Abstract
This work presents a sensitive method for the determination of formaldehyde. It is based on the use of modified alumina nanoparticles for its preconcentration, this followed by a new and simple catalytic kinetic method for its determination. Alumina nanoparticles were chemically modified by immobilization of 2,4-dinitrophenylhydrazine via sodium dodecyl sulfate as a surfactant. The formaldehyde retained on the modified adsorbent was then desorbed and determined via its catalytic effect on the oxidation of thionine by bromate ion. Factors affecting the preconcentration and determination of formaldehyde have been investigated. Formaldehyde can be detected in the range from 0.05 to 38.75 μg L−1, and no serious interferences have been observed. The method has been successfully applied to the quantitation of formaldehyde in water, food, and certain biological samples.

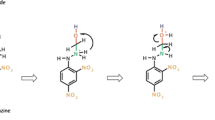
Schematic representation of the grafting 2,4-dinitrophenylhydrazine on the SDS coated alumina nanoparticles for the preconcentration and determination of formaldehyde based on its catalytic effect on oxidation reaction of thionine by bromated ions. This method can be used to the determination of formaldehyde in different real samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Formaldehyde (FA) is considered as one of the most significant industrial hazards and environment pollutants and can be found in the air, natural food, some skin-care products as well as preservatives in processed foods, especially dried foods and frozen foods [1, 2]. This compound has been classified as a probable human carcinogen by the International Agency for Research on Cancer; and by the U.S. Environmental Protection Agency. Furthermore, the World Health Organization (WHO) has established a maximum daily dose reference (RfD) of 0.2 mg kg−1 per day for this compound [3–5]. The evidences show that endogenous FA is present in urine, blood and all cells including the brain. Because of the adverse health effects, exposures to FA have been an issue of serious concern. In recent years, there has been a tendency in the industries to restrict and regulate the use of FA in their products. Whereas, regarding the significant role of FA in industrial processes due to its high reactivity and relatively low cost, it is still widely found in some products.
Therefore, researches on the determination of trace amounts of FA have great importance [6]. Chromatographic and spectrophotometric methods are among the most common choices. In general, in an aqueous environment, most chromatographic and spectrometric methods for the determination of FA often require time-consuming chemical reaction with various reagents to form derivatives. Of these, 2,4-dinitrophenylhydrazine (DNPH) is mostly used to react with FA to form the hydrazone [7]. However, DNPH can react with many aldehydes and ketones, and the DNPH derivatization reaction takes 1 h for a complete reaction [8, 9]. The chromotropic acid method [8, 9], MBTH (3-methyl-2-benzothiazolone hydrazone) method [10, 11], AHMT (4-amino-3-hydrazino-5-mercapto-1,2,4-triazole) [12, 13] and pararosaniline method [14, 15] are popular colorimetric methods for the detection of FA. In these methods serious problems are present; for example, the chromotropic acid method requires concentrated H2SO4, which is potentially hazardous and corrosive, and the requirement of heating the resulting solution for about 1 h in a steam bath (100°C), makes its utilization less attractive in routine analysis [9]. The MBTH method has been less commonly used because it is very expensive, unstable and can react easily with other aldehydes [16, 17]. The AHMT method needs a very strong base as the reaction medium, which is not desirable especially as carbonate formation will occur. In the method using pararosaniline-based Schiff reaction, color development is relatively slow and sensitivity is not so good [18]. Most of the methods for the determination of FA were reviewed in 2001 [19].
The general trend of modern analytical chemistry is towards the elaboration of simple, ecologically safe, sensitive, and selective methods for the determination of trace components combining previous concentration methods and further determination by physical or physico-chemical methods. Among the separation/preconcentration methods, solid-phase extraction (SPE) has become the most frequently used technique for trace species analysis [20–22]. In order to achieve more effective extractions, new types of sorbents, e. g., human hair, bone powder, nanomaterials, various polymers and various microorganisms (biosorbents) are currently being developed. In recent years, new solid nano-sized materials have become more important due to their special physical and chemical properties [23–25].
Nano alumina is characterized by strong adsorption capacity, attributed to its high surface area, porosity, degree of surface reactivity, mechanical strength and low temperature modification. However, non- modified alumina nanoparticles are hydrophilic and do not show ideal adsorption capacity for organic compounds [24]. Retention of FA in non-modified alumina nanoparticles is not quantitative owing to the weak interaction between this organic compound and the hydrophilic surface of alumina, thus chemical or physical modification of the surface of γ-Al2O3 nanoparticles with certain functional groups containing some donor atoms is necessary [21, 26].
