Abstract
Open surgical repair (OSR) for thoracoabdominal aortic aneurysms (TAAAs) is maximally invasive and associated with high rates of operative mortality and perioperative complications including spinal cord ischemia (SCI), despite improvements in surgical techniques and perioperative care. Elderly patients, patients with a history of aortic surgery, and patients with severe comorbidities are often considered ineligible for this surgery and endovascular treatment may be their only treatment option. Total endovascular aneurysm repair (t-EVAR) without debranching surgery does not require thoracotomy and laparotomy and could improve the outcomes of these patients. t-EVAR includes fenestrated EVAR (f-EVAR), multi-branched EVAR (b-EVAR), and physician-modified fenestration endograft (PMFG). Although these techniques have achieved lower mortality rates than OSR, there are concerns about perioperative complications including limb ischemia, SCI, and long-term outcomes such as endograft migration and endoleaks (ELs). This article provides an overview of available endovascular devices for TAAAs and reviews the short and mid-term results of t-EVAR, as well as alternative options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Open surgical repair (OSR) is the gold standard of treatment for thoracoabdominal aortic aneurysms (TAAAs). However, despite improvements in surgical techniques, spinal cord protection, and peri-operative critical care support, mortality and perioperative complication rates remain high [1]. In particular, spinal cord ischemia (SCI) is a serious complication that remains unresolved. Other complications include organ ischemia, renal failure, pulmonary hemorrhage, and pneumonia. In fact, several reports have indicated that the 30-day mortality rate of OSR is approximately 5–19% [1, 2]. According to the Japanese Association for Thoracic Surgery and the Japanese Society for Vascular Surgery, the 30-day mortality rate of OSR is 6–10%. In addition to the high mortality rates, the incidence of SCI in patients with Crawford type II TAAA ranges from 2 to 27%, with an average of 10% [3]. Patients with a history of aortic surgery and those with severe comorbidities, such as cardiac disease and chronic obstructive pulmonary disease (COPD), are at increased surgical risk [4, 5]. Because of its highly invasive nature, many high-risk patients are deemed ineligible for OSR. Thus, fenestrated EVAR (f-EVAR; Cook Medical Inc., Bloomington, IN, USA; Fig. 1), multi-branched EVAR (b-EVAR) (t-Branch; Cook Medical Inc.; Fig. 2), and physician-modified fenestrated endograft (PMFG) have been developed as alternative options for high-risk OSR patients. This review article provides an overview of the various treatment modalities for TAAAs, with special emphasis on the total EVAR (t-EVAR) technique including f-EVAR, b-EVAR, and PMFG, as well as hybrid procedures, and their clinical outcomes.
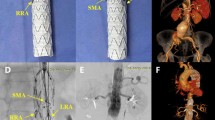
a Fenestrated stent graft (Cook Medical Inc., Bloomington, IN, USA). b Covered stents were deployed to bridge the fenestrated stent graft and each visceral branch. c The short overlap of the fenestration site between the main stent graft and the covered stent of the visceral arteries. d, e Enhanced computed tomography of a thoracoabdominal aortic aneurysm (TAAA) (Crawford type III): d preoperatively, e after treatment with fenestrated endovascular aneurysm repair (EVAR)
a Multibranched stent graft (t-Branch, Cook Medical Inc., Bloomington, IN, USA). The branching portion of the stent graft has a waist. b Covered stents were deployed to bridge the multibranched stent graft and each visceral branch. c, d Enhanced computed tomography of a thoracoabdominal aortic aneurysm (TAAA) (Crawford type I): d preoperatively, e after treatment with t-Branch
Current status of conventional open surgical repair
OSR is highly invasive and one of the most challenging operative techniques for vascular surgeons because it involves opening both the thoracic and abdominal cavities, then reconstructing the visceral branches and repairing the aneurysm. In a high-volume center, the 30-day mortality was estimated to be 5–19%, which increased to 20–40% for emergency cases. The postoperative complication rate ranged from 10 to 16% and the most common postoperative complication was paraplegia. [5,6,7] The early mortality rate depends on the location and extent of the TAAA and ranges from 5 to 8% for Crawford type I, 8–13% for Crawford type II, 8–21% for Crawford type III, and 2–6% for Crawford type IV at experienced centers [8,9,10,11]. Coselli et al. [5] reported that severe perioperative complications occurred most frequently following the treatment of Crawford type II TAAAs.
Current endovascular technology
The endovascular options for preserving abdominal visceral branches include f-EVAR, b-EVAR, and PMFG. While PMFG and hybrid procedures such as debranching EVAR can be performed in emergency surgery, custom-made f-EVAR and b-EVAR require time to procure and are not an option for emergency surgery. Custom-made fenestrated and multi-branched stent grafts (SGs) seem to be ideal for elective TAAA surgery. Off-the-shelf devices have been developed to overcome this procurement delay.
