Abstract
This work describes the design of a conductometric biosensor intended to monitor aquatic environments. The biosensor is based on the measurement of Alkaline Phosphatase Activity (APA) of the microalgae Chlorella vulgaris. This activity is inhibited in the presence of heavy metals. The purpose of this article is to obtain a tool for detection of heavy metals through inhibition of APA. The biosensor is composed of two parts: two platinum interdigitated electrodes which form the transducer, and the microalgae, the bioreceptor. The microalgae were immobilized on self-assembled monolayers (SAMs) of alkanethiolate. The change in the local conductivity of the electrodes following the addition of the substrate allowed us to measure alkaline phosphatase activity of Chlorella vulgaris. Good repeatability (RSD < 5%) and reproducibility between different biosensors (RSD < 10%) were obtained. Lifetime is estimated to be 17 days. The originality of this work consists in the immobilization of algal cells on self-assembled monolayers. The advantage of the SAMs is that they do not form a physical barrier between the algae and the constituents of the reaction medium (whether the reaction substrate, or the toxic components are to be detected). In addition, these sensors permit to obtain good measurement repeatability. Detection limit of cadmium reaches ppb levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The microalgae Chlorella vulgaris has been used in different works to produce whole cell biosensors to monitor toxic pollutants in aquatic media [1, 2]. This unicellular green algae was chosen due to its considerable ecological advantages (it is ubiquist in all dulcicol environments and is able to accumulate large quantities of pollutants). The first biosensor using whole algal cells was developed by Pandard et al.(1993) [2]. Its operation was based on measuring the oxygen produced by immobilized algae. In earlier works we developed optical [1], conductometric [3] and amperometric [4] sensors based on measuring chlorophyll fluorescence and the activity of enzymes located on the external membrane of algae [3, 5] in order to detect toxic compounds in aquatic media. Numerous works have been shown the interest of enzymatic activity measurements to monitor water pollution [3, 6, 7].
In this paper we developed a biosensor based on APA measurements. This activity can detect metal ion concentrations between 1 and 10 ppb [8]. This sensor is not specific for a single metal, but it provides a global response to the presence of heavy metals. This biosensor can be considered as an early warning system, and was used to measure concentrations of heavy metals in the order of ppb in real samples as urban water [9]. The main problem encountered is the low reproducibility of the results obtained by the different sensors which can be explained by the heterogeneity of the layers of immobilized algae. Regarding the immobilization processes used, mention can be made of physical adsorption which is the simplest technique to implement. However, desorption can occur during variations of pH, ionic force, temperature or the presence of a solvent [10]. Physical trapping in a polymer matrix (polyacrylamide [11], Polypyrrole [12],) or in a gelling agent (agar [13], K-carrageenan [14], Alginate [15], sol–gel [16], BSA [17]) is frequently used, since it does not denature the biological element and it permits protecting metabolic activities. Nonetheless, the resulting matrix can form a diffusion barrier that restricts the accessibility of the substrate and/or inhibitors of algal cells. The Self Assembly Monolayers (SAMs) have been exploited to provide model surfaces in order to permit examining cellular behaviour for bioanalysis and tissue engineering [18]. This technique permits immobilizing proteins [19], antigens/antibodies [20], DNA [21] and yeasts [18] on various substrates (Au, Ag, SiO2, ZrO2, etc.). The bioreceptor in this case is linked to the tranducers by a covalent link, thus it is free in the reaction medium instead of being trapped in a matrix.
The objective of this work is to study the feasibility of immobilizing algal cells on SAMs in the design of a sensor based on the measurement of the alkaline phosphatase activity of Chlorella vulgaris and to improve reproducibility of responses. This technique is developed with algal cells for the first time. The repeatability of the measurements performed using the same sensor, the reproducibility between sensors and the lifetime of the device will be considered after which the improvements contributed by this technique will be discussed.
