Abstract
Background
The aim of this study was to evaluate the incidence and clinical characteristics of splenic infarction (SI) in gastric cancer patients who have undergone gastrectomy.
Methods
For this study, the medical records of 1084 patients were reviewed and 877 patients were ultimately enrolled. The times of symptom onset, diagnosis of SI, and complete resolution on CT were calculated from the day of the operation. Based on the wedge shape of the SI in all cases, the total volume of the SI was measured based on that of a corn kernel.
Results
Thirty-six patients (4.10%) were diagnosed with SI after gastrectomy; four of these patients (0.45%) developed complications associated with the SI. Total gastrectomy and extended lymph node dissection were risk factors for development of SI. Patients with complications exhibited inflammatory signs between 7 and 10 days after surgery. The mean volume of the SI was 4025.69 mm3. The mean time to complete resolution on the CT scan was 327 days postoperatively. In 30 cases, small branched arteries from the splenic artery that could have caused the SI were retrospectively detected on the preoperative CT scans.
Conclusion
Although the incidence of the SI was low, large volume of the SI is associated with complication development. Measuring the infarction volume via a CT scan may be useful to decide on the treatment strategy. Preoperative 3-D reconstruction of the splenic artery tributaries may help reduce the risk of inadvertent SI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radical gastrectomy including lymph node dissection is a standard treatment for gastric cancer. The most common postoperative complications associated with radical gastrectomy include bleeding, anastomosis or duodenal stump leakage, intra-abdominal abscesses, wound complications, pancreatic fistulas, stomach necrosis, and obstructive ileus [1]. Splenic infarction (SI) following gastrectomy is not uncommon, but usually resolves spontaneously, so it tends to be overlooked after gastrectomy [2]. Even radiologists often forget to examine the spleen in daily routine radiology [3]. Severe complications related to SI such as splenic hemorrhage, abscess formation, or rupture can be fatal, necessitating splenectomy and total gastrectomy. However, early diagnosis is often difficult due to the nonspecific symptomatic presentation [2]. Some single case reports of SI following Nissen fundoplication or gastrectomy have been published [4,5,6,7], but the overall incidence of SI after gastrectomy in gastric cancer patients has not been well established. The aim of this study was to evaluate the clinical characteristics and overall incidence of SI as a possible cause of a complication after gastrectomy in gastric cancer patients.
Materials and methods
Patients
For this retrospective analysis, the gastric cancer database and electronic medical records from January 2013 to July 2015 were reviewed for 1087 patients who underwent surgery for gastric cancer at the Department of Surgery at Seoul St. Mary’s Hospital. All of the cases had been histologically confirmed as gastric adenocarcinoma via endoscopic biopsies and abdominal computed tomography (CT) scans had been taken preoperatively. The exclusion criteria were as follows: patients who underwent non-curative surgery for gastric cancer such as palliative surgery or gastric bypass (85 patients), those who were lost to follow-up within 1 year after surgery (47 patients), those who underwent splenectomy and gastrectomy simultaneously (37 patients), and those who have not taken CT scan as regular follow-up schedule within 6 months (41 patients). Total of 877 patients were ultimately analyzed in this study.
Operations
All patients underwent conventional R0 resection according to the Japanese Gastric Cancer Treatment Guidelines 2010 (ver. 3). Billroth I, Billroth II, or Roux-En-Y anastomoses were performed for subtotal gastrectomy, whereas only Roux-En-Y anastomoses were conducted for total gastrectomy. Depending on the extent of lymph node (LN) dissection, the patients underwent D1+, D2, or D2+ dissection.
CT scan
For preoperative evaluation, patients underwent stomach pre-op CT including perigastric artery 3-D reconstruction. CT images of arterial phase were reconstructed using maximal intensity projection (MIP) with planes of the left gastric artery and the splenic artery and 3D volume rendering.
For patients with cancer staged above IIA according to the 7th UICC/AJCC TNM classification, the first postoperative CT scan was performed within 1 month for baseline assessment of the efficacy of adjuvant chemotherapy, and for up to 1 year, abdominal CT was repeated every two cycles of chemotherapy at the response checkups, which were every 2 or 3 months depending on the regimen. After 1 year, CT was performed every 3 months for 5 years. For the patients with stage I cancer, CT was performed every 6 months for 5 years. Patients who exhibited symptoms of postoperative complications such as fever, chills, abdominal pain, or vomiting underwent abdominal CT. Patients who had not taken CT within 6 months after the surgery were excluded to minimize the possibility of hidden SI with rapid resolution before first CT follow-up.