Fenestrated EVAR (f-EVAR)
Since the late 1990s, f-EVAR has been performed to secure the proximal landing zone for type Ia ELs [12]. In 2001, successful f-EVAR for pararenal AAA was reported [13, 14] and in 2005, f-EVAR for TAAA was reported [15]. Subsequently, in 2006, f-EVAR was introduced in our department [16]. The most common type of fenestrated SG is composed of a Zenith platform (Cook Medical Inc., Bloomington, IN, USA). The number of fenestrations is determined by the number of reconstructed branches and an iCAST (Atrium Medical Corp., Hudson, NH, USA) balloon-expandable covered stent is often used for bridging between the fenestration and the visceral branches.
Device design and sizing are of paramount importance for successful f-EVAR, as well as for accurate intraoperative positioning of the fenestration to the accompanying visceral branches. Since the extent of SG coverage must be weighed against the risk of spinal cord ischemia (SCI) [17], preoperative designing is necessary to obtain a sealing zone that is not excessive or insufficient. Although there are several methods of preoperative planning, centerline analysis is used most commonly, particularly for complex TAAAs and its usefulness and accuracy have been described by several authors [18, 19]. The calculation of an aortic centerline is performed in a semi-automatic manner using a dedicated workstation. Operators need to assess whether the center line runs along the proper path and modify it if necessary. The operator must draw the centerline manually in cases of insufficient contrast enhancement for detecting arterial flow automatically [20].
The clinical outcomes of f-EVAR have been reported from various institutions (Table 1) [17, 21,22,23,24,25,26,27,28,29,30,31]. Current reports on f-EVAR have demonstrated that the 30-day mortality is 1.4–7.8% and that technical success was achieved in 87–98% of cases. The rate of SCI was 2–10% and the visceral vessel patency rate at 1 year was 90–98%, with estimated overall survival at 2 years of 78–92%. Crawford type II TAAA was associated with higher mortality and longer hospitalization than Crawford type III TAAA. The risk factors for poor long-term survival following TAAA treatment were age, chronic pulmonary obstructive disease, and Crawford type II TAAA [21,22,23,24,25,26,27,28,29,30,31].
Since type III ELs often occur following f-EVAR, patients undergoing f-EVAR required secondary intervention more frequently. This is because the junction between the main body of the SG and the bridging-covered stent for the visceral arteries is very short. The rate of freedom from secondary intervention has been reported as 79–96.7% at 1 year and 63–88.0% at 3 years, which is not satisfactory [17, 25, 27, 30, 32]. Since the main device is commonly inserted via the common femoral artery (CFA) and the required number of sheaths for branched reconstruction is inserted from the contralateral CFA, common complications include limb ischemia and myonephropathic metabolic syndrome (MNMS) because of the prolonged lower extremity ischemia [17, 33]. The longer operative time related to technical difficulties results in longer ischemic time of the lower extremities, warranting greater attention in this situation.
Endovascular procedure of fenestrated EVAR (Fig. 3)
The bilateral femoral arteries are surgically exposed and the devices are inserted via the femoral artery after systemic heparinization. Custom-made fenestrated SGs have many radiopaque markers that denote the direction of the SG as well as the location of the fenestration. Subsequently, the SG is semi-deployed in the optimal direction and clock position, while the radiopaque markers are checked with fluoroscopic guidance. At this point, the custom-made SG is designed so that its diameter does not extend over the entire length of the SG because of the diameter-reducing tie [16, 17]. Theoretically, the SG position and direction can be adjusted until the removal of the diameter-reducing tie. The guiding sheath is inserted from the contralateral femoral artery and cannulated into the main SG from its distal opening, and then into each visceral branch through the fenestrations, using a guidewire and an appropriate catheter. The SG is then deployed completely by removing the diameter-reducing tie. Finally, covered stents are delivered into the visceral arteries, via the guiding sheaths, and deployed to bridge between the fenestrated SG and each visceral branch.
a Intraoperative angiography of a thoracoabdominal aortic aneurysm (TAAA) (Crawford type III). b The stent graft was deployed after the operator established its optimal direction and position while checking the radiopaque markers and performing angiography. c The guiding sheath was inserted into the abdominal visceral branches via the contralateral femoral artery or brachial artery. d Covered stents via guiding sheaths were inserted to the visceral arteries and deployed to bridge the fenestrated stent graft and each visceral branch. e The position of the fenestrated site was confirmed by the radiopaque markers. f Postoperative angiography of a TAAA (Crawford type III) after treatment with fenestrated endovascular aneurysm repair (EVAR)
Multi-branched EVAR (t-Branch)
The first multi-branched EVAR (b-EVAR) of TAAA was performed in 2001 [34] using a “home-made” modular SG with caudally directed cuffs for branch attachment. A multi-branched TAAA SG became commercially available in 2008, [35,36,37,38] and gained popularity rapidly. The t-Branch (Cook Medical Inc.), which is the most commonly used multi-branched SG, was designed with four directional sleeves for the celiac axis, superior mesenteric artery, and both renal arteries. It is estimated that just over 50% of the TAAA population are potential candidates for the device in a single-stage procedure, with even greater suitability when performed in a staged manner [35]. The t-Branch is designed with a smaller waist at the site of the main body-attached visceral sleeve. Although the t-Branch is an off-the-shelf device, the number of side branches, the diameter of the side branches (6 mm or 8 mm), and the proximal and distal diameters can be modified by the surgeons. The t-Branch for TAAAs was introduced in our department in 2012 [16, 17]. The clinical outcomes of the t-Branch have been reported by various institutions (Table 2) [17, 23, 39,40,41,42,43]. Current t-Branch reports report that the 30-day mortality is 4.0–9.1%, with technical success achieved in 82–98.9%. The occurrence rate of SCI ranges from 3–35.7%, and the visceral vessel patency rate at 1 year is 95–99%. The estimated overall survival at 1 year is documented as 82–88%.
