes in environmental water and human urine samples.
Experimental
Instrumentation
PCBs and PBDEs were analyzed by an Agilent 1200 HPLC equipped with a manual injection and variable wavelength detector (www.agilent.com.cn). A Zorbax Eclipse XDB-C18 column (150 mm × 4.6 mm, 5 μm particle size) was used and all injections were performed manually with 20.0-μL sample loop. The operating conditions were as follows: mobile phase, methanol–water, 85:15 (v/v); flow rate, 1.2 mL min−1; column temperature, 25 ± 1 °C and the wavelength of detection, 226 nm. The TDL-50C centrifuge was used for separation of the organic phase from water (www.xiongdiyiqi.com, Henan Xiongdi Instrument Co. Ltd., China). The screw cap conical bottom glass test tubes (10 mL), used as extraction vessels, were heated at 500 °C in a furnace (www.carbolite.com, Carbolite, UK; model CWF 1200) to remove organic compounds before use.
Reagent and standards
2,2′,4,5,5′-pentachlorominated biphenyl (CB-101), 2,2′,3,4,4′,5′-hexachlorominated biphenyl (CB-138), 2,2′,4,4′,5,5′-hexachlorominated biphenyl (CB-153) and 2,2′,3,4,4′,5,5′- heptachlrobiphenyl (CB-180) were purchased from Accustandard (www.accustandard.com, New Haven, CT, USA). Each compound was dissolved in acetonitrile to prepare a 100 μg mL−1 stock solution.
PBDE congeners (50 μg mL−1 in isooctane, 1 mL) such as 2,2′,4,4′-tetrabrominated diphenyl ether (BDE-47), 2,2′,4,4′,5-pentabrominated diphenyl ether (BDE-99), 2,2′,4,4′5,6′-hexbrominated diphenyl ether (BDE-154) and 2,2′,3,4,4′,5′,6-heptabrominated diphenyl ether (BDE-183) were obtained from Sigma-Aldrich (www.sigmaaldrich.com, St. Louis, MO, USA). Because of the limited water solubility of isooctane, acetonitrile was selected as intermediate solvent. One milliliter of each solution of PBDE congener was mixed and evaporated under a gentle nitrogen flow. Then, the residue was redissolved in 10 mL acetonitrile to obtain a standard stock solution with a concentration of 5 μg mL−1.
All the working standard solutions were prepared by serial dilutions of the stock solution with ultra Milli-Q water (www.millipore.com, Millipore, Molsheim, France) prior to analysis. The HPLC-grade acetonitrile and methanol were purchased from Merck Company (www.merck.com, Darmstadt, Germany). Acetone and tetrahydrofuran were all of analytical grade and redistilled prior to use. 1-Octyl-3-methylimidazolium hexafluorophosphate ([C8MIM][PF6]) were purchased from Shanghai Chengjie Chemical Co., Ltd (www.shyfhx.com, Shanghai, China).
Sample preparation
Water samples were collected from Qiantang River, which is the largest river in Zhejiang Province, China, and stored in amber bottles at 4 °C until analysis. Blank urine samples were collected from healthy individuals and stored in polytetrafluoroethylene flasks at −20 °C until analysis. Prior to the TA-IL-DLLME procedures, each sample was adjusted to pH 1.0 with diluted hydrochloric acid and filtered through a 0.45-μm membrane to remove particulate matter.
TA-IL-DLLME procedures
Aliquots of 5.0 mL sample solution containing PCBs and PBDEs (pH 1.0) were placed in a 10 mL screw cap conical bottom glass test cube. One milliliter of methanol (disperser solvent) containing 40.0 μL [C8MIM][PF6] (extraction solvent) was injected rapidly into the sample solution by using a 1.00-mL syringe. A cloudy solution (water, methanol, [C8MIM][PF6]) formed in the test tube. Then the conical tube was heated at 40 °C for 3 min in a water bath. The IL was dissolved completely, and the analytes will partition into the IL phase. The tube was thereafter cooled at −20 °C for 3 min and the solution became turbid. Then the solution was centrifuged at 3500 rpm for 8 min, and the dispersed fine droplets of [C8MIM][PF6] were deposited at the bottom of conical test tube (about 15.0 μL). The sedimented phase (15 μL) was removed using a 50-μL microsyringe. The ionic liquid extract is too viscous to be injected directly in the HPLC system, thus it was diluted with equal volume of methanol, and 10 μL were injected into the HPLC system for analysis.