Recently, carbons or layered materials containing amino groups which can react with aldehydes are reported as the adsorbents in FA removal [27–29]. We have recently reported immobilization of amino groups onto the alumina nanoparticles using DNPH [21, 24]. Therefore, it is anticipated that modification of alumina nanoparticles with amino-containing compounds can enhance their extraction capacity for FA because of the cooperation of physical adsorption and the increased chemical interaction between amino groups on the surface of alumina nanoparticles and FA molecules.
One goal of our research is to prepare an effective, selective and cost effective nano-adsorbent for the extraction and preconcentration of FA from various real samples. Furthermore, in order to determine FA concentration during suggested extraction process, we applied a simple, sensitive, rapid and inexpensive catalytic kinetic spectrophotometric method for monitoring of FA concentration in samples via its catalytic effect on oxidation of thionine by bromate ions in acidic media. The ease of the method is noteworthy. The preconcentration and determination steps are rapid and require no difficult laboratory procedures. The kinetic methods are simple, sensitive, rapid and inexpensive in comparison with HPLC and other reported methods [30–34]. Also, the comparative data from some recent papers on catalytic kinetic methods of FA determination for the figures of the merit are summarized in Table S1 (Electronic Supplementary Material, ESM).
Experimental
Apparatus and chemicals
An Agilent 8453 diode array UV–Vis spectrophotometer (Agilent, USA, www.agilent.com) equipped with 1.0 cm path length quartz cells was used to obtain absorbance spectra and absorbance curves at fixed wavelength in a given time period. Infrared spectra were recorded with a Fourier transform infrared (FT-IR) spectrometer (FT-IR, Perkin Elmer, spectrum 100, USA, www.perkinelmer.com). Samples were gently powdered and diluted in nonabsorbent KBr matrices to identify the functional groups and chemical bonding of the coated materials.
Scanning electron microscopy (SEM) was performed to measure the particle size and shape (SEM-EDX, XL30 and Philips Netherland, www.philips.com). Surface area and porosity were defined by N2 adsorption–desorption porosimetry (77 K) using a porosimeter (Bel Japan, Inc.). A Metrohm model 713 (Herisau, Switzerland, www.metrohm.com) pH-meter with a combined glass electrode was used for pH measurements. The investigations of column method were performed using a peristaltic pump (Ismatec, MCP, ISM404B, Switzerlands).
All the reagents used were of analytical grade. Double distilled water (DDW) was used throughout. Laboratory glassware was kept overnight in a 10% (v/v) HNO3 solution and then rinsed with DDW. Alumina nanoparticles (average size of 40 nm, γ-type (X-ray analysis), spherical shape, 99.9% purity) were purchased from Plasmachem GmbH (Germany, www.plasmachem.com). 2,4-Dinitrophenylhydrazine (DNPH) and sodium dodecyl sulfate (SDS) were purchased from Merck (Darmstadt, Germany, www.merck.de). pH adjustments were performed with 0.01–1.0 mol L−1 HCl or NaOH solutions.
A 2,000 μg mL−1 stock solution of FA was prepared by diluting 5.0 mL (37%) FA solution (Merck) to 1,000 mL with DDW and standardized by sulfite standard solution using a titrimetric method [35]. This solution was stored at 4°C. FA working standard solutions were prepared daily from the stock standard solution by appropriate dilution with water. All experiments with FA must be performed in a well-ventilated area such as a fume hood. Sulfuric acid solutions were prepared by dissolving calculated amount of concentrated acid (Merck). Thionine reagent (3.484 × 10−4 mol L−1) was prepared by dissolving 0.010 g in DDW and diluting to 100 mL. Bromate solution (0.125 mol L−1) was prepared by dissolving 2.0876 g of KBrO3 (Merck) in DDW in a 100 mL volumetric flask.
For each sample and blank, five replicate measurements were made and the mean signals were used.
Preparation of the solid sorbent
2,4-Dinitrophenylhydrazine was selected as a modifying agent to graft functional amine groups on the γ-Al2O3 nanoparticles by mixing the suspension of the nanoparticles, SDS and DNPH. In detail, the amine group grafted samples were prepared as follows: a 2.0 g sample of alumina nanoparticles was suspended in 50 mL of water and mixed with 100 mg of SDS. Then, 20 mL of DNPH solution (0.90 g DNPH in concentrated HCl+acetonitrile) were added. The suspension was stirred at 60°C for 3 h. The mixture was followed by evaporation of the solvent, washing, air-drying, and storing in a closed bottle for subsequent uses.
Samples preparation
Food samples were purchased from retail stores in Hamedan, Iran. Each food sample (2–5 g) was mixed with 20–30 mL of DDW in a conical tube and shaking for 15 min to release the FA from the food sample. The supernatant was filtered through a filter syringe [1]. The filtrate was measured as will be described in the next section.