Materials and methods
Material
Paranitrophenyl phosphate (pNPP) from Sigma-Aldrich (http://www.sigmaaldrich.com) was used as the substrate for measuring alkaline phosphatase activity. 3-mercaptopropionic acid (HSCH2CH2COOH, MPA) was purchased from Fluka (http://www.sigmaaldrich.com). Cd(NO3)2 salt (of analytical grade) was used to inhibit alkaline phosphatase activity. All the reagents used were of analytic quality and used without any treatment.
An SR830 synchronous amplifier (Stanford Research Systems, http://www.thinksrs.com) was used. Photomicrographs of immobilized algal cells were taken with an epi-fluorescent Axioskop microscope equipped with an excitation filter of 450–490 nm, an emission filter 530–750 nm and a CCD camera (Cohu). Image acquisition was performed with the analysis software Komet 4.0 (Kinetic Imagining Ltd.).
Total enlargement was ×400 with a lens (×40) and an eyepiece (×10).
Cell culture and measurement of alkaline phosphatase activity
The strain of freshwater algae Chlorella vulgaris (CCAP 211/12) came from the “Culture Collection of Algae and Protozoa”, Cumbria (UK). This strain was transplanted weekly under sterile conditions in an LC medium (standard AFNOR NT 90 304). Preconditioning for 21 days in a medium without phosphate was required [22] to measure phosphatase activity which was measured with paranitrophenyl phosphate (pNPP) used as a substrate. The reaction medium was buffered at pH 8.5 (Tris 0.1 mol l−1).
Sensor design
The conductometric transducer was composed of two pairs of platinum interdigitated electrodes manufactured by the Institute of Chemo and Biosensorics (Münster, Germany). The two pairs of platinum interdigitated electrodes, 150 nm thick, were placed on a Pyrex substrate. An intermediate layer of Ti 50 nm thick was used to improve adhesion of the platinum on the substrate. The width of the thimbles composing the interdigitated electrodes, and the distance between them was 10 μm and they were about 1 mm long, thereby providing a sensitive zone on each of the two electrodes of about 1 mm2. To define the sensitive part of the sensor, the central part of the latter was covered with epoxide resin [23].
The measurements were based on the detection of conductivity variations at the sensitive zones related to the movement of ions between the anode and the cathode.
As with other enzymes, the alkaline phosphatase of Chlorella vulgaris leads to catalytic reactions generating ionic species resulting in measurable changes of conductivity [3].
Immobilization of microalgae
The interdigitated electrodes were first cleaned by ultrasound for 10 minutes in water, then subjected to chemical reduction by immersion in Piranha solution (H2O2/H2SO4, 1:3 v/v) for 15 minutes (Caution: this solution reacts violently with a lot of organic residues, so it must be handled with care). The electrodes were then rinsed in absolute ethanol and dried in a nitrogen flow.
The same experiments were performed with gold interdigitated electrodes with the same characteristics as those of platinum, but they were not used because they did not resist to the treatment with piranha solution.
The pretreated electrodes were immersed in an aqueous solution of 3-mercaptopropionic acid (MPA) at 2 mmol l−1 for 10 h at room temperature. After the formation of SAMs, the modified platinum electrodes were rinsed in ultra-pure water to eliminate the MPA physically adsorbed on the surface of the electrode. 0.5 μL of algal culture was deposited on the electrodes. To do this an active culture (preconditioning without phosphate) was used for the deposit on one of the two electrodes and an inactive culture (no preconditioning) was used for the deposit on the second electrode, which was therefore the reference electrode. The deposit was kept on the electrodes from 12 to 14 hours and these were then washed with ultrapure water to remove the excess cells just physically adsorbed. Between the experiments, the electrodes were kept at 4 °C in a culture medium without phosphate.
Measurements
The conductometric measurements were based on a differential measurement between the working electrode (on which the active culture were deposited) and the reference electrode (on which the inactive culture were deposited). Using a generator, a sinusoidal excitation signal of a frequency of 100 kHz could be applied to the two exciting electrodes. The output signals measured at the receiving electrodes depended on the impedance of the system.