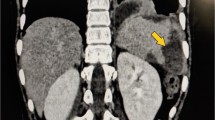
Via CT, we calculated the time of diagnosis of splenic infarction following the operation and that of complete resolution. On the preoperative CT scans, we also assessed the small artery branches from the splenic artery that could have contributed to SI (Fig. 1a, b).
Volumetry of the SI
Since the SI was wedge-shaped in most cases, its volume was calculated using a formula to calculate the volume of a corn kernel; V = 1/3 × area of base × height.
The area of the SI base was measured using region-of-interest (ROI), a programmed system on the PACS (MARO-view), at the transverse section of the CT scan, and the height was measured longitudinally (Fig. 2a, b). In the case of more than one lesion, the volumes were added.
Volume of SI was calculated with the area of the SI base and the height of SI. a The area of base was measured using region-of-interest (ROI), b height was measured on coronal view. Follow-up CT scan showed complete resolution of the SI, c postoperative SI of the lower-pole periphery, d infarcted tissue has shrunk with atrophic change
Statistical analysis
The χ 2 test or Fisher’s exact test was used to evaluate between-group differences in categorical variables, and a P value less than 0.05 was deemed to indicate statistical significance.
To adjust for the bias inherent in an analysis of outcomes stratified by different CT timing, we used propensity score matching analysis. The propensity scores were estimated by using a nonparsimonious multiple logistic regression models. Postoperative first CT follow-up day was selected to calculate the propensity score. In our propensity analyses, a 1:2 matching ratio was used. Univariate analysis was performed using the Kaplan–Meier method with the log-rank; multivariate analysis of survival was performed using a Cox proportional hazards model. All results were analyzed using SPSS Version 22 (SPSS, Chicago, IL, USA).
Results
Out of 877 patients, CT scan revealed an SI in 36 patients (4.10%) after gastrectomy. Of those, four patients developed Clavien–Dindo classification III or IV complications associated with the SI, such as abscess formation or necrosis of the remnant stomach. The overall incidence of complications due to SI was 0.45%, which is very low.
Clinicopathological characteristics of total patients are shown in supplement Table 1. There are significant differences in age, extent of gastrectomy, surgical approach, operation time, estimated blood loss, and pathologic stages between the patients who had SI and had not (Supplement Table 1, P = 0.030, 0.005, 0.003, 0.020, 0.030, and 0.005, respectively). After 1:2 propensity score matching, female sex, total gastrectomy, and D2, D2+ LN dissection were significantly frequent in 36 patients who had SI (Table 1, P = 0.039, 0.032, and 0.021, respectively). Univariate and multivariate analysis revealed that total gastrectomy and D2, D2+ LN dissection were risk factors for the SI development (Table 2, P = 0.009 and 0.011, respectively).
Table 3 shows the clinical features of the SI. Of 44 lesions in 36 patients, eight patients had two lesions each, while 28 patients had a single lesion. The lower-pole periphery (antero-inferior) and upper-pole periphery (median) were the most common and second most common sites (65.9, 29.5%, respectively). The mean volume of the SI was 4025.69 mm3. Four lesions corresponded to complicated SIs, the mean volume of which was significantly larger than that of non-complicated SIs (12,412.2 vs 1318.4 mm3, P < 0.001). The mean time to complete resolution on the CT scan was 327 days following the operation. The preoperative CT scans of 30 cases showed small branched arteries from the splenic artery, which could have been occluded or ligated, possibly preventing the SI. One patient had another thromboembolic complication postoperatively (deep vein thrombosis), but had no symptoms of either SI or deep vein thrombosis (Table 3).
Case series
This study presents four cases of complications related to SI (4 of 36, 11.11%). In these cases, patients showed late onset febrile symptoms, either fever or leukocytosis, after 7 days postoperatively. Other than these four patients, who had no clinical symptoms of the SI did not undergo any of additional treatments but only routine follow-up study.