Method performance
Enrichment factor (EF) is defined as the ratio of the analyte concentration in the sedimented phase (Csed) and the initial concentration of analyte (C0) in the sample. Csed is obtained from a suitable calibration graph. EF can be calculated based on the following equation [23]:
The relative extraction recovery (ER%) is defined as the percentage of total analyte amount extracted to the sedimented phase [23]:
where Vsed and Vaq are the volumes of sedimented phase and sample solution, respectively.
Results and discussion
Selection of extraction solvent
The type of extraction solvent used in DLLME is essential for obtaining an efficient extraction. This solvent should have a lower solubility in water, higher extraction capability for analytes, and good chromatography behavior [20]. According to the above criteria, the ionic liquid, [C8MIM][PF6], was selected as extraction solvent in this study. It had been proved that [C8MIM][PF6] was used to extract some typical environmental pollutants, including polycyclic aromatic hydrocarbons, phthalates, phenols and aromatic amines with satisfactory results [24, 25].
Selection of disperser solvent
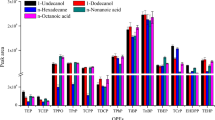
In DLLME procedures, disperser solvent should be miscible with both water and extraction solvent. Therefore, methanol, tetrahydrofuran, acetonitrile and acetone were tested as disperser solvent. Under the same conditions, a series of sample solutions were tested using 1.0 mL disperser solvent, containing different volumes of the extraction solvents to achieve a 15.0 ± 0.5 μL sedimented phase. Therefore, 40.0, 37.0, 33.0 and 45.0 μL [C8MIM][PF6] was added to 1.0 mL of methanol, tetrahydrofuran, acetonitrile and acetone, respectively. The results, as shown in Fig. 1, demonstrated that methanol exhibited the highest extraction efficiency (83.4–102.9%). As a result, methanol was chosen as the disperser solvent in subsequent experiment.
Effect of disperser solvent on extraction efficiency of PCBs and PBDEs obtained from TA-IL-DLLME. Conditions: water sample volume, 5.0 mL, concentration level at 10.0 ng mL−1; extraction solvent, [C8MIM][PF6]; disperser solvent volume, 1.0 mL; centrifugation time, 8 min; pH, 1.0; temperature, 40 °C; data for triplicate extractions
Effect of volume of extraction solvent
If the volume proportion of [C8MIM][PF6] to methanol is improper, the mixed process between them needs much time to obtain a uniform mixture and, in turn, affect the mass transfer of analyte from the sample to the microdrop [26]. In this investigation, with the increase of [C8MIM][PF6] volume from 30.0 to 55.0 μL, the volume of sedimented phase increased from 5.0 to 26.0 μL, while EF decreased from 344.4 to 166.4 for PCBs, and from 549.0 to 217.6 for PBDEs, respectively. As can be seen from Fig. 2, the range of extraction recovery increased gradually when the volume of [C8MIM][PF6] was increased from 30.0 to 40.0 μL, while it remained constant from 40.0 to 55.0 μL. Therefore, 40.0-μL [C8MIM][PF6] was chosen as the optimum volume.