Type III ELs are unlikely to occur with the t-Branch device because the main device has structural sleeves to visceral arteries, thereby providing a longer overlap zone between the bridging stent [14, 17, 33]. It was reported that the rate of freedom from secondary intervention 1 year after t-Branch intervention was 79–100% [17, 23, 39, 40, 43]. Another advantage of the t-Branch procedure over f-EVAR is that it is associated with a lower risk of lower extremity ischemia and subsequent compartment syndrome because the SG delivery sheath is removed following the deployment of the main body and prior to visceral cannulation, which is the time-consuming step. Thus, the lower extremity ischemic time is shorter than that for the fenestrated device [17, 33].
We reported previously that the chance of SCI increased remarkably when the risk factors, such as the procedure (t-Branch), a maximum short axis of ≥ 65 mm, a coverage length of ≥ 360 mm, and ≥ 5 sacrificed intercostal arteries, were combined [17]. In particular, a high incidence of SCI following t-Branch has been observed in patients with these high-risk factors. We perform spinal cord drainage for these patients in our department, but a more effective way of preventing SCI is required. Staged surgery, in which the main device is deployed as the first step and visceral stenting is performed a few days or weeks later, has been suggested as an effective measure for preventing SCI. Several studies have proposed that a staged approach was effective in reducing the rate of SCI to approximately 5% [44,45,46]. However, there is concern about aneurysm rupture during the staged approach interval. Therefore, it is recommended that the side branch reconstruction be performed within 2–4 weeks, depending on the size of the TAAA.
Cerebral infarction sometimes occurs during the t-Branch procedure, possibly caused by embolization secondary to the insertion of a long sheath and pull-through wire via the upper extremities. Thus, specific attention should be paid to the quality of the arch and to the appropriate selection of patients for the t-Branch device [17]. To prevent intraoperative embolic stroke, it may be useful to stabilize the plaque with the administration of a statin prior to surgery, although further study on this is warranted [47].
Endovascular procedure of multi-branched EVAR (t-Branch) (Fig. 4)
The femoral artery is surgically exposed unilaterally for insertion of the main device, and a 4 Fr sheath is inserted percutaneously into the contralateral femoral artery. The left axillary artery is exposed surgically, and a 6-Fr sheath is placed. The SG deployment is performed while adjusting to the clock position. The t-Branch procedure does not require the same precise deployment of the main body as that for the f-EVAR since the branched sleeve is positioned 10–20 mm above the orifice of the visceral vessels and there is enough room for adjustment and cannulation. The SG and delivery sheath are removed after deployment and before visceral cannulation and the femoral artery arteriotomy site is closed; thus, allowing perfusion of the lower limb. A pull-through wire is inserted between the axillary sheath and the 4 Fr femoral sheath using a snare device. The 6 Fr sheath in the left axillary artery is exchanged with a 10-Fr Ansel sheath (Cook Medical Inc.) and guided into the SG over the pull-through wire. Since tension is applied to both ends of the pull-through wire, the Ansel sheath tracts easily around the acute aortic arch curvature. The pull-through wire is kept in position to maintain the stability of the Ansel sheath during delivery of the bridge stents. A covered stent such as Fluency (Bard peripheral vascular; Bard, Inc., Tempe, AZ, USA) or Viabahn (W. L. Gore & Associates, Flagstaff, AZ, USA) is deployed to each visceral branch. The bridging covered stent is lined with a self-expandable stent to prevent kinking [17, 33].
