Abstract
We impregnated Rayon-based activated carbon fibers (ACFs) by p-aminobenzoic acid (PABA) and systematically investigated their porous structure, surface chemistry, and formaldehyde removal behavior. Using standard nitrogen adsorption analysis, we found that the specific surface area, the micropore volume, and the total pore volume decreased with increasing concentration of PABA. Through elemental analysis and X-ray photoelectron spectroscopy, it was found that some nitrogen-containing functional groups presented on the surface of modified Rayon ACFs. The modified Rayon-based ACFs showed much higher adsorption capacity and longer breakthrough time for formaldehyde than did as-prepared Rayon-based ACF. We proposed that the improvement of formaldehyde removal by modified ACFs was attributed to the combined effects of physisorption contributed by pore structures and chemisorption contributed by the N-containing functional groups, whereas there was only physisorption between the as-prepared ACF and formaldehyde molecules.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, air pollution caused by formaldehyde, a toxic and odorous gas, has attracted growing concern. Formaldehyde is pungent to the mucosa of the eye, nose, and respiratory tract. Worse, over 50 ppm formaldehyde in air can cause headache, nausea, coryza, lung cancer, and even death (Medinsky and Bond 2001). Therefore, formaldehyde should be removed effectively from air and our surrounding environment.
So far, the most widely used method to remove odorous or poisonous substances (gases or liquids) is filtration and adsorption by porous materials. Boonamnuayvitaya et al. (2005) investigated formaldehyde adsorption by activated carbon prepared from coffee residues, but the adsorption capacity was somewhat low. Prado et al. (2006) used biotrickling filters to remove formaldehyde and methanol at concentrations not exceeding a few hundreds of milligrams per cubic meter. However, this method was not very effective for removal of formaldehyde at higher concentration.
Activated carbon fiber (ACF), one of the most promising adsorbents for the treatment of volatile organic chemicals (VOC) or odorous molecules in air, is characterized by strong adsorption capacity, attributed to its high surface area, porosity, and degree of surface reactivity (Byrne and Marsh 1995; Carrott and Freeman 1991). However, normal ACFs manufactured by steam or CO2 activation are hydrophobic and do not show ideal adsorption capacity for polar or polarizable molecules. The adsorption capacity of ACFs for formaldehyde is not effective in ambient air either (Economy et al. 1992), owing to the weak interaction between polar formaldehyde and the hydrophobic surface of porous carbon (Ozaki and Oya 1997). Song et al. (2007) studied the removal of formaldehyde at low concentration using various activated carbon fibers and found that polyacrylonitrile (PAN)-ACF showed the greatest formaldehyde adsorption capacity, while the formaldehyde adsorption capacity of Rayon-based ACF was less than expected and lower than needed.
Carbonyl groups in the formaldehyde molecule can react with primary amine to imines, according to the following reaction mechanism (Pine et al. 1980):

It is considered that surface functional groups can be controlled by modification of surface properties such as selective adsorptivity and reactivity (Marquez-Alvarez et al. 1996a, b; Hattori and Kaneko 2001; Kaneko et al. 1989). A low concentration of surface functional groups in ACFs can enhance their adsorption capacity for polar molecules. According to Eq. (1), it is anticipated that impregnation of ACFs with amino-containing compounds can enhance their removal capacity for formaldehyde because of the cooperation of physical adsorption and the increased chemical interaction between amino groups on the surface of ACFs and formaldehyde molecules. Tanada et al. (1999) found that the presence of nitrogen on the carbon surface facilitates formaldehyde removal in formalin, but the amount of formaldehyde adsorbed onto activated carbon with amino groups is only about 1–2 mg/g. Matsuo et al. (2008) studied the behavior of silylated graphite oxide for removal of formaldehyde vapors, but the investigated concentration of formaldehyde was still very low (about 2.14 ppm) and the adsorbed amount for formaldehyde could only reach ~76.0 mmol/g.