The signals measured were processed using the look-in method, i.e. they were filtered using a low bandwidth centred on the excitation frequency then transmitted to the synchronous detection which provided the difference of conductivity between the working electrode and the reference electrode. The measurements were performed in daylight at room temperature in a 2 mL glass cell. The biosensor was laid in an agitated buffer solution of Tris–HCl, pH 8.5. After the signal stabilised, different quantities of the substrate were added in the measurement glass cell. The differential signal was recorded by an SR830 synchronous amplifier.
Toxicity measurements
For Toxicity measurements, dS was measured for a definite substrate concentration. The biosensor was then preincubated in a test solution for 30 min. After washing, dS before (dSbefore) and after exposure (dSafter) to the test solution were compared and the residual activity rate was calculated.
Results and discussion
Pt–SAM–algae cell layers formation on the electrode
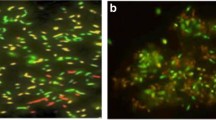
The film prepared with the algal cells was washed with ultrapure water several times to eliminate the cells just adsorbed physically. After this rigorous washing, microscopic analysis showed the algal cells immobilized on the surface of the platinum, leading to the assumption that the microalgae were bound covalently. An unmodified electrode has been prepared and the Fig. 1 shows that in this case the algae were not preferentially adsorbed on the platinum substrates.
The terminal carboxylic group of alkanethiolate SAM is often used to immobilize proteins due to its reactivity with certain chemical groups of biomolecules [24]. The interaction between the algal cells and the surface of the SAMs probably resulted from the reaction between certain terminal mdash; NH2 groups of proteins interbedded in the membrane of the algal cells and the −COOH group of the SAM layer. Consequently we obtained a −CONH group, as shown in Fig. 2.
Microscopic view of immobilized algal cells
Figure 3 shows a photograph using epifluorescence microscopy of the single algal layer immobilized on the thimbles of the interdigitated electrodes. In this study, the algal cells were immobilized on a platinum surface by SAMs. This procedure permits good covalent bonding, though the vital functions of the cell still require checking. Contrary to the often used method of physical trapping with a gelling agent (such as alginate, BSA, etc.), this immobilization technique eliminates any diffusional barrier.
As shown in Fig. 3, the microalgae are fixed only on the platinum but faults in the monolayer appear. This can be explained by cracks in the platinum substrate as has been observed by several authors when working on a gold substrate [25, 26] and/or by the size of the cells (about 5 μm). Indeed, crowding can lead to “holes” in the algal monolayer.
Measurement of alkaline phosphatase activity
Figure 4 shows that the sensor fabricated permits monitoring the alkaline phosphatase activity of Chlorella vulgaris cells immobilized in monolayers. The kinetics obtained has a Michaelian curve, as seen in previous works [3] performed with other immobilization techniques. Bonding the algal cells on SAMs does not therefore appear to disturb their alkaline phosphatase activity. In this case a loss of activity was observed for substrate concentrations higher than 70 μmol l−1. This is probably inhibition due to excess of substrate. In the following the activity measurements were performed for substrate concentrations lower than 70 μmol l−1. This phenomenon could not be observed by the conductometric biosensor based on the immobilization of microalgae with BSA reticulated by glutaraldehyde. It is possible to assume that the diffusion of the substrate occurs inside the BSA gels thereby implying trapping of the substrate, making it more difficult for the latter to reach the algal cells. In such a case the concentration of substrate in contact with the algae is always lower than the concentration of the test medium. Regarding the chemical immobilization, it permits direct contact between the enzymes and the substrate. Thus all the substrate comes into contact with the enzyme.
The conductometric biosensors were developed in this study in order to be used for the detection of pollutants, especially metal ions. The first results obtained (Fig. 5) show that the biosensor is sensitive to the presence of cadmium with a detection limit of 1 ppb.