a Intraoperative angiography of a thoracoabdominal aortic aneurysm (TAAA) (Crawford type I). b t-Branch deployment was performed while adjusting to the clock position. c The 10-Fr Ansel sheath (Cook Medical Inc., Bloomington, IN, USA) was advanced into the stent graft via a pull-through wire. d Each branch was stented with a covered stent. e The bridging endograft was routinely lined with a self-expandable stent to prevent dissection and kinking. f Postoperative angiography of a TAAA (Crawford type I) after treatment with t-Branch
Physician-modified fenestration stent-graft (PMFG)
The physician-modified graft utilizes Cook TX2 (Cook Medical Inc.) or Zenith devices. The device is unsheathed on a back table under sterile conditions. Reinforced fenestrations are created with Atrium SST PTFE (Atrium Medical Corp.) and platinum coils. Permanent and temporary diameter-reducing ties are created according to the technique described by Oderich [48]. Once the modifications are complete, the device is reinserted into its original delivery sheath [49]. Several investigators have reported the results of using PMFGs in the management of complex aortic aneurysms; predominantly, juxtarenal aneurysms [50]. There have been only a few limited series or individual cases reported of PMFGs being used in the treatment of TAAA (Table 3) [51,52,53,54,55,56]. The 30-day mortality is reported to range from 2 to 9%, with the technical success achieved in 88–98% of cases [49, 50, 54, 56]. To our knowledge, the largest experience comes from the Mayo Clinic [57]. Oderich et al., compared the results of 30 PMFGs with 16 hybrid debranching repairs for high-risk patients with complex aortic aneurysms. The proportion of TAAA patients in the PMFG group vs. in the hybrid groups was 47% vs. 81%. The PMFGs were associated with less blood loss, less fluid requirements, and shorter total operative time than the hybrid repairs. Postoperative mortality rates were 3.3% in the PMFG group vs. 19% in the hybrid group.
TEVAR with celiac artery coverage for TAAAs
Several studies with short-term follow-up have demonstrated that coverage of the celiac artery is an acceptable and alternative endovascular approach for selected patients, particularly those with Crawford type I TAAA with collateralization between the celiac artery (CA) and superior mesenteric artery (SMA) [58,59,60]. Angiography is recommended for assessing collateral circulation before the planned coverage of the celiac artery. Multiple collateral pathways flow from the SMA to the branches of the CA origin. Pancreaticoduodenal arcades and the dorsal pancreatic arteries are well-known collateral arteries. [61]. Branches of the gastroduodenal artery and inferior pancreaticoduodenal arteries arising from the SMA communicate to form the anterior and posterior pancreaticoduodenal arcades. The incidence of complications of visceral ischemia during TEVAR with celiac artery coverage is reported as 6–11% and that of death resulting from associated visceral ischemia is reported as 3–6% [58,59,60]. Therefore, it is important to evaluate the symptoms of mesenteric and liver ischemia during the perioperative period since selective CA and SMA angiography alone might not accurately predict adequate collateralization.
Hybrid surgery for TAAAs
Hybrid surgery, comprised of an initial open visceral debranching procedure followed by endovascular aneurysm exclusion, is performed worldwide [62,63,64,65,66,67]. These procedures avoid the extensive double-cavity exposure, aortic cross-clamping, and mechanical circulatory support that are associated with open TAAA repair. They also offer the theoretical advantage of being less invasive than OSR. Although the visceral debranching procedure requires laparotomy, this approach is considered less invasive because the organ ischemic time can be reduced and thoracotomy or aortic clamping is not required. Two Japanese studies reported good short- and long-term outcomes with a 2.3–5% operative mortality rate [66, 67]. However, other studies reported 30-day mortality rates ranging from 12.3 to 34.2% [63,64,65, 68,69,70,71], suggesting no improvement in perioperative mortality (Table 4). Moulakakis et al. [68] performed a meta-analysis of outcomes of 528 hybrid TAAA repairs from 14 studies and found a substantial mortality rate of 14.3% and a complication rate of 7.0% for spinal cord ischemia, 4.5% for mesenteric ischemia, and 7.0% for permanent renal failure. Mesenteric ischemia after hybrid surgery remains a concern and may range from 4.5 to 20% [63, 68,69,70]. Chiesa et al. [71] reported that severe angulation of the SMA bypass graft is predictive of these ischemic complications. In the advent of t-Branch SG and other multi-branched SGs, the number of hybrid procedures is expected to decline.