Effect of volume of extraction solvent ([C8MIM][PF6]) on extraction efficiency of PCBs and PBDEs obtained from TA-IL-DLLME. Extraction conditions: water sample volume, 5.0 mL, concentration level at 10.0 ng mL−1; disperser solvent (methanol) volume, 1.0 mL; extraction solvent volume, 30.0, 35.0, 40.0, 45.0, 50.0 and 55.0 μL; centrifugation time, 8 min; pH, 1.0; temperature, 40 °C; data for triplicate extractions
Effect of volume of disperser solvent
After choosing methanol as disperser solvent, it is necessary to optimize its volume. At low volume, methanol can’t disperse IL properly and cloudy solution is not formed completely, leading to low extraction efficiency. On the contrary, the solubility of PCBs and PBDEs in water increases at high volume of methanol, and thus the extraction efficiency decreases too. To obtain optimized volume of methanol, a series of experiments were conducted by using different volumes of methanol (0.5, 0.8, 1.0, 1.5 and 2.0 mL) containing 35.0, 37.0, 40.0, 51.5 and 61.0 μL [C8MIM][PF6], respectively. It is necessary to change the volume of [C8MIM][PF6] by changing the volume of methanol in order to obtain constant volume of sedimented phase (15.0 ± 0.5 μL). Fig. 3 shows the curves of extraction recoveries of PCBs and PBDEs versus the volume of methanol. According to Fig. 3, 1.0 mL methanol was chosen as the optimum volume.
Effect of volume of disperser solvent (methanol) on extraction efficiency of PCBs and PBDEs obtained from TA-IL-DLLME. Extraction conditions: water sample volume 5.0 mL, concentration level at 10.0 ng mL−1; disperser solvent (methanol) volume, 0.5, 0.8, 1.0, 1.5 and 2.0 mL; extraction solvent ([C8MIM][PF6]) volume 35.0, 37.0, 40.0, 51.5 and 61.0 μL; centrifugation time, 8 min; pH, 1.0; temperature, 40 °C; data for triplicate extractions
Effects of extraction temperature and sample pH
Temperature acts an important role as an assisted factor in the extraction process, which can affect the mass transfer rates of analytes and increase the contact area between IL and aqueous solution [21]. In this investigation, the extraction efficiencies of analytes for [C8MIM][PF6] at 30 °C were lower than those at 40 °C (with exception of CB-101 and BDE-99). However, a decreasing trend was observed with further increase of temperature from 40 °C (data not shown in figure). Therefore, 40 °C was selected as the optimum temperature.
In water samples, the sample pH (1.0 ~ 11.0) had not a remarkable effect on the extraction of PCBs and PBDEs, while it was not the case in urine samples. The acidic conditions aid to remove large molecular proteins, to reduce the biomolecular interference, and to enhance extraction efficiency. The best extraction efficiency was observed at pH 1.0 in urine samples. After taking all factors into consideration, the pH value was adopted at pH 1.0 in water or urine samples.
Analytical feature of the method
For the purpose of quantitative analysis, the calibration curve was obtained under the optimized TA-IL-DLLME-HPLC conditions. The precision of this method was evaluated by carrying out five independent measurement of the studied compounds at 10 ng mL−1. As listed in Table 1, the relative standard deviation (n = 5) and limits of detection (S/N = 3) were in the range of 1.0–5.6% and 0.1–0.4 ng mL−1, respectively. The linear range was between 0.5 and 500 ng mL−1 for PCBs and PBDEs. The correlation coefficients ranged from 0.9992 to 0.9999. Furthermore, the relatively higher EF values (278–343) were also obtained under the optimized conditions.
Real water and urine sample analysis
Water and urine samples, collected from Qiantang River (Hanzhou, China) and from healthy individuals, respectively, were used to test the applicability and accuracy of the method. The residues of CB-101, CB-138, BDE-99 and CB-153 were found to be 8.2, 4.7, 0.9 and 5.3 ng mL−1 in water samples, respectively. However, it was detected to be at below detectable level (<0.1 ng mL−1) in all urine samples. Then the analytes were fortified to the water and urine samples at two levels (10 and 100 ng mL−1). As summarized in Tables 2 and 3, the ER values were in the range of 84.0–110.3% for 10-ng mL−1 treatments, and of 81.0–127.1% for 100-ng mL−1 treatments, respectively. Therefore, it can be concluded that TA-IL-DLLME pretreatment technique is feasible for quantitative and routine multi-residue analyses of PCBs and PBDEs in real water and urine samples. The chromatograms in blank and spiked urine samples are displayed in Fig. 4.