Tap water sample was taken after flowing for 10 min from water tap in our faculty. Reservoir water sample was collected from local household reservoir; which is known to store water for several weeks. Stream water sample was collected from Alvand stream, Hamedan. Rain water sample was collected in rainy days of Aug 2011 in Ganjnameh, Hamedan. After collecting the samples, the bottles were capped carefully to avoid spillage, and were agitated by hand for 1 min. Before use, all the environmental water samples were filtered through 0.45 μm micropore membranes and stored in polypropylene bottles at 4°C away from light. Samples must be analyzed within 7 days of sampling. However, since aldehydes are subjected to decay in stored samples, all samples should be analyzed as soon as possible [36].
As the urinary sample is one of the main ways for the detection of endogenous FA, in this work, we sought to determine whether FA levels in the urine were possible with suggested method. Five healthy controls and five patients with common aging-related diseases (hypertension or diabetes) were also evaluated. Informed consent was obtained for all participants, and for illiterate patients, informed consent was obtained from relatives. This clinical investigation was approved by the Ethics Committee of Nader Clinic of Tehran, Iran. Patients with renal disease and urine abnormalities were excluded from the study. Morning urine samples of patients were taken before breakfast. Urine samples from healthy controls with neither any neurological disorders nor known alcohol and drug abuse, were also taken. Urine was collected from each participant and immediately placed on ice and stored in freezer. To avoid the samples being thawed too many times, the urine was freshly analyzed or divided into equal parts to be stored for only one time use. After centrifugation (3,000 rpm, 10 min), supernatant fractions of urine samples were analyzed by suggested method [37].
Procedures
Batch procedure
For the batch adsorption experiments, a 100 mL sample solution containing 0.05–38.75 μg L−1 of FA and 70 mg modified nanoparticles were put into a 250 mL beaker and the pH of the mixture was adjusted to the desired value with 0.1 mol L−1 HCl and/or 0.1 mol L−1 NaOH. Then the mixture was shaken vigorously for 15 min to facilitate adsorption of the FA onto the adsorbents. After that, modified adsorbent suspension was centrifuged. The adsorbent was then washed with a 2.5 mL solution of 0.5 mol L−1 sulfuric acid followed by 1 mL of DDW. The eluate was transferred into a 5 mL volumetric flask. Then 0.4 mL of 3.484 × 10−4 mol L−1 thionine and 0.7 mL of 0.125 mol L−1 bromate solution were added in sequence. The solution was diluted to the mark with DDW. The zero time was taken as the moment at which the last drop of bromate solution was added and the solution was mixed well for 20 s. After that, a portion of the solution was transferred into a 1.0 cm quartz cell and the decrease in the absorbance was measured at 598 nm (ΔAS) for first 300 s after initiation of the reaction, with time intervals of 20 s at 25°C. The blank reaction was performed according to the same procedure without addition of FA and the decrease in the absorbance was labeled as ΔA0.
Column SPE procedure
To assess the sorption efficiency, 70 mg of adsorbent was packed in a glass column plugged with a small portion of glass wool at both ends with diameter of 7 mm. Under optimum conditions, a 100 mL of aqueous solution with FA concentration of 0.05–38.75 μg L−1, was passed through the sorbent bed at a flow rate of 6 mL min−1. The column was then washed with a 2.5 mL solution of 0.5 mol L−1 sulfuric acid followed by 1 mL of double distilled water. The FA concentration in the collected eluate was determined spectrophotometrically according to the suggested method. In both the experiments (batch and column), the color of adsorbent changed from orange to olive green.
Results and discussion
Mechanism of the extraction and desorption
Carbonyl group in the FA molecule can react with primary amine to produce imine, according to the following reaction mechanism [20, 27]:

FA was regenerated by washing the adsorbent with acid;

According to Eq. (1), reaction of FA with the amino groups on the surface of the alumina, can cause a significant increase in the amount of FA adsorbed onto the modified nano-alumina. These reasons might be effective in efficient extraction of FA: (1) the active hydrogen atom of adsorbent might react with FA to form alcohol [38]; (2) the nitrogen atoms in the DNPH-γ-Al2O3 structure possess partially negative charge (δ-) for its strong electronegativity, while carbon atom of FA shows slightly positive charge (δ+) for its weaker electronegativity than oxygen atom. So the nitrogen atoms in the DNPH-γ-Al2O3 structure and carbon atom in the carbonyl group of FA will attract each other for the electrostatic gravitation, which will lead to better FA adsorption capacity over non-modified alumina nanoparticles. These interactions led to change color of adsorbent from orange to olive green.