Initial and mid-term results of fenestrated and branched EVAR for TAAAs: the Jikei experience of 113 patients
Between July 2006 and June 2018, 113 patients underwent elective f-EVAR, b-EVAR (t-Branch), and PMFG for TAAAs in our institution. The mean age was 75.1 ± 6.9 years and the mean maximum diameter of the aneurysm was 57.3 ± 9.0 mm. f-EVAR and t-Branch were performed for patients at high risk for OSR, such as those with heart disease and COPD and those who had undergone prior aortic surgery. The devices used were fenestrated SG in 77 patients, PMFG in 19 patients, and multi-branched SG (t-Branch) in 17 patients, and all patients underwent a single-stage procedure. The mean operative time was 396 ± 152.4 min, the mean intraoperative blood loss was 1616 ml, and the technical success rate was 96.6%. In one patient with a fenestrated SG, since the SMA scallop did not align with the SMA orifice and resulted in SMA ischemia, a mini laparotomy was performed with stent deployment in a retrograde manner, from the ilio-colic artery and then to the superior mesenteric artery orifice, to maintain its patency. The 30-day mortality rate was 2.7% (n = 3) and the causes of death were myonephropathic metabolic syndrome (MNMS) (n = 2; f-EVAR) and cerebral infarction (n = 1; t-Branch). Spinal cord ischemia was identified in 11 patients (9.7%), as paraplegia in 6 and paraparesis in 5; and lower extremity compartment syndrome was identified in 3 patients (2.7%). Notably, five of the patients with paraplegia and four of those with paraparesis were in the t-Branch group, and three with lower extremity compartment syndrome were in the f-EVAR group. The average postoperative hospital stay was 16.1 days, the mean follow-up period was 35.9 months (range 0–140), and 24 patients (21.2%) needed additional treatments involving postoperative ELs in the long term. The majority of reinterventions were performed for Type III Els, (n = 14, 12.3%), mostly after f-EVAR (n = 13). The incidences of freedom from aneurysm-related death and from secondary intervention at 1, 3, and 5 years were 97.3%, 94.5%, and 94.5%, respectively, and 88.9%, 75.9% and 63.3%, respectively. Intentional celiac artery coverage during TEVAR for Crawford type I TAAA was performed in 33 patients, achieving good celiac artery flow via the superior mesenteric artery collaterals. There were no fatal complications after TEVAR with celiac artery coverage and all patients were discharged from hospital.
Conclusions
Because of the lack of long-term data and regulatory approval for most t-EVAR procedures, conventional OSR remains the standard therapy for TAAAs for low-risk patients, despite the less than satisfactory short and long-term outcomes. Development and proliferation of t-EVAR is expected in light of the promising short and long-term outcomes described in this review article. The complications of f-EVAR were serious, including MNMS and a high rate of secondary intervention for type III EL. In contrast, although more durable, the incidence of SCI following b-EVAR (t-Branch) was alarmingly high compared with f-EVAR and further refinements in devices as well as surgical technique, including a staged procedure, are warranted. As yet, there is no completely reliable device and it is important to select the best treatment option for each individual patient depending on their condition. Therefore, it is best to keep every treatment modality in the surgeon’s armamentarium to manage TAAAs.
References
Coselli JS, Bozinovski J, LeMaire SA. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg. 2007;83:862–4 (discussion S890–862).
Rigberg DA, McGory ML, Zingmond DS, Maggard MA, Agustin M, Lawrence PF, et al. Thirty-day mortality statistics underestimate the risk of repair of thoracoabdominal aortic aneurysms: a statewide experience. J Vasc Surg. 2006;43:217–23.
Ferreira M, Lanziotti L, Monteiro M. Branched devices for thoracoabdominal aneurysm repair: early experience. J Vasc Surg. 2008;48:S30–6.
Suzuki S, Davis III CA, Miller III CC, Huynh TT, Estrera AL, Porat EE, et al. Cardiac function predicts mortality following thoracoabdominal and descending thoracic aortic aneurysm repair. Eur J Cardiothorac Surg. 2003;24:119–124 (discussion 124).
Coselli JS, LeMaire SA, Conklin LD, Köksoy C, Schmittling ZC. Morbidity and mortality after extent II thoracoabdominal aortic aneurysm repair. Ann Thorac Surg. 2002;73;1107–1115 (discussion 1115–1116).
LeMaire SA, Price MD, Green SY, Zarda S, Coselli JS. Results of open thoracoabdominal aortic aneurysm repair. Ann Cardiothorac Surg. 2012;1:286–92.
Coselli JS, Conklin LD, LeMaire SA. Thoracoabdominal aortic aneurysm repair: review and update of current strategies. Ann Thorac Surg 2002; 74:S1881–S1884 discussion S1892–S1898).
Conrad MF, Crawford RS, Davison JK, Cambria RP. Thoracoabdominal aneurysm repair: a 20-year perspective. Ann Thorac Surg. 2007;83:S856–61.
Patel VI, Ergul E, Conrad MF, Cambria M, LaMuraglia GM, Kwolek CJ, et al. Continued favorable results with open surgical repair of type IV thoracoabdominal aortic aneurysms. J Vasc Surg. 2011;53:1492–8.
Kulik A, Castner CF, Kouchoukos NT. Outcomes after thoracoabdominal aortic aneurysm repair with hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2011;141:953–60.
Zoli S, Roder F, Etz CD, Brenner RM, Bodian CA, Lin HM, et al. Predicting the risk of paraplegia after thoracic and thoracoabdominal aneurysm repair. Ann Thorac Surg. 2010;90:1237–45.
Faruqi RM, Chuter TA, Reilly LM, Sawhney R, Wall S, Canto C, et al. Endovascular repair of abdominal aortic aneurysm using a pararenal fenestrated stent-graft. J Endovasc Surg. 1999;6:354–8.
Stanley BM, Semmens JB, Lawrence-Brown MM, Goodman MA, Hartley DE. Fenestration in endovascular grafts for aortic aneurysm repair: new horizons for preserving blood flow in branch vessels. J Endovasc Ther. 2001;8:16–24.