Rayon-based ACFs have much higher specific surface area and richer surface functional groups compared with PAN- or pitch-based ACFs. In this study, we modified Rayon-based ACFs by p-aminobenzoic acid (PABA) and investigated the effect of impregnation on their formaldehyde-removal behavior. We also propose, in accordance with different analysis techniques, a possible mechanism for adsorption and removal of formaldehyde by the modified Rayon-based ACFs.
Experimental procedures
Sample preparation
Activated carbon fibers used in this study were prepared by steam activation of Rayon-based carbon fiber in our laboratory. In brief, the original Rayon fiber fabrics were carbonized at 800–1,000°C in inert nitrogen atmosphere and simultaneously activated with steam vapor at the same temperature for 30–60 min to obtain the as-prepared Rayon ACFs. The adsorbent Rayon-based ACFs modified with PABA were made by the following procedure: (1) different concentration of PABA and hot water were mixed and stirred for 30 min to obtain aqueous solution, then (2) 1 g Rayon-ACFs was modified with the aqueous solution for 20 min, followed by drying at 100°C in air.
The as-prepared ACF was designated Rayon-based ACF0; the modified ACFs samples were designed PABA (##), where the numbers indicate the concentration of the PABA aqueous solution.
Adsorption of vapors from formaldehyde solution and water vapor
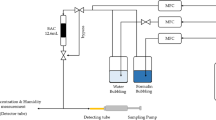
The static adsorption capacity of vapors from formaldehyde solution and water vapor was determined by a desiccator method. A bottle of formaldehyde solution was placed in a desiccator with valves to produce saturated formaldehyde vapors. As a comparison, the adsorption capacity of the ACFs for water vapor only was also investigated in this study. In order to investigate which component (water and formaldehyde) was adsorbed on the ACFs during the process of static adsorption, we carried out water pre-adsorption on all studied samples first. Furthermore, by suitable manipulation of the valves, the vapor pressure of water was controlled close to the level of its partial pressure in the vapor from the formaldehyde solution. Then the samples were placed in the desiccator to adsorb the formaldehyde vapors from the formaldehyde solution.
Dynamic adsorption of vapors from formaldehyde solution and water vapor by the samples was determined by nitrogen gas bubbling method using a DuPont Instrument 951 thermogravimetric analyzer. The adsorption temperature was 30°C and the desorption temperature was 150°C. Before this determination, all samples were degassed at 100°C over 24 h.
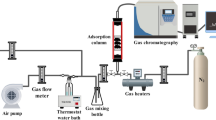
A laboratory fixed-bed unit and experimental procedures were set up for continuous-flow study to obtain equilibrium adsorption data. The adsorption column was 20 mm in length and 10 mm in diameter. A thermal conductivity detector (TCD) of a gas chromatograph (GC-103, Shanghai Analysis Instrument) was used to monitor the concentration of the gas stream for formaldehyde and water vapors. The flow rate of argon was set at 0.5 cm3/s. In these experiments, the mass of all the samples was 1.0 g.
Characterization
Porosity and specific surface area
The porosity and specific surface area of the studied samples were obtained by a micromeritics ASAP 2000 accelerated surface area and porosimetery apparatus (Micromeritics Ins. Cor., Georgia, USA) by volumetric measurement at 77.4 K at relative pressure from 10−6 to 1. High-purity (99.99%) nitrogen was used; before the measurement, all samples were degassed at 100°C for 2 h. The micropore surface area and volume were obtained by Dubinin–Astakhov method (Dubinin 1966, 1987). The pore size distribution of the samples was calculated by employing the regularization method according to density functional theory (DFT) (Ryu et al. 1999; Kruk et al. 1996).
Elemental analysis
An Analysensysteme (Elementar Vario EL GmbH Germany) was applied for elemental analysis. The nitrogen, carbon, and hydrogen contents were determined directly, while the oxygen content was calculated by difference.
X-ray photoelectron spectroscopy
The surface chemistry of the studied samples was analyzed by a VG ESCALB5 X-ray photoelectron spectroscopy (XPS) spectrometer (VG Scientific, The Surface Analysis Company, UK) with Al Kα X-ray radiation and an analyzer with pass energy of 50 eV. Deconvolution was carried out with a nonlinear least-squares curve-fitting program with a combined Gaussian–Lorentzian peak function. During data collection the pressure in the analysis chamber was maintained below 1.0 × 10−7 Pa.