Surface characterizations of adsorbent
Detailed surface characterizations of modified γ-alumina are presented in our previous papers [21, 24]. Successful surface modification with DNPH moieties was verified with infrared spectroscopic analysis of the material. Comparison of the KBr FT-IR spectra of DNPH, modified γ-alumina and γ-alumina nanoparticles indicated that the DNPH molecules modified the γ-alumina nanoparticles [21, 24]. A comparison between the characteristic spectral bands indicated that the surface DNPH-γ-Al2O3 contained –NH– functional group as a result of the immobilization procedure. On the other hand, comparing the IR-spectra of the modified alumina phase after FA sorption with the FA free one clearly demonstrate that the nitrogen containing groups are the main active donating sites to FA. This is based on: (a) the shift of N-H stretching vibrations around 25–40 cm−1 characteristic to FA binding to this functional sites and (b) the appearance of stretching frequency band at 1,618 cm−1 characteristic to FA binding to nitrogen functional sites and formation of imine (Fig. 1).
The SEM images show that the non-modified alumina nanoparticles had a mean diameter of 53 nm and the DNPH modified nanoparticles had a mean diameter of 75 nm. This shows that the alumina nanoparticles have been completely coated by DNPH. The specific surface area of alumina nanoparticles and the modified one, obtained by BET analysis, was found to be 42.62 m2 g−1, and 30.38 m2 g−1, respectively. Obviously, more functional groups were grafted on the small nanoparticles due to their large specific surface areas. The decrease in BET surface area of modified γ-Al2O3 with DNPH is due to the bulk size of the organic ligand, which blocks the surface of the alumina nanoparticles. This decrease in surface area can also be due to aggregation after surface modification. According to BET theory, this decrease indicates the decrease in interaction between adsorbent-N2 molecules and the particle surface after modification with DNPH.
Variables affecting the preconcentration process
Effect of pH
The effect of pH on the preconcentration of 2.5 μg L−1 of FA was studied over the range from 2.0 to 10.0 using either 0.01–0.1 M NaOH or HCl. The experimental results showed that the adsorption performance was the best at the pH range 4.5–8.5 (Fig. S1, ESM). The extraction efficiency decreased at pH values lower than 4.5. At pHs <4.5, the hydrogen ions (H+) protonate the nitrogen-containing functional groups and therefore, FA adsorption decreases in strong acidic solutions. Also, low FA extraction at acidic pHs is due to the fact that the reactivity of an unsaturated compound such as FA with adsorbent is very low. By considering the results and in order to apply industrial usages, pH 7.0 was selected as the optimized pH for all subsequent experiments.
Effect of the amount of sorbent
The effect of the amount of sorbent on preconcentration of FA was studied in the range 10–100 mg. In the present work, by increasing the amounts of the DNPH modified γ-alumina nanoparticles the extraction efficiency increased due to increase in the surface area and accessible sites to the sorption of the analytes. Thus, quantitative extraction of the FA was achieved using only 70 mg of this adsorbent within 30 min under the optimum pH. When higher amounts of sorbent were used, the recovery dropped slightly because more eluent volume is required for complete elution of the analyte. Therefore, in the subsequent experiments, 70 mg of the adsorbent was added to 50 mL of the sample solution.
Effect of shaking time
The shaking time is an important factor in determining the equilibration rate. In this work, different shaking times (from 5 to 60 min) were studied. The results showed that the 99% uptakes of FA were achieved within 15 min in solution of 2.5 μg L−1 FA (Fig. S2, ESM). It also indicated that DNPH modified γ-alumina nanoparticles had rapid adsorption kinetics for FA.
Effect of flow rate
An important parameter in extraction by column is the flow rate of the sample solution, which influence the sorption of analyte to the sorbent surface and the analysis time. Therefore, the effect of the flow rate of sample solution was examined for column system under the optimum conditions (pH, eluent, etc.) by passing 50 mL of sample solution through the column with a peristaltic pump. The flow rates were adjusted in the range 1.0–9.0 mL min−1. It was found that the sorption of the analyte decreased when the flow rate was higher than 6.0 mL min−1. Thus, a flow rate of 6.0 mL min−1 was employed in this work.
Effect of the nature of the eluent and eluent volume
The nature of the eluent is of prime importance and should optimally meet three criteria: efficiency, selectivity and compatibility. With attention to Eq. (2), one can expect that elution will be favored in acidic solutions. So various concentrations of acid and other eluants were used for desorption of the retained FA (Table 1). The obtained results (Table 1) showed that 2.5 mL of 0.5 mol L−1 H2SO4 solution was sufficient for complete elution of retained FA. The flow rate for desorption of FA was varied from 0.5 to 4.0 mL min−1. The results showed that target analyte can be quantitatively eluted at flow rates below 2.0 mL min−1.