Anderson JL, Berce M, Hartley DE. Endoluminal aortic grafting with renal and superior mesenteric artery incorporation by graft fenestration. J Endovasc Ther. 2001;8:3–15.
Anderson JL, Adam DJ, Berce M, Hartley DE. Repair of thoracoabdominal aortic aneurysms with fenestrated and branched endovascular stent grafts. J Vasc Surg. 2005;42:600–7.
Ohki T. Branched stent graft for the treatment of thoracoabdominal aneurysms. Nihon Geka Gakkai Zasshi. 2011;112:26–31.
Baba T, Ohki T, Kanaoka Y, Maeda K, Ohta H, Fukushima S, et al. Clinical outcomes of spinal cord ischemia after fenestrated and branched endovascular stent grafting during total endovascular aortic repair for thoracoabdominal aortic aneurysm. Ann Vasc Surg. 2017;44:146–57.
Higashiura W, Kichikawa K, Sakaguchi S, Tabayashi N, Taniguchi S, Uchida H. Accuracy of centerline of flow measurement for sizing of the Zenith AAA endovascular graft and predictive factor for risk of inadequate sizing. Cardiovasc Intervent Radiol. 2009;32:441–8.
Velazquez OC, Woo EY, Carpenter JP, Golden MA, Barker CF, Fairman RM. Decreased use of iliac extensions and reduced graft junctions with software-assisted centerline measurements in selection of endograft components for endovascular aneurysm repair. J Vasc Surg. 2004;40:222–7.
Banno H, Kobeiter H, Brossier J, Marzelle J, Presles E, Becquemin JP. Inter-observer variability in sizing fenestrated and/or branched aortic stent-grafts. Eur J Vasc Endovasc Surg. 2014;47:45–52.
Marzelle J, Presles E, Becquemin JP, WINDOWS trial participants. Results and factors affecting early outcome of fenestrated and/or branched stent grafts for aortic aneurysms: a multicenter prospective study. Ann Surg. 2015;261:197–206.
Cochennec F, Kobeiter H, Gohel MS, Majewski M, Marzelle J, Desgranges P, et al. Impact of intraoperative adverse events during branched and fenestrated aortic stent grafting on postoperative outcome. J Vasc Surg. 2014;60:571–8.
Bisdas T, Donas KP, Bosiers MJ, Torsello G, Austermann M. Custom-made versus off-the-shelf multibranched endografts for endovascular repair of thoracoabdominal aortic aneurysms. J Vasc Surg. 2014;60:1186–95.
Budtz-Lilly J, Wanhainen A, Eriksson J, Mani K. Adapting to a total endovascular approach for complex aortic aneurysm repair: outcomes after fenestrated and branched endovascular aortic repair. J Vasc Surg. 2017;66:1349–56.
Verhoeven EL, Katsargyris A, Bekkema F, Oikonomou K, Zeebregts CJ, Ritter W, et al. Editor’s choice- Ten-year experience with endovascular repair of thoracoabdominal aortic aneurysms: results from 166 consecutive patients. Eur J Vasc Endovasc Surg. 2015;49:524–31.
Grimme FA, Zeebregts CJ, Verhoeven EL, Bekkema F, Reijnen MM, Tielliu IF. Viscera stent patency in fenestrated stent grafting for abdominal aortic anerurysm repair. J Vasc Surg. 2014;59:298–306.
Metcalfe MJ, Holt PJ, Hinchliffe RJ, Morgan R, Loftus IM, Thompson MM. Fenestrated endovascular aneurysm repair: graft complexity does not predict outcome. J Endovasc Ther. 2012;19:528–35.
Amiot S, Haulon S, Becquemin JP, Magnan PE, Lermusiaux P, Goueffic Y, et al; Association Universitaire de Recherche en Chirurgie Vasculaire. Fenestrated endovascular grafting: the French multicentre experience. Eur J Vasc Endovasc Surg. 2010;39:537–544.
Shahverdyan R, Gray D, Gawenda M, Brunkwall J. Single centre results of total endovascular repair of complex aortic aneurysms with custom made Anaconda fenestrated stent grafts. Eur J Vasc Endovasc Surg. 2016;52:500–8.
Freyrie A, Massoni CB, Pini R, Mascoli C, et al. Endovascular repair of thoracoabdominal aortic aneurysm in high-surgical risk patients: fenestrated and branched endografts. Ann Vasc Surg. 2017;40:170–7.
Haulon S, Amiot S, Magnan PE, Becquemin JP, Lermusiaux P, Koussa M, Association Universitaire de Recherche en Chirurgie Vasculaire (AURC), et al. An analysis of the French multicentre experience of fenestrated aortic endografts: medium-term outcomes. Ann Surg. 2010;251:357–62.