Results and discussion
Nitrogen adsorption isotherms
Nitrogen adsorption isotherms for the samples modified by different concentrations of PABA aqueous solutions are of type I according to the IUPAC classification (Sing et al. 1985), as shown in Fig. 1. All the adsorption isotherms at relative pressure below 0.05 are still of type I, suggesting that the samples did not lose extensive microporosity on impregnation with PABA aqueous solutions.
The porous structure parameters of the modified and untreated ACF samples are shown in Table 1. Compared with ACF0, the specific surface area, micropore area (S mic), micropore volume (V mic), and total pore volume at relative pressure 0.95 (V t) of the modified samples decreased with increasing concentration of PABA aqueous solution, while the median pore diameter (MPD) increased. The specific surface area and total pore volume of the sample modified by the 0.1 mol/L (relatively high concentration) PABA aqueous solution obviously decreased, which implied that impregnation with PABA aqueous solution could lead to destruction of part of the micropore wall, or that PABA molecules could plug part of the pores in ACFs and thus lead to a decrease of the specific surface area and increase of the median pore diameter.
Pore size distributions
The pore size distributions (PSDs) of the modified ACFs, as shown in Fig. 2, were calculated by employing the regularization methods according to density functional theory (DFT). ACF0 possesses a reasonable amount of micropores, a small fraction of mesopores, but no macropores. PABA (0.02) and PABA (0.04) are also microporous and possess some fraction of mesopores. The micropore volume of PABA (0.02) is obviously lower than that of ACF0, and the micropore volume of PABA (0.04) is lower than that of PABA (0.02) and ACF0. PABA (0.10) is also microporous, with a small fraction of mesopores but without macropores. These findings suggest that the concentration of the PABA aqueous solution plays an important role in the pore size distribution of the modified Rayon-based ACFs.
Surface chemistry by elemental analysis and X-ray photoelectron spectroscopy
The elemental analysis data (Table 2) showed that the contents of both hydrogen and oxygen in the modified PABA (0.02), PABA (0.04), and PABA (0.1) were lower than those in ACF0. On the contrary, the content of nitrogen in the modified samples was higher than that of ACF0. With increasing concentration of the PABA aqueous solution, the nitrogen content increased to some extent in the modified ACFs. PABA (0.1) contained the highest nitrogen content, which might be attributed to the impregnation of a high concentration of the NH2-containing compound PABA.
We used Fourier-transform infrared spectroscopy (FTIR) to analyze the surface chemistry of the Rayon-based ACFs under study. However, we could not measure an obvious surface chemistry difference between the as-prepared and impregnated Rayon ACFs. This may be attributed to the fact that both kinds of ACF samples are black and that it is not easy to detect the surface functional groups. So, we used X-ray photoelectron spectroscopy to investigate the chemical state of the ACFs in a surface layer with a depth of about 5 nm (Briggs and Sherwood 1986; Takahashi and Ishitani 1984). The amounts determined for C, O, and N were calculated from the corresponding peak areas divided by the appropriate sensitivity factors (Figueiredo et al. 1999). XPS data for ACF0, PABA (0.02), and PABA (0.1) are given in Table 3. XPS data also indicated that the nitrogen content in the modified samples was higher than that in ACF0, which was in agreement with the elemental analysis data. It was also shown that the nitrogen-containing functional group was mainly located on the surface of the modified Rayon-based ACFs. PABA (0.1) contained higher nitrogen than did PABA (0.02) and ACF0, and the nitrogen content in PABA (0.02) was higher than that in ACF0, which implied that impregnation by higher concentration of PABA could lead to higher load of nitrogen-containing functional groups.