Effect of sample volume
Due to the low concentration of FA in real samples, the FA contents samples with large volumes should be taken into smaller volumes to obtain maximum preconcentration factor. Hence, the maximum sample volume was optimized by the investigation of the recoveries of FA in various sample volumes in the range 25–150 mL containing the same amount of FA. The recoveries were found to be stable up to 100 mL and then they started to decrease.
Optimization of catalytic kinetic procedure variables
In acidic media bromate can oxidize thionine at a very slow rate. Trace amounts of FA catalyze the reaction and cause a significant increase in reaction rate (Fig. 2).
Absorption spectra for the thionine and bromate mixture in acidic media in the absence of formaldehyde (a) and in the presence of formaldehyde (b). Conditions: sulfuric acid, 0.25 mol L−1; thionine, 2.79 × 10−5 mol L−1; bromate, 1.75 × 10−2 mol L−1 and temperature 25°C with scan time intervals of 20 s to 300 s
The reaction variables were optimized in order to maximize the sensitivity and precision of the suggested catalytic kinetic method. In order to find the optimal conditions for the catalytic determination of FA, the effects of type and concentration of acid, concentration of the reagents, temperature, time and ionic strength were examined, in both the catalyzed and uncatalyzed reactions.
As the reaction took place in acidic media, the effect of various acids such as sulfuric, hydrochloric and phosphoric acid with the same concentration on the determination of FA was studied. The results showed that greater sensitivity was obtained with sulfuric acid. The effect of sulfuric acid concentration on the determination of FA was studied within the range 0.10–0.50 mol L−1. The sensitivity increased by increasing acid concentration up to 0.25 mol L−1, whereas higher amounts of acid do not affect it (Fig. S3, ESM). Therefore 0.25 mol L−1 sulfuric acid was used for the future studies. The results showed that maximum sensitivity can be achieved in a wide range of acid concentration.
The effect of thionine concentration on the sensitivity was investigated within the range 6.97 × 10−6 to 2.79 × 10−5 mol L−1. The results show that the difference between absorbance changes for sample and blank solutions (Δ(ΔA) = ΔAs–ΔAb), increases with increasing thionine concentration (Fig. 3). From the analytical point of view, thionine concentration should be used that provides an absorbance in the range of minimum photometric error. Therefore, 2.79 × 10−5 mol L−1 of thionine was chosen as the most suitable concentration.
The influence of bromate concentration on the reaction rate was studied in the range of 2.5 × 10−3–2.5× 10−2 mol L−1 with 0.25 mol L−1 sulfuric acid and 2.79 × 10−5 mol L−1 thionine at room temperature. The results showed that by increasing the bromate concentration up to 1.5 × 10−2 mol L−1, the sensitivity (Δ(ΔA)) increased, whereas concentrations of bromate higher than 2.0 × 10−2 mol L−1 caused a decrease in sensitivity (Fig. S4, ESM). This effect was due to the fact that at higher concentrations of bromate the blank reaction was so fast and therefore, the sensitivity decreased. Thus, the concentration of 1.75 × 10−2 mol L−1 as the optimum concentration was selected for bromate.
The effect of the temperature on the rate of catalyzed and blank reactions was studied within the range 20–45°C at the optimum reagent concentrations. The reaction rate and the sensitivity increased negligibly with temperature; however, a temperature of 25°C was adopted in the recommended procedure because of its convenience for operation and its relatively low blank value.
The ionic strength effect on the analytical signal used in construction calibration curve (Δ(ΔA)) was studied in the concentrations of 0.0–2.0 mol L−1 using Na2SO4 solution and optimal concentrations of other reagents. The results show that the catalytic effect was independent on the ionic strength up to 2.0 mol L−1 (maximum value tested) of sodium sulfate. This observation can be probably attributed to the non-ionic nature of reaction transition state.
The time for measuring the change in absorbance was also optimized and it was found that sensitivity increased up to 6 min, but at 5 min the reaction had good sensitivity, so 5 min was selected.
The catalytic system
Thionine is a cationic dye that has been used in spectrophotometric methods. For example, it has been used in the kinetic determination of silver(I), copper(II) and etc. [39, 40]. In this investigation, it was observed that the reaction of thionine and bromate at room temperature and in an acidic media was slow (reaction 3 and Fig. 2a). In the presence of trace amounts of FA, the reaction rate increased significantly due to the catalytic effect of FA. Bromate ion produces Br2 in the presence of FA in acidic media (reactions 4 and 5) [41]. The produced Br2 reacts with thionine and changes it to a colorless substance and a catalytic cycle is formed (reaction 6). Further studies indicated that catalytic effect increases linearly by increase in FA concentration. As a result, thionine (Th) absorbance at λ max (598 nm) decreases with time (Fig. 2b).