Dossabhoy SS, Simons JP, Diamond KR, Flahive JM, Aiello FA, Arous EJ, et al. Reinterventions after fenestrated or branched endovascular aortic aneurysm repair. J Vasc Surg. 2018;68:669–81.
Baba T, Ohki T, Kanaoka Y, Maeda K, Toya N, Ohta H, et al. Clinical outcomes of total endovascular aneurysm repair for aortic aneurysms involving the proximal anastomotic aneurysm following initial open repair for infrarenal abdominal aortic aneurysm. Ann Vasc Surg. 2018;49:123–33.
Chuter TA, Gordon RL, Reilly LM, Pak LK, Messina LM. Multi-branched stent-graft for type III thoracoabdominal aortic anerurysm. J Vasc Interv Radiol. 2001;12:391–2.
Gasper WJ, Reilly LM, Rapp JH, Grenon SM, Hiramoto JS, Sobel JD, et al. Assessing the anatomic applicability of the multibranched endovascular repair of thoracoabdominal aortic aneurysm technique. J Vasc Surg. 2013;57:1553–8 (discussion 1558).
Chuter TA, Rapp JH, Hiramoto JS, Schneider DB, Howell B, Reilly LM. Endovascular treatment of thoracoabdominal aortic aneurysms. J Vasc Surg. 2008;47:6–16.
Park KH, Hiramoto JS, Reilly LM, Sweet M, Chuter TA. Variation in the shape and length of the branches of a thoracoabdominal aortic stent graft: implications for the role of standard off-the-shelf components. J Vasc Surg. 2010;51:572–6.
Sweet MP, Hiramoto JS, Park KH, Reilly LM, Chuter TA. A standardized multi-branched thoracoabdominal stent-graft for endovascular aneurysm repair. J Endovasc Ther. 2009;16:359–64.
Silingardi R, Gennai S, Leone N, Gargiulo M, Faggioli G, Cao P, Italian mbEVAR study group, et al. Standard “off-the-shelf” multibranched thoracoabdominal endograft in urgent and elective patients with single and staged procedures in a multicenter experience. J Vasc Surg. 2018;67:1005–16.
Gallitto E, Gargiulo M, Freyrie A, Pini R, Mascoli C, Ancetti S, et al. Off-the-shelf multibranched endograft for urgent endovascular repair of thoracoabdominal aortic aneurysms. J Vasc Surg. 2017;66:696–704.
Tsilimparis N, Fiorucci B, Debus ES, Rohlffs F, Kölbel T. Technical aspects of implanting the t-Branch off-the-shelf multibranched stent-graft for thoracoabdominal aneurysms. J Endovasc Ther. 2017;24:397–404.
Bosiers MJ, Bisdas T, Donas KP, Torsello G, Austermann M. Early experience with the first commercially available off-the-shelf multibranched endograft (t-branch) in the treatment of thoracoabdominal aortic aneurysms. J Endovasc Ther. 2013;20:719–25.
Hu Z, Li Y, Peng R, Liu J, Jia X, Liu X, et al. Multibranched stent-grafts for the treatment of thoracoabdominal aortic aneurysms: a systematic review and meta-analysis. J Endovasc Ther. 2016;23:626–33.
Mascoli C, Vezzosi M, Koutsoumpelis A, Iafrancesco M, Ranasinghe A, Clift P, et al. Endovascular repair of acute thoraco-abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2018;55:92–100.
Kasprzak PM, Gallis K, Cucuruz B, Pfister K, Janotta M, Kopp R. Editor’s choice—temporary aneurysm sac perfusion as an adjunct for prevention of spinal cord ischemia after branched endovascular repair of thoracoabdominal aneurysms. Eur J Vasc Endovasc Surg. 2014;48:258–65.
Schurink GW, De Haan MW, Peppelenbosch AG, Mess W, Jacobs MJ. Spinal cord function monitoring during endovascular treatment of thoracoabdominal aneurysms: implications for staged procedures. J Cardiovasc Surg (Torino). 2013;54:117–24.
Kawahara T, Nishikawa M, Kawahara C, Inazu T, Sakai K, Suzuki G. Atorvastatin, etidronate, or both in patients at high risk for atherosclerotic aortic plaques: a randomized, controlled trial. Circ. 2013;127:2327–35.
Oderich GS. Technique of adding a diameter-reducing wire to the modified TX2 fenestrated stent graft. Vascular. 2010;18:350–5.
Sweet MP, Starnes BW, Tatum B. Endovascular treatment of thoracoabdominal aortic aneurysm using physician-modified endografts. J Vasc Surg. 2015;62:1160–7.
Starnes BW. Physician modified endovascular grafts for the treatment of elective, symptomatic, or ruptured juxtarenal aortic aneurysms. J Vasc Surg. 2012;56:601–7.
Oderich GS, Fatima J, Gloviczki P. Stent graft modification with mini-cuff reinforced fenestrations for urgent repair of thoraco-abdominal aortic aneurysms. J Vasc Surg. 2011;54:1522–6.