Case 1
A 79-year-old female patient underwent totally laparoscopic distal gastrectomy with D2-12a LN dissection and B-II reconstruction for recovery of gastrointestinal continuity. She recovered uneventfully until POD 7, when she developed left abdominal pain and mild leukocytosis with a left shift (WBC count 12,030). CT scan revealed SI with abscessation at the lower-pole periphery (Supplement Fig. 1a). She recovered with 5 days of oral antibiotics and was discharged uneventfully.
Case 2
A 58-year-old male patient underwent open total gastrectomy with Roux-En-Y anastomosis and D1+ LN dissection. CT was performed on POD 8 due to fever of 38 °C and WBC count of 16,300. CT showed a small pocket of fluid with an air-fluid level near the esophagojejunostomy site and an SI with abscess at the upper medial side. The two lesions were thought to be separate entities. The patient underwent stent insertion at the EJ anastomosis and pigtail catheter insertion at the splenic abscess. Streptococcus viridans was isolated from the drained fluid, and intravenous tazocin and metronidazole were initiated. At POD 37, follow-up CT and radiographs with gastrografin showed regression of the fluid and no leakage from the anastomosis. The stent and pigtail drain were removed and the patient was discharged on POD 42.
Case 3
A 47-year-old male patient presented due to left upper abdominal pain with tenderness on physical examination 10 days after open total gastrectomy with Roux-En-Y anastomosis and D2-10 LN dissection. Abdominal CT revealed two SI lesions at the antero-inferior and upper medial side of the spleen. The antero-inferior lesion had formed an abscess with an air-fluid level. Intravenous antibiotics were administered for 13 days, and the patient was discharged on POD 17 in good condition.
Case 4
An obese 60-year-old male patient with a body mass index of 29 underwent robot-assisted distal gastrectomy with D1+ LN dissection and B-I anastomosis. Immediately post-op just before general anesthesia was to be ended, his blood pressure suddenly dropped to 60/40. Open exploratory laparotomy revealed a few bleeding foci on the spleen capsule, which were controlled by electrocoagulation. After controlling of the hemorrhage, no parenchymal color change in the spleen was observed. Total blood loss was estimated as 2000 cc during the operation. His vital signs remained stable until POD 3, when he developed mild abdominal discomfort and a fever of 38.0 °C. On POD 7, the WBC count was elevated at 13,250 and continued to increase until POD 8. CT detected an SI and remnant stomach necrosis (Supplement Fig. 1b). Abdominal exploratory laparotomy revealed severe necrosis of the remnant stomach and the spleen, necessitating total gastrectomy. Anastomotic leakage and duodenal stump leakage occurred subsequently, and due to sepsis, a covered stent was placed at the EJ site and a Foley catheter was inserted into the duodenal stump to stop the leakage. The patient recovered and was discharged on POD 36.
Discussion
Although SI is uncommon in general patients, its etiology has been relatively well studied. Previous studies reported that the main causes of SI are thromboembolism, either due to cardiovascular or hypercoagulability problems; acute infection or hematologic diseases leading to rapid enlargement of the spleen; vasculitis; and extrinsic or intrinsic obstruction of the splenic vessels [2, 8,9,10]. These were initially determined via autopsy as the symptoms of SI are nonspecific, making it difficult to diagnose [11].
SI after gastric surgery is not relatively uncommon; small SIs are occasionally seen during surgery after ligation of the perigastric vessels, especially the left gastroepiploic vessels and short gastric vessels that dissect the stomach, but these usually resolve spontaneously without complications unless they are large.
The splenic artery constitutes the main blood supply of the spleen and branches into two typical polar arteries: the superior artery, which sometimes communicates with the short gastric, superior, middle, and inferior terminal arteries, and the inferior artery. It can branch off near the splenic hilum or branch off distant from the hilum. The middle branch exists in 20% of the population [12]. Because of the variable nature of the splenic artery and limited collateral arteries, SI can occur due to occlusion of these peripheral arteries after gastric surgery [3].