Because nitrogen-containing functional groups in the modified Rayon-based ACF samples played an important role in their formaldehyde-removal ability, XPS N1S spectra of ACF0 and PABA (0.02) were investigated. The XPS N1S spectra for the two samples ACF0 and PABA (0.02) are presented in Fig. 3. It was found that the two spectra were very different. The spectrum for PABA (0.02) showed a much higher half-width value than did ACF0, while the spectrum for ACF0 showed a broad peak and lower half-width value, suggestive of different chemical structures of nitrogen in the two samples. In order to clarify the forms of different nitrogen-containing functional groups for the two Rayon-based ACFs, curve-fitting procedures were performed according by using a nonlinear least-squares curve-fitting program with a combined Gaussian-Lorentzian peak function. The N1S envelope contained three chemically shifted peaks in both samples. The binding energy values of the peaks for the two samples are given in Table 4 (Raymundo-Pinero et al. 2003). The two samples both presented pyridine-like nitrogen at ~398 eV and amine moieties (NH-) nitrogen at ~400 eV, respectively. The relative intensity for amine moieties (NH-) in PABA (0.02) was much higher than that in ACF0. However, ACF0 presented quaternary (NH +3 )-like nitrogen at ~402 eV with relative intensity 23.04% whereas PABA (0.02) presented iminium ion (NH2 +) at ~403 eV with relative intensity 14.21% (Bansal et al. 1977; Jansen and van Bekkum 1995). These changes indicated that there were chemical transformations of the N species during the impregnation process. The increased relative intensity for amine moieties (NH-) nitrogen functional groups and the existence of iminium ion (NH +2 ) in the modified sample PABA (0.02) provide important evidence of its formaldehyde-removal properties. According to Eq. (1), it can be conjectured that formaldehyde can react with NH- and NH +2 functional groups on the surface and pore walls of PABA (0.02) by enhanced chemical interaction, and hence PABA (0.02) presented high removal capacity for formaldehyde.
Effect of impregnation conditions on static removal capacity for vapors from formaldehyde solution and water vapor
Because pure formaldehyde is not stable in air, 37–40% (weight concentration) formaldehyde solution was used in this study. Impregnation conditions for Rayon-based ACFs in PABA aqueous solution can affect the formaldehyde-removal property of ACFs. The relationship between the concentration of PABA aqueous solution and the adsorption capacity for vapors from formaldehyde solution and water vapor on the samples under study are shown in Fig. 4 (for the same impregnation time of 24 h). With the concentration of PABA aqueous solution increasing from 0.002 to 0.02 mol/L, the adsorption capacity for formaldehyde increased obviously. For vapors from formaldehyde solution, the adsorption capacity increased from 560 to 932 mg/g and for water vapor from 460 to 678.5 mg/g. However, for higher concentrations of PABA aqueous solution, the adsorption capacity for vapors from formaldehyde solution dramatically decreased, which reveals that the use of an appropriate concentration of PABA loaded on the ACF surface could enhance the adsorption capacity for polar formaldehyde molecules, but that overload of PABA might plug the pores in Rayon-based ACF samples, decreasing the contact probability between the pore surface and formaldehyde molecules and hence dramatically decreasing their adsorption capacity for formaldehyde. Thus, a suitable dispersion of PABA played a very important role in the adsorption property of ACFs for removal formaldehyde. The sample modified by 0.02 mol/L PABA showed the highest adsorption capacity for formaldehyde and water vapor compared with samples modified by lower and higher concentration. Therefore, in the following experiments we chose 0.02 mol/L as the optimum PABA aqueous solution concentration for modification of Rayon-based ACFs.
The impregnation time in the PABA aqueous solution may also affect the formaldehyde-removal property of the modified Rayon-based ACFs. Figure 5 shows the effect of impregnation time in PABA aqueous solution on the static capacity for vapor absorption from formaldehyde solution and water vapor. With the impregnation time increasing from 30 min to 4 h, the adsorption capacity of the modified samples for formaldehyde solution and water vapor obviously increased: for formaldehyde solution from 580 to 932 mg/g and for water vapor from 350 to 480 mg/g. However, when the impregnation time was over 4 h, the adsorption capacity of the modified Rayon-based ACDs stayed nearly constant, which might imply a possible saturation effect.