The reaction rate was monitored spectrophotometrically by measuring the decrease in the absorbance at 598 nm. In reactions (4)–(6), the bromide ion liberated again after the catalytic reduction reaction reacts with bromate ion. The rate equation for the uncatalyzed-reaction can be written as following:
Due to the overall reaction, the catalyzed and uncatalyzed reactions occur simultaneously, the rate equation for the reaction can be written:
In which, k′ and k′′ are the rate constants for the uncatalyzed and catalyzed reactions, respectively, a, b, c, d, a′, b′, c′ are the order of the reaction with respect to the each reactant. Because the concentrations of thionine and BrO -3 are greater than FA, it is assumed that the reaction is pseudo first-order.
Interferences study
The investigation of possible interferences including various ions, several organic and inorganic compounds was conducted with regard to possible chemical interferences and the problem of selectivity. The effect of various interferences on the preconcentration and determination of 25 μg L−1 of FA was studied. Tolerance ratio was defined as the ratio of the concentration of the ion causing less than ±5% relative error in the FA determination to the concentration of FA for each interfered species. It was found that their interferences with the determination of FA in studied samples were negligible even when interfering substances were added at higher concentrations than commonly existing ones (Table 2). Therefore, the suggested method had a good selectivity, and was not subject to the interferences in the determination of FA in real samples.
Analytical characteristics
The calibration graph was obtained by plotting the difference between absorbance changes for catalyzed and blank reactions, Δ(ΔA), versus FA concentration using the developed method under the optimal conditions. From the results of experiments, 5 min was chosen as the optimal time, because it provided the best correlation coefficient and sensitivity. The calibration graph was linear in the range 0.05–38.75 μg L−1 of FA for 100 mL sample. The equation of the line was Δ(ΔA) = 1.11 × 10−3 CFA + 0.049 (r = 0.998), where CFA is the FA concentration in μg L−1. The correlation coefficient indicated a good linear correlation between Δ(ΔA) and concentration of FA. Each point in the calibration graph was the average of five replicates. The experimental limit of detection (3 S/m, three times the blank standard deviation divided by the slope of the calibration graph) was 0.013 μg L−1. The relative standard deviation for 10 replicate measurements of 0.25, 4.0 and 27.5 μg L−1 of FA solution was 2.8%, 2.2 and 1.4%, respectively.
Kinetic determination of FA after preconcentration in real samples
To evaluate the analytical applicability of the method, the recommended procedure was applied to the determination of FA in tap water, reservoir water, stream water and rain water, urine samples and food samples. The FA in the samples was determined by standard methods of analysis for hygienic chemists, that is, the acetylacetone method [28, 41]. For comparing the results of this new kinetic method with those of standard procedure, we applied the t-test to compare the mean of results. It can be concluded from Table 3 that there is no significant difference between the results obtained by the two methods for P = 0.05. The results show that the method is suitable for the analysis of real samples.
In order to evaluate the validity of the suggested method for the determination of FA, a recovery test was performed. The samples were spiked with known amounts of FA solutions. Significantly good recoveries from 97.0% to 103% were obtained from the determination of FA in real samples (Tables 3, 4 and 5). The results show that the method is suitable for the analysis of real samples.
Conclusion
A sensitive technique for the determination of formaldehyde in various real samples has been developed. The DNPH modified alumina nanoparticles can be used as a good extractor of FA under simple conditions. Preconcentration by this method significantly improves the limit of quantification over other methods. Also, preconcentration using DNPH-γ-Al2O3 is one of the most sensitive approaches for formaldehyde detection without requiring to the derivatization methods, while being considerably more rapid. After desorption of retained FA, its determination was performed by a simple, rapid and cost-effective catalytic kinetic method. An important feature of this study is that we grafted functional group on the surface of inorganic substrate, γ-Al2O3 nanoparticles. These functionalized nanoparticles exhibited high sorption ability for FA from real samples. This novel design strategy provided the superior advantages for the nano-sorbent in practical application. Considering that amine group can adsorb FA from samples, the amine grafted γ-Al2O3 nanoparticles were employed to extract FA. For both of column and batch experiments, the results indicate that the modified adsorbent provides better sorption ability in comparison to naked-alumina.
It can be found that the presence of large amounts of Na+, Ca2+ and acetaldehyde species did not substantially reduce the sorption capacities of amine grafted nanoparticles for FA. It means that the co-existed competitive cations only have slight influence on the sorption ability of the nano-sorbent. These results suggest that the sorbent is a good sorbent for practical application where competitive ions exist. Also, comparing with other reported spectrophotometric methods, the suggested method was characterized by low cost, high sensitivity and simple system configuration. These made the system promising to be used in routine analytical applications [2, 16, 31–34, 42].