Chromatograms in (a) blank, (b) spiked urine samples at the concentration level of 10.0 ng mL−1 and (c) spiked urine samples at the concentration level of 100 ng mL−1, which were all obtained using TA-IL-DLLME technique. (1) BDE-47; (2) CB-101; (3) CB-138; (4) BDE-99; (5) CB-153; (6) BDE-154; (7) CB-180; (8) BDE-183
Comparison of TA-IL-DLLME with other methods
The presented method was compared with other methods such as liquid-liquid extraction, solid-phase microextraction and single-drop microextraction with reference to relative standard deviation, limit of detection, EF, and required sample volume. As summarized in Table 4, the required sample volume is very low (only 5 mL) in the TA-IL-DLLME procedure, while liquid-liquid extraction and solid-phase microextraction procedures requires 500 and 30 mL, respectively [26, 27]. This technique also provides much higher EF (100–400) in comparison with that of single-drop microextraction (ca. 10) [28], and the comparable relative standard deviation and limit of detection were obtained among the four pretreatment techniques described above. Additionally, the use of ionic liquid ([C8MIM][PF6]) involves some advantages such as reduction of exposure to toxic solvents, and possibility of obtaining more reproducible results since evaporation of extraction solvent is not required [22]. Although the direct DLLME method for PCBs, developed by Razaei et al. in 2008 [15], has slightly higher EF (378–540) than the presented method, it requires toxic chlorobenzene as extraction solvent. Zhang and co-workers in 2010 reported the TA-IL-DLLME for the pretreatment of anthraquinones in solid herbal medicines [21], but the method required refluxing for 60 min with 25 mL methanol firstly, and then combined with microextraction procedures, leading to time-consuming and tedious processes. Besides, the method described here has lower IL usage, high EF and can be also used in urine samples as compared with IL-DLLME [29], IL cold-induced aggregation DLLME [30] and IL head-space single-drop microextraction [31]. In conclusion, the presented method in this paper reveals an excellent prospect in the field of sample pretreatment of PBDEs and PCBs in water or urine samples.
Conclusions
In this work, the TA-IL-DLLME technique was successfully applied for separation and preconcentration of eight PCBs and PBDEs from water and urine samples prior to multi-residue analyses by HPLC. Compared with other conventional pretreatment techniques (e.g. liquid-liquid extraction and microwave-assisted extraction), the presented method displays a higher precision, better separation efficiency and much lower sample volume required. In addition, an IL, [C8MIM][PF6], is used as extraction solvent, which is safer and more environmentally friendly. This extraction procedure is noticeable due to its lower IL usage in comparison with IL-DLLME technique for the determination of PCBs. In conclusion, the presented technique has a great potential for the multi-residue analyses of PCBs and PBDEs at trace levels in environmental water and human urine samples.