Oderich GS, Mendes BC, Correa MP. Preloaded guidewires to facilitate endovascular repair of thoraco-abdominal aortic aneurysm using a physician modified branched stent graft. J Vasc Surg. 2014;59:1168–73.
Oderich GS, Ricotta JJ 2nd. Modified fenestrated stent grafts: device design, modifications, implantation, and current applications. Perspect Vasc Surg Endovasc Ther. 2009;21:157–67.
Ricotta JJ 2nd, Tsilimparis N. Surgeon-modified fenestrated-branched stent grafts to treat emergently ruptured and symptomatic complex aortic aneurysms in high risk patients. J Vasc Surg. 2012;56:1535–42.
Jim J, Sanchez LA, Rubin BG. Use of a surgeon-modified branched thoracic endograft to preserve an aortorenal bypass during treatment of an intercostal patch aneurysm. J Vasc Surg. 2010;52:730–3.
Cochennec F, Kobeiter H, Gohel M, et al. Early results of physician modified fenestrated stent grafts for the treatment of thoracoabdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2015;50:583–92.
Oderich GS. Reporting on fenestrated endografts: surrogates for outcomes and implications of aneurysm classification, type of repair, and the evolving technique. J Endovasc Ther. 2011;18:154–6.
Vaddineni SK, Taylor SM, Patterson MA, Jordan WD Jr. Outcome after celiac artery coverage during endovascular thoracic aortic aneurysm repair: preliminary results. J Vasc Surg. 2007;45:467–71.
Mehta M, Darling RC 3rd, Taggert JB, Roddy SP, Sternbach Y, Ozsvath KJ, Kreienberg PB, et al. Outcomes of planned celiac artery coverage during TEVAR. J Vasc Surg. 2010;52:1153–8.
Rose MK, Pearce BJ, Matthews TC, Patterson MA, Passman MA, Jordan WD. Outcomes after celiac artery coverage during thoracic endovascular aortic aneurysm repair. J Vasc Surg. 2015;62:36–42.
Rao AS, Rhee RY. Coverage of the celiac artery during TEVAR: is it ever appropriate? Semin Vasc Surg. 2009;22:152–8.
Huang B, Yuan D, Zhao J, Ma Y. Hybrid treatment of a thoracoabdominal aortic aneurysm in china: report of the first successful case. Surg Today. 2012;42:1219–24.
Moulakakis KG, Mylonas SN, Avgerinos ED, Kakisis JD, Brunkwall J, Liapis CD. Hybrid open endovascular technique for aortic thoracoabdominal pathologies. Circulation. 2011;124:2670–84.
Chiesa R, Tshomba Y, Melissano G, Marone EM, Bertoglio L, Setacci F, et al. Hybrid approach to thoracoabdominal aortic aneurysms in patients with prior aortic surgery. J Vasc Surg. 2007;45:1128–35.
Benrashid E, Wang H, Andersen ND, Keenan JE, McCann RL, Hughes GC. Complementary roles of open and hybrid approaches to thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2016;64:1228–38.
Kuratani T, Kato M, Shirakawa Y, Shimamura K, Sawa Y. Long-term results hybrid endovascular repair for thoraco-abdominal aortic aneurysms. Eur J Cardiothorac Surg. 2010;38:299–304.
Shuto T, Wada T, Miyamoto S, Kamei N, Hongo N, Mori H. Ten-year experience of the thoraco-abdominal aortic aneurysm treatment using a hybrid thoracic endovascular aortic repair. Interact Cardiovasc Thorac Surg. 2018;26:951–6.
Moulakakis KG, Mylonas SN, Antonopoulos CN, Liapis CD. Combined open and endovascular treatment of thoracoabdominal aortic pathologies: a systematic review and meta-analysis. Ann Cardiothorac Surg. 2012;1:267–76.
van de Graaf RA, Grüne F, Hoeks SE, Ten Raa S, Stolker RJ, Verhagen HJ, et al. One-year follow-up after hybrid thoracoabdominal aortic repair. Vasc Endovasc Surg. 2017;51(1):23–7.
Rosset E, Ben Ahmed S, Galvaing G, Favre JP, Sessa C, Lermusiaux P. Editor’s choice–hybrid treatment of thoracic, thoracoabdominal, and abdominal aortic aneurysms: a multicenter retrospective study. Eur J Vasc Endovasc Surg. 2014;47:470–8.
Chiesa R, Tshomba Y, Logaldo D, Kahlberg A, Baccellieri D, Apruzzi L. Possible graft-related complications in visceral debranching for hybrid B dissection repair. Ann Cardiothorac Surg. 2014;3:393–9.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Takao Ohki is a paid consultant for WL Gore & Associates. The other authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baba, T., Ohki, T. & Maeda, K. Current status of endovascular treatment for thoracoabdominal aortic aneurysms. Surg Today 50, 1343–1352 (2020). https://doi.org/10.1007/s00595-019-01917-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-019-01917-3