Analysis of response variability
Three measurements were performed on the same biosensor for each substrate concentration. In all cases the standard deviation did not exceed 5% (Fig. 4). This variability is lower than that observed up to now in previous works in which it could reach 8% [3]. Immobilization on a monolayer therefore appears to improve this problem of reproducibility due to more homogenous distribution of the cells on the surface of the transducer.
Figure 6 shows that the immobilization of the algae on SAMs appears to also lead to a reduction of response variability between the different sensors in comparison to classical immobilization techniques such as BSA [8]. The variability of the measurements performed here between the different sensors did not exceed 10%.
In classical immobilization techniques (BSA, alginate, etc), algae form on the surface of the transducer a layer with a certain thickness. In this case, the substrate may not react only with the cells on the surface of the layer, but penetrate, according to the experimental conditions, more or less well into the thickness of the layer. Since the algae are deposited manually on the surface of the transducer, the thickness of the cell layer varies from one sensor to another, obviously leading to the problem of variability. Immobilization in a monolayer eliminates this problem.
Sensor stability through time
Figure 7 shows the response of the sensor monitored regularly during a period of 30 days under the same storage conditions as those described in “Immobilization of microalgae” subsection. Three different zones appeared. From D 0 to D 5: the response fell slightly until the fifth day. This could have been due either to a release of certain algal cells that were poorly fixed to the electrode, or to a cellular adaptation to the new medium. From D 6 to D 22 the response was stable. From D 23 to D 30 the response fell constantly. This can be explained by cellular death or again by partial release of the algal cells. These results were obtained on three different sensors and show that after a brief period of declining activity, the response of the sensor was stable over a period of 17 days.
Conclusion
The results obtained show that it is possible to immobilize whole algae on SAMs on the surface of a conductometric biosensor.
In this work, we have demonstrated that immobilization on a monolayer improves the repeatability (RSD < 5%) and the reproducibility (RSD < 10%) of the response. The lifetime of the sensor is 17 days. The objective of this work was to find a mode of immobilization that does not lead to interference with the toxic elements to be detected as was the case in previous studies. The absence of interactions between the mode of immobilization and the reaction medium is necessary to obtain a sensor sensitive and specific to a family of pollutants. The first results obtained show that the biosensor is sensitive to the presence of cadmium with a detection limit of 1 ppb. Other enzymatic activities could be studied, for example esterase activities, for identification of different families of pollutants as pesticides in the natural environment at thresholds useful to decision-makers.
References
Durrieu C, Tran-Minh C (2002) Optical algal biosensor using alkaline phosphatase for determination of heavy metals. Ecotoxicol Environ Saf 51(3):206–209
Pandard P, Vasseur P, Rawson DM (1993) Comparison of two types of sensors using eukaryotic algae to monitor pollution of aquatic systems. Water Res 27(3):427–431
Chouteau C et al (2004) Development of novel conductometric biosensors based on immobilised whole cell Chlorella vulgaris microalgae. Biosens Bioelectron 19(9):1089–1096
Ionescu RE, Abu-Rabeah K, Cosnier S, Durrieu C, Chovelon JM, Marks RS (2006) Amperometric algal Chlorella vulgaris cell biosensors based on alginate and polypyrrole-alginate gels. Electroanalysis 18(11):1041–1046
Vedrine C et al (2003) Optical whole-cell biosensor using Chlorella vulgaris designed for monitoring herbicides. Biosens Bioelectron 18(4):457–463