Table 5 lists the pre-adsorption data with water vapor for the Rayon-based ACFs under study. The amount of formaldehyde was calculated by the difference c = b − a. From Table 5, it can be seen that all the samples under study pre-adsorbed with water vapor still showed high adsorption capacity for vapors from formaldehyde solution, which may be evidence that their increased adsorption capacity should be attributable to the adsorption of formaldehyde, because for a certain adsorbate (in this case water), under a certain pressure and temperature, the adsorption capacity of a certain adsorbent should be fixed.
Dynamic adsorption and desorption for vapors from formaldehyde solution and water vapor
By the nitrogen gas bubbling methods, dynamic adsorption and desorption curves for formaldehyde solution and water vapor by Rayon-based ACF0 and PABA (0.02) were investigated (Fig. 6). PABA (0.02) showed 282.2 mg/g adsorption equilibrium capacity for vapors from formaldehyde solution, which was 1.72 times higher than that of ACF0 (163.3 mg/g). The adsorption rate for PABA (0.02) was higher than that of ACF0. It took about 30 min to reach the equilibrium state for PABA (0.02) for vapors from formaldehyde solution, while this time was about 40 min for ACF0. The adsorption equilibrium capacity for water vapor in both samples ACF0 and PABA (0.02) was much lower than that for vapors from formaldehyde solution, which was 140 mg/g in PABA (0.02) and 93 mg/g in ACF0. It is proposed that, due to the enhanced interaction of adsorbate and adsorbent, the increased relative intensity for amine moieties (NH-) nitrogen functional groups and the iminium ion (NH +2 ) in the modified sample were beneficial for removal of formaldehyde and for increased adsorption rate for formaldehyde by the modified Rayon-based ACFs.
In the desorption curves, PABA (0.02) showed about 8.6% remaining vapor from formaldehyde solution after desorption for some time, while Rayon-based ACF0 showed almost no remaining vapor from formaldehyde, which might imply that there was chemical adsorption between formaldehyde and PABA (0.02). For water vapor desorption, both samples PABA (0.02) and ACF0 showed almost no remaining vapor, meaning that only physical adsorption occurred between water vapor and these two samples.
Breakthrough curves
Figure 7 shows breakthrough curves for formaldehyde and water vapors at 30°C for PABA (0.02) and ACF0. PABA (0.02) showed a higher breakthrough time of 3,850 min/g for formaldehyde than did ACF0 (2,200 min/g), meaning that PABA (0.02) showed greater reactivity with formaldehyde than did ACF0. This might be attributed to the reaction between the amine moieties (NH-) and NH +2 functional groups in PABA (0.02) and –C=O (carbonyl) functional groups of formaldehyde. Some researchers also reported that hydrogen bonding between –C=O (carbonyl)-containing materials such as aldehyde and functional groups on ACFs and aldehyde–aldehyde functional groups could be formed during the adsorption process (Kaneko 1996).
The breakthrough curves for formaldehyde and water vapors at 50°C for PABA (0.02) and ACF0 are shown in Fig. 8. PABA (0.02) showed a much longer breakthrough time of 8,000 min/g for formaldehyde, which was over 3.6 times higher than that for ACF0 (about 2,200 min/g), and also much longer than that at 30°C for PABA (0.02). At higher temperature the reactivity of PABA (0.02) with formaldehyde became stronger, further demonstrating the chemical reaction between formaldehyde and the modified Rayon-based ACFs, and also implying that chemical adsorption occurred between formaldehyde and the PABA-modified Rayon-based ACFs. Both samples had a longer breakthrough time for formaldehyde than for water vapor at both temperatures, which implies that, after impregnated by NH2-containing PABA aqueous solutions, the adsorption selectivity of the ACFs for formaldehyde could be improved to some extent.