References
Weng X, Chon CH, Jiang H, Li DQ (2009) Rapid detection of formaldehyde concentration in food on a polydimethylsiloxane (PDMS) microfluidic chip. Food Chem 114:1079–1082
Afkhami A, Rezaei M (1999) Sensitive spectrophotometric determination of formaldehyde by inhibition of the malachite green–sulfite reaction. Microchem J 63:243–249
Teshima N, Fernandez SKM, Ueda M, Nakai H, Sakai T (2011) Flow injection spectrophotometric determination of formaldehyde based on its condensation with hydroxylamine and subsequent redox reaction with iron(III)-ferrozine complex. Talanta 84:1205–1208
U.S. Environmental Protection Agency (1989) Office of air and radiation, report to congress on indoor air quality. Vol. (II), assessment and control of indoor air pollution
Burini G, Coli R (2004) Determination of formaldehyde in spirits by high-performance liquid chromatography with diode-array detection after derivatization. Anal Chim Acta 511:155–158
Zhou ZL, Kang TF, Zhang Y, Cheng SY (2009) Electrochemical sensor for formaldehyde based on Pt–Pd nanoparticles and a Nafion-modified glassy carbon electrode. Microchim Acta 164:133–138
Oliva-Teles MT, Paiga P, Delerue-Matos CM, Alvim-Ferraz MCM (2002) Determination of free formaldehyde in foundry resins as its 2,4-dinitrophenylhydrazone by liquid chromatography. Anal Chim Acta 467:97–103
Jones SB, Terry CM, Lister TE, Johnson DC (1999) Determination of submicromolar concentrations of formaldehyde by liquid chromatography. Anal Chem 71:4030–4033
Rivero RT, Topiwala V (2004) Quantitative determination of formaldehyde in cosmetics using a combined solid-phase microextraction–isotope dilution mass spectrometry method. J Chromatogr A 1029:217–222
Toda K, Yoshioka KI, Mori K, Hirata S (2005) Portable system for near-real time measurement of gaseous formaldehyde by means of parallel scrubber stopped-flow absorptiometry. Anal Chim Acta 531:41–49
Matthews TG, Howell TC (1981) Visual colorimetric formaldehyde screening analysis for indoor air well. J Air Pollute Control Assoc 31:1181–1184
Kawamura K, Kerman K, Fujihara M, Nagatani N, Hashiba T, Tamiya E (2005) Development of a novel hand-held formaldehyde gas sensor for the rapid detection of sick building syndrome. Sensor Actuat B 105:495–501
Qoesenberry MS, Lee YC (1996) A rapid formaldehyde assay using purpald reagent: Application under periodation conditions. Anal Biochem 234:50–55
Bosque-Sendra JM, Pescarolo S, Cuadros-Rodríguez L, García-Campaña AM, Almansa-López EM (2001) Optimizing analytical methods using sequential response surface methodology. Application to the pararosaniline determination of formaldehyde. Fresenius J Anal Chem 369:715–718
Munoz MP, de Villena Rueda FJM, Diez LMP (1989) Determination of formaldehyde in air by flow injection using pararosaniline and spectrophotometric detection. Analyst 114:1469–1471
Ensafi AA (1999) Abassi S (1999) Sensitive reaction rate method for the determination of low levels of formaldehyde with photometric detection. Fresenius J Anal Chem 363:376–379
Pereira EA, Dasgupta PK (1997) Measurement of atmospheric formaldehyde using a drop collector and In-situ colorimetry. Int Environ J Anal Chem 66:201–213
Girousi ST, Golia EE, Voulgarapoulos AN, Maroulis AJ (1997) Fluorometric determination of formaldehyde. Fresenius J Anal Chem 358:667–668
Li J, Dasgupta PK, Genfa Z, Hutterli MA (2001) Measurement of atmospheric formaldehyde with a diffusion scrubber and light-emitting diode–liquid-core waveguide based fluorometry. Field Anal Chem Technol 5:2–12
Kiba N, Sun LM, Yokose S, Kazue MT, Suzuki TT (1999) Determination of nano-molar levels of formaldehyde in drinking water using flow-injection system with immobilized formaldehyde dehydrogenase after off-line solid-phase extraction. Anal Chim Acta 378:169–175
Afkhami A, Saber-Tehrani M, Bagheri H, Madrakian T (2011) Flame atomic absorption spectrometric determination of trace amounts of Pb(II) and Cr(III) in biological, food and environmental samples after preconcentration by modified nano-alumina. Microchim Acta 172:125–136
Afkhami A, Madrakian T (2002) Kinetic–spectrophotometric determination of selenium in natural water after preconcentration of elemental selenium on activated carbon. Talanta 58:311–317