Dzyadevych SV et al (2005) Early-warning electrochemical biosensor system for environmental monitoring based on enzyme inhibition. Sens Actuators B Chem 105(1):81–87, Piet Bergveld Special Issue
Marrakchi M et al (2005) A novel proteinase K biosensor based on interdigitated conductometric electrodes for proteins determination in rivers and sewers water. Sens Actuators B Chem 111–112:390–395, Eurosensors XVIII 2004—The 18th European Conference on Solid-State Transducers
Chouteau C et al (2005) A bi-enzymatic whole cell conductometric biosensor for heavy metal ions and pesticides detection in water samples. Biosens Bioelectron 21(2):273–281
Durrieu C et al (2007) Wole cell algal biosensor for urbain waters monotoring. in NOVATEC. Lyon
Albareda-Sirvent M, Merkoci A, Alegret S (2000) Configurations used in the design of screen-printed enzymatic biosensors. a review. Sens Actuators B Chem 69(1–2):153–163
Peter J et al ( 1996) Detection of chlorinated and brominated hydrocarbons by an ion sensitive whole cell biosensor. Biosens Bioelectron 11(12):1215–1219
Cosnier S, Gondran C, Senillou A (1999) Functionalized polypyrroles: a sophisticated glue for the immobilization and electrical wiring of enzymes. Synth Met 102(1–3):1366–1369
Aksu Z, Egretli G, Kutsal T (1998) A comparative study of copper(II) biosorption on Ca-alginate, agarose and immobilized C. vulgaris in a packed-bed column. Process Biochem 33(4):393–400
Calik G et al (1999) Growth and [kappa]-carrageenan immobilization of Pseudomonas dacunhae cells for -alanine production. Enzyme Microb Technol 24(1–2):67–74
Kumar P, Satyanarayana T (2007) Optimization of culture variables for improving glucoamylase production by alginate-entrapped Thermomucor indicae-seudaticae using statistical methods. Bioresour Technol 98(6):1252–1259
Nguyen-Ngoc H, Tran-Minh C (2006) Sol–gel process for vegetal cell encapsulation. Mater Sci Eng C 27(4):607–611
Dzyadevych SV et al (2002) Development of enzyme biosensor based on pH-sensitive field-effect transistors for detection of phenolic compounds. Bioelectrochem 55(1–2):79–81
Chen H et al (2005) Detection of Saccharomyces cerevisiae immobilized on self-assembled monolayer (SAM) of alkanethiolate using electrochemical impedance spectroscopy. Anal Chim Acta 554(1–2):52–59
Gau J-J et al (2001) A MEMS based amperometric detector for E. coli bacteria using self-assembled monolayers. Biosens Bioelectron 16(9–12):745–755
Hleli S et al (2006) Atrazine analysis using an impedimetric immunosensor based on mixed biotinylated self-assembled monolayer. Sens Actuators B Chem 113(2):711–717, special issue—in honour of Professor Karl Cammann
Zhao Y-D et al (1999) DNA-modified electrodes; part 4: optimization of covalent immobilization of DNA on self-assembled monolayers. Talanta 49(4):751–756
Fitzgerald GP, Nelson TC (1966) Extractive and enzymatic analyses for limiting or surplus phosphorous in algae. Phycology 2:32–37
Wang X et al (2006) Development of a conductometric nitrate biosensor based on methyl viologen/Nafion(R) composite film. Electrochem Commun 8(2):201–205
Choi SH, Lee JW, Sim SJ (2005) Enhanced performance of a surface plasmon resonance immunosensor for detecting Ab-GAD antibody based on the modified self-assembled monolayers. Biosens Bioelectron 21(2):378–383
Bucher J-P, Santesson L, Kern K (1994) Thermal healing of self-assembled organic monolayers: hexane- and octadecanethiol on Au(111) and Ag(111). Langmuir 10(4):979–983
Guo L-H et al (1994) Effect of gold topography and surface pretreatment on the self-assembly of alkanethiol monolayers. Langmuir 10(12):4588–4593
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guedri, H., Durrieu, C. A self-assembled monolayers based conductometric algal whole cell biosensor for water monitoring. Microchim Acta 163, 179–184 (2008). https://doi.org/10.1007/s00604-008-0017-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-008-0017-2