Adsorption mechanism
Pure formaldehyde is not stable in air, and paraformaldehyde can form easily through the following reaction (Pine et al. 1980):
Paraformaldehyde is highly condensed and can be adsorbed on surfaces where formaldehyde cannot be adsorbed (Kaneko 1996). According to Eq. (1), there exists a cooperative interaction between the carbonyl group of formaldehyde and the amino functional groups of PABA, so formaldehyde molecules were firstly adsorbed on the surface of the modified Rayon-based ACFs, then went into their pores, where the formaldehyde molecules may form paraformaldehyde by strong adsorption potential and chemical interaction, thus improving the adsorption capacity for formaldehyde on the modified Rayon-based ACFs. PABA (0.02) showed higher adsorption capacity for formaldehyde and water vapors than did ACFs modified by lower and higher concentrations, which implies that both physical adsorption and chemical adsorption occurred in the modified Rayon-based ACFs. Higher concentration of PABA could plug the pores in the ACFs and make their total available micropore volume decrease, while too low a concentration of PABA could not provide enough adsorption/reaction sites for formaldehyde in Rayon-based ACFs. In the micropore spaces, Rayon-based ACFs modified by the NH2-containing compound PABA could react with formaldehyde by strong chemical interaction, and formaldehyde might form paraformaldehyde by strong adsorption potential and chemical interaction (Kaneko 1996), hence the adsorption capacity for formaldehyde on the modified ACFs was obviously improved.
Conclusions
Modification by the NH2-containing molecule PABA resulted in a significant increase in the adsorption capacity and elongation of the breakthrough time of Rayon-based ACFs used for formaldehyde removal. The adsorption of formaldehyde on ACFs strongly depends on their pore structure and surface chemistry. A low concentration of surface groups can enhance adsorption of the polar formaldehyde molecule on modified porous carbon. The amount of formaldehyde adsorbed by the modified Rayon-based ACFs increased on the introduction of amino groups at a suitable concentration because of cooperative interaction between the carbonyl functional group of formaldehyde, the amino functional groups of NH2-containing PABA-modified Rayon-based ACFs, and the formed aldehyde–aldehyde functional groups. Rayon-based ACFs modified by too high a concentration of PABA showed low adsorption capacity for formaldehyde, which may be caused by pores in the modified ACFs being plugged up, thus decreasing their available micropore volume, while too low a concentration of PABA cannot provide enough adsorption/reaction sites for formaldehyde in the ACFs. The modified Rayon-based ACFs showed much longer breakthrough times for formaldehyde than did the original as-prepared Rayon-based ACF0, indicating reaction between the increased amine moieties (NH-) and the added NH +2 functional groups in the modified ACFs and carbonyl functional groups of formaldehyde. Increased temperature can enhance the adsorption capacity of the modified Rayon-based ACFs for formaldehyde, which implies that there exist both physical and chemical adsorption between formaldehyde and NH2-containing PABA-modified Rayon-based ACFs. In the adsorption and removal process, formaldehyde may be firstly adsorbed on the surface, then move into the pores, where formaldehyde molecules may form paraformaldehyde by strong adsorption potential and chemical interaction, finally leading to improvement of the adsorption capacity for formaldehyde.