References
Shimizu K, Ogawa K, Thiele JJ, Lee JB, Bae S, Sato S (2008) Increased levels of urinary nitrite and nitrotyrosine in Yusho victims 40 years after accidental poisoning with polychlorinated biphenyls in Nagasaki, Japan. J Appl Toxicol 28:1040–1044
Kalantzi OI, Geens T, Covaci A, Siskos PA (2011) Distribution of polybrominated diphenyl ethers (PBDEs) and other persisitent organic pollutants in human serum from Greece. Environ Int 37:349–353
Tue NM, Sudaryanto A, Minh TB, Isobe T, Takahashi S, Viet PH, Tanabe S (2010) Accumulation of polychlorinated biphenyls and brominated flame retardants in breast milk from women living in Vietnamese e-waste recycling sites. Sci Total Environ 408:2155–2162
She J, Petreas M, Winkler J, Visita P, McKinney M, Kopec D (2002) PBDEs in the San Francisco Bay Area: measurements in harbor seal blubber and human breast adipose tissue. Chemosphere 46:697–707
Schiavone A, Kannan K, Horii Y, Focardi S, Corsolini S (2010) Polybrominated diphenyl ethers, polychlorinated naphthalenes and polycyclic musks in human fat from Italy: Comparison to polychlorinated biphenyls and organochlorine pesticides. Environ Pollut 158:599–606
Esteban M, Castaño A (2009) Non-invasive matrices in human biomonitoring: A review. Environ Int 35:438–449
Kazda R, Hajšlová J, Poustka J, Čajka T (2004) Determination of polybrominated diphenyl ethers in human milk samples in the Czech Republic: Comparative study of negative chemical ionization mass spectrometry and time-of-flight high resolution mass spectrometry. Anal Chim Acta 520:237–243
Li QQ, Loganath A, Chong YS, Obbard JP (2005) Determination and occurrence of polybrominated diphenyl ethers in maternal adipose tissue from inhabitants of Singapore. J Chromatogr B 819:253–257
Carro AM, Lorenzo RA, Fernández F, Rodil R, Cela R (2005) Multi-residue screening of chlorinated and brominated compounds from aquaculture samples using matrix solid-phase dipersion-gas chromatography–mass spectrometry. J Chromatogr A 1071:93–98
Xiao Q, Hu B, Duan J, He M, Zu W (2007) Analysis of PBDEs in soil, dust, spiked lake water, and human serum samples by hollow fiber-liquid phase microextraction combined with GC-ICP-MS. J Am Soc Mass Spectrom 18:1740–1748
Herrera-Herrera AV, Asensio-Ramos M, Hernández-Borges J, Rodríguez-Delgado MÁ (2010) Dispersive liquid-liquid microextraction for determination of organic analytes. Trends Analyt Chem 29:728–751
Hu J, Li YY, Zhang W, Wang HL, Huang CJ, Zhang MH, Wang XD (2009) Dispersive liquid-liquid microextraction followed by gas chromatography-electron capture detection for determination of polychlorinated biphenyls in fish. J Sep Sci 32:2103–2108
Hu J, Fu LY, Zhao XN, Liu XJ, Wang HL, Wang XD, Dai L (2009) Dispersive liquid-liquid microextraction combined with gas chromatography-electron capture detection for the determination of polychlorinated biphenyls in soils. Anal Chim Acta 640:100–105
Liu XJ, Hu J, Huang CJ, Wang HL, Wang XD (2009) Determination of polybrominated diphenyl ethers in aquatic animal tissue using cleanup by freezing-dispersive liquid-liquid microextraction combined with GC-MS. J Sep Sci 32:4213–4219
Rezaei F, Bidari A, Birjandi AP, Milani-Hosseini MR, Assadi Y (2008) Development of a dispersive liquid-liquid microextraction method for the determination of polychlorinated biphenyls in water. J Hazard Mater 158:621–627
Liu XJ, Li JW, Zhao ZX, Zhang W, Lin KF, Huang CJ, Wang XD (2009) Solid-phase extraction combined with dispersive liquid-liquid microextraction for the determination for polybrominated diphenyl ethers in different environmental matrices. J Chromatogr A 1216:2220–2226
Li YY, Wei GH, Hu J, Liu XJ, Zhao XN, Wang XD (2008) Dispersive liquid-liquid microextraction followed by reversed phase-high performance liquid chromatography for the determination of polybrominated diphenyl ethers at trace levels in landfill leachate and environmental water samples. Anal Chim Acta 615:96–103