Shishehbore MR, Afkhami A, Bagheri H (2011) Salicylic acid functionalized silica-coated magnetite nanoparticles for solid phase extraction and preconcentration of some heavy metal ions from various real samples. Chem Cent J 5:41–51
Afkhami A, Madrakian T, Ahmadi R, Bagheri H, Tabatabaee M (2011) Chemically modified alumina nanoparticles for selective solid phase extraction and preconcentration of trace amounts of Cd(II). Microchim Acta. doi:10.1007/s00604-011-0660-x
Afkhami A, Saber-Tehrani M, Bagheri H (2010) Modified maghemite nanoparticles as an efficient adsorbent for removing some cationic dyes from aqueous solution. Desalination 263:240–248
Hiraide M, Iwasawa J, Hiramatsu S, Kawaguchi H (1995) Use of surfactant aggregates formed on alumina for the preparation of chelating sorbents. Anal Sci 11:611–615
Rong H, Liu Z, Wu Q, Pan D, Zheng J (2010) Formaldehyde removal by Rayon-based activated carbon fibers modified by P-aminobenzoic acid. Cellulose 17:205–214
Tanada S, Kawasaki N, Nakamura T, Araki M, Isomura M (1999) Removal of formaldehyde by activated carbons containing amino groups. J Colloid Interf Sci 214:106–108
Matsuo Y, Nishino Y, Fukutsuka T, Sugie Y (2008) Removal of formaldehyde from gas phase by silylated graphite oxide containing amino groups. Carbon 46:1159–1174
Bahram M, Afkhami A (2008) Recent applications of kinetic methods in multi-component analysis. J Iran Chem Soc 5:352–366
Abbasi S, Esfandyarpour M, Taher MA, Daneshfar A (2007) Catalytic–kinetic determination of trace amount of formaldehyde by the spectrophotometric method with a bromate–Janus green system. Spectrochim Acta A 67:578–581
Mohamed AA, Mubarak AT, Marestani ZMH, Fawy KF (2008) Highly sensitive and selective catalytic determination of formaldehyde and acetaldehyde. Talanta 74:578–585
Cui XJ, Fang GZ, Jiang L, Wang S (2007) Kinetic spectrophotometric method for rapid determination of trace formaldehyde in foods. Anal Chim Acta 590:253–259
Keyvanfard M (2010) Catalytic spectrophotometric determination of formaldehyde based on its catalytic effect on the reaction between bromate and cresyl violet. Asian J Chem 22:6708–6714
Maeda Y, Hu XC, Itou S, Kitano M, Takenaka N, Bandow H, Munemori M (1994) Continuous determination of gaseous formaldehyde by a chemiluminescence method. Analyst 119:2237–2240
U.S. Environmental Protection Agency (1999) Determination of carbonyl compounds in drinking water by fast gas chromatography. 12–14
Ghanbari H, Ghanbari K, Beheshti I, Munzar M, Vasauskas A, Averback P (1998) Biochemical assay for AD7C-NTP in urine as an Alzheimer’s disease marker. J Clin Lab Anal 12:285–288
Afkhami A, Bagheri H, Madrakian T (2011) Alumina nanoparticles grafted with functional groups as a new adsorbent in efficient removal of formaldehyde from water samples. Desalination. doi:10.1016/j.desal.2011.07.052
Bagheri H, Tabatabaee M, Kerdegari AA, Shahvazian M, Shishehbore MR (2010) Highly selective catalytic spectrophotometric determination of copper in water samples. Asian J Chem 22:661–668
Tabatabaee M, Nateghi MR, Mosavi SJ (2006) Kinetic-spectrophotometric determination of trace amounts of silver using the oxidation of thionine with peroxodisulfate. Anal Sci 22:1601–1603
Wang S, Cui XJ, Fang GZ (2007) Rapid determination of formaldehyde and sulfur dioxide in food products and Chinese herbals. Food Chem 103:1487–1493
Zhen YQ, Wang S, Xiao WX, Yuan HY, Xiao D (2007) A fluorescent dosimeter for formaldehyde determination using the Nash reagent in silica gel beads. Microchim Acta 159:305–310
Acknowledgement
This research is supported by the Bu-Ali Sina University Research Council and Center of Excellence in Development of Chemical Methods (CEDCM).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 113 kb)
Rights and permissions
About this article
Cite this article
Afkhami, A., Bagheri, H. Preconcentration of trace amounts of formaldehyde from water, biological and food samples using an efficient nanosized solid phase, and its determination by a novel kinetic method. Microchim Acta 176, 217–227 (2012). https://doi.org/10.1007/s00604-011-0715-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0715-z