References
Bansal RC, Dhami TL, Parkash S (1977) Surface characteristics and surface behavior of polymer carbons-I: associated oxygen and hydrogen. Carbon 15:157–162
Boonamnuayvitaya V, Sae-ung S, Tanthspanichakoon W (2005) Preparation of activated carbons from coffee residue for the adsorption of formaldehyde. Sep Purif Tech 42:159–168
Briggs D, Sherwood PMA (1986) X-ray photoelectron spectroscopic studies of carbon fibers VII- Electrochemical treatment in ammonium salt electrolytes. Carbon 24:357–363
Byrne JF, Marsh H (1995) Introductory overview. In: Patrick JW (ed) Porosity in carbons, characterization and applications. Halsted, New York, pp 1–48
Carrott PJM, Freeman JJ (1991) Evolution of micropore structure of activated charcoal cloth. Carbon 29:499–506
Dubinin MM (1966) Porous structure and adsorption of active carbon. In: JrPL Walker (ed) Chemistry and physics of carbon. Marcel Dekker, New York, pp 51–120
Dubinin MM (1987) Adsorption properties and microporous structure of carbonaceous adsorbents. Carbon 25:593–598
Economy J, Foster K, Jung H (1992) Tailoring carbon fibers for adsorbing volatiles. Chemtech 22:597–603
Figueiredo JL, Pereira MFR, Freitas MMA, Orfao JJM (1999) Modification of the surface chemistry of activated carbon. Carbon 37:1379–1389
Hattori Y, Kaneko K (2001) Adsorptive properties of metal oxide dispersed carbon materials and characterization of metal oxide fine particles by XAFS. J Synchron Radiat 8:525–527
Jansen RJJ, van Bekkum H (1995) XPS of nitrogen-containing functional groups on activated carbon. Carbon 33:1021–1027
Kaneko K (1996) Adsorption on new and modified inorganic sorbents: micropore filling mechanism in inorganic sorbents. In: Dabrowski A, Tertykh VA (eds) Studies in surface science and catalysis. Elsevier Science, Amsterdam, pp 573–598
Kaneko K, Kosugi N, Kuroda H (1989) Characterization of iron oxide-dispersed ACFs with iron K-edge XANES and with water adsorption and Fe K-edge XANES and EXAFS. J Chem Soc, Faraday Trans I 85:869–881
Kruk M, Jaroniec M, Bereznitski Y (1996) Adsorption study of porous structure development in carbon blacks. J Colloid Interface Sci 182:282–288
Marquez-Alvarez C, Rodriguez-Ramos I, Guerrero-Ruiz A (1996a) NO removal over carbon supported copper catalysts. I. Reactivity of NO with graphite and activated carbon. Carbon 34(1–2):339–346
Marquez-Alvarez C, Rodriguez-Ramos I, Guerrero-Ruiz A (1996b) NO removal over carbon supported copper catalysts. II. Evaluation of catalytic properties under different reaction conditions. Carbon 34:1509–1514
Matsuo Y, Nishino Y, Fukutsuka T, Sugie Y (2008) Removal of formaldehyde from gas phase by silylated graphite oxide containing amino groups. Carbon 46:1159–1174
Medinsky MA, Bond JA (2001) Sites and mechanisms for uptake of gases and vapors in the respiratory tract. Toxicology 160:165–172
Ozaki J, Oya A (1997) Addition of functions to ACFs—designing of materials with taking account of surface functions. Hyomen 35:535–543 (In Japanese)
Pine SH, Hendrickson JB, Carm DJ, Hammond GS (1980) Organic chemistry. McGraw-Hill, New York
Prado O′J, Veiga MC, Kennes C (2006) Effect of key parameters on the removal of formaldehyde and methanol in gas-phase biotrickling filters. J Hazard Mater B 138:543–548
Raymundo-Pinero E, Cazorla-Arnoros D, Linares-Solano A (2003) The role of different nitrogen functional groups on the removal of SO2 from flue gases by N-doped activated carbon powders and fibers. Carbon 41:1925–1932
Ryu ZY, Zheng JT, Wang MZ, Zhang BJ (1999) Characterization of pore size distributions on carbonaceous adsorbents. Carbon 37:1257–1264
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 57:603–619
Song Y, Qiao WM, Yoon SH, Mochida I, Guo QG, Liu L (2007) Removal of formaldehyde at low concentration using various activated carbon fibers. J Appl Polym Sci 106:2151–2157
Takahashi T, Ishitani A (1984) XPS studies by use of the digital difference spectrum technique of functional groups on the surface of carbon fiber. Carbon 22:43–46
Tanada S, Kawasaki N, Nakamura T, Araki M, Isomura M (1999) Removal of formaldehyde by activated carbons containing amino groups. J Colloid Interface Sci 214:106–108
Acknowledgments
The authors thank the National Natural Science Foundation of China (no. 50403005) for partial financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rong, H., Liu, Z., Wu, Q. et al. Formaldehyde removal by Rayon-based activated carbon fibers modified by P-aminobenzoic acid. Cellulose 17, 205–214 (2010). https://doi.org/10.1007/s10570-009-9352-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-009-9352-7