Li YY, Hu J, Liu XJ, Fu LY, Zhang X, Wang XD (2008) Dispersive liquid-liquid microextraction followed by reversed phase HPLC for the determination of decabrominated diphenyl ether in natural water. J Sep Sci 31:2371–2376
Liu Y, Zhao E, Zhu W, Gao H, Zhou Z (2009) Determination of four heterocyclic insecticides by ionic liquid dispersive liquid-liquid microextraction in water samples. J Chromatogr A 1216:885–891
Abdolmohammad-Zadeh H, Sadeghi GH (2010) Combination of ionic liquid-based dispersive liquid-liquid microextraction with stopped-flow spectrofluorometry for the preconcentration and determination of aluminum in natural waters, fruit juice and food samples. Talanta 81:778–785
Zhang HF, Shi YP (2010) Temperature-assisted ionic liquid dispersive liquid-liquid microextraction combinated with high performance liquid chromatography for the determination of anthraquinones in Radix et Rhizoma Rhei samples. Talanta 82:1010–1016
Pena MT, Casais MC, Mejuto MC, Cela R (2009) Development of an ionic liquid based dispersive liquid-liquid microextraction method for the analysis of polycyclic aromatic hydrocarbons in water samples. J Chromatogr A 1216:6356–6364
Rezaee M, Yamini Y, Faraji M (2010) Evolution of dispersive liquid-liquid microextraction method. J Chromatogr A 1217:2342–2357
Peng JF, Liu JF, Hu XL, Jiang GB (2007) Direct determination of chlorophenols in environmental water samples by hollow fiber supported ionic liquid membrane extraction coupled with high-performance liquid chromatography. J Chromatogr A 1139:165–170
Liu JF, Chi YG, Jiang GB (2005) Screening the extractability of some typical environmental pollutants by ionic liquids in liquid-phase microextraction. J Sep Sci 28:87–91
Zaater M, Tahboub Y, Qasrawy S (2005) Monitoring of polychlorinated biphenyls in surface water using liquid extraction, GC/MS, and GC/ECD. Anal Lett 38:2231–2245
Cortazar E, Zuloaga O, Sanz J, Raposo JC, Etxebarria N, Fernández LA (2002) Multisimplex optimization of the solid-phase microextraction gas chromatographic-mass spectrometric determination of polycyclic aromatic hydrocarbons, polychlorinated biphenyls and phthalates from water samples. J Chromatogr A 978:165–175
Li YY, Wei GH, Wang XD (2007) Determination of decabromodiphenyl ether in water samples by single-drop microextraction and RP-HPLC. J Sep Sci 30:2698–2702
Han DD, Row KH (2011) Trends in liquid-phase microextraction, and its application to environmental and biological samples. Microchim Acta. doi:10.1017/s00604-011-0678-0
Jiang XY, Zhang H, Chen XQ (2011) Determination of phenolic compounds in water samples by HPLC following ionic liquid dispersive liquid-liquid microextraction and cold-induced aggregation. Microchim Acta 175:341–346
Zhao FQ, Lu S, Du W, Zeng BZ (2009) Ionic liquid-based headspace single-drop microextraction coupled to gas chromatography for the determination of chlorobenzene derivatives. Microchim Acta 65:29–33
Acknowledgements
This work was jointly funded by National Natural Science Foundation of China (21077079), Public Benefit Project of Zhejiang Province (2011C23112, 2011C37006), Key Project of Zhejiang Environmental Protection Department (2011B25) and Project of Wenzhou Science and Technology Bureau (H20100053, H20100054, S20100034).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Esm 1
(DOC 1982 kb)
Rights and permissions
About this article
Cite this article
Zhao, A., Wang, X., Ma, M. et al. Temperature-assisted ionic liquid dispersive liquid-liquid microextraction combined with high performance liquid chromatography for the determination of PCBs and PBDEs in water and urine samples. Microchim Acta 177, 229–236 (2012). https://doi.org/10.1007/s00604-012-0776-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-012-0776-7