Since the splenic artery usually has no communications, theoretically SI should be atrophied due to a lack of blood supply [12]. To figure the mechanism of the recovery from SI, comparison between the preoperative CT and postoperative CT at the time of disappearance of SI was performed. Majority of the infarcted tissue showed shrinkage after SI, so that it seems to be absorbed and atrophied rather than recovered through re-vascularization (Fig. 2c, d). However, since there was no adequate study on this topic, further research is needed to find out the mechanism of it.
Several studies have shown that sonography is ineffective for diagnosing SI, while contrast CT scan is the best diagnostic tool and has the advantage of detecting possible complications associated with SI, such as thromboembolism, hemorrhage, or abscess formation [2, 13]. Additionally, in the present study, the preoperative CT scans from 30 out of 36 cases showed small branched arteries from the splenic artery, which could have caused the SIs. Therefore, when preoperative CT shows branched arteries arising distant from the splenic hilum, all vessels near the spleen should be carefully dissected to prevent SI.
To date, there are few published case reports of SI following Nissen fundoplication or gastrectomy in either morbidly obese patients or cancer patients [4,5,6,7]. Hwang et al. published the three-year surgical outcome of laparoscopy-assisted gastrectomy in 197 patients with gastric cancer and reported only one case of SI [14]. Additionally, Kayihan et al. reported five cases of SI among 25 patients who underwent radical gastrectomy based on CT scan findings [3], but the number of patients was too small to analyze the overall incidence of SI or its complication rate.
To the best of our knowledge, the current study of the CT results of 877 patients is the largest analysis of its type, as well as the first report describing that complicated SI is associated with larger volume of it. Therefore, when a surgeon observes an SI at the periphery of the spleen in the surgical field, detailed description in the operation record with careful monitoring for postoperative complications is recommended.
The symptoms of SI are nonspecific and occur relatively late. According to a 30-year review of general patients by Nores et al. [15] of 59 patients with SI, 69% had various symptoms including abdominal pain, fever and chills, and constitutional symptoms, which are difficult to differentiate from other surgical complications. Early diagnosis of SI is therefore relatively difficult, and fatal complications often arise due to delayed diagnosis. In our study, four patients with a complicated SI had more than one symptom of an inflammatory reaction such as fever, abdominal pain, or leukocytosis within 7–10 days after surgery, whereas surgical complications such as anastomotic leakage or septic fluid collection usually manifest sooner, within 3–5 days. These patients underwent contrast CT scans on the day their symptoms were noted and were diagnosed with abscess-forming SIs. Three patients were treated with antibiotics and catheter drainage, while one patient had to undergo total gastrectomy due to remnant stomach necrosis. Therefore, a complicated SI should always be a differential in the later immediate postoperative period and contrast CT should be performed.
This study had a few limitations. First, since this was retrospective study, detailed information from the surgical fields was not available. Second, the overall incidence of SI was very low, as was the incidence of complicated SI, which hindered evaluation of risk factors; in addition, asymptomatic SIs may not have been detected despite regular follow-up CT three to 6 months postoperatively. However, due to the exclusion of the patients who had not undertaken first CT within 6 months, and 312 days of mean resolution time of the SI on our result, there might be minimum of hidden SI cases. Furthermore, our study contained the largest number of patients to date with an overall incidence of SI of 4.10%, which correlates to the incidence of 1.0–6.9% in the literature. And for further evaluation, large-scale prospective studies are required.
Conclusion
In conclusion, total gastrectomy and extended LN dissection are risk factors of SI development. Although the incidence of SI is relatively low and that of complicated SI is lower still, the condition can lead to fatal complications. Therefore, it should be considered as a cause of an inflammatory reaction, especially 7 days following gastrectomy. In addition, SIs with larger volume on a CT scans are more likely to develop complications and preoperative 3-D reconstruction of the splenic artery tributaries may help to reduce the risk of inadvertent SI.
References
Zilberstein B, Martins BC, Jacob CE et al (2004) Complications of gastrectomy with lymphadenectomy in gastric cancer. Gastric Cancer 7:254–259
Antopolsky M, Hiller N, Salameh S et al (2009) Splenic infarction: 10 years of experience. Am J Emerg Med 27:262–265
Akin K, Kosehan D, Cengiz AY et al (2012) Splenic infarction following conventional open gastrectomy in patients with gastric malignancy: a CT-based study. Abdom Imaging 37:609–615
Hajime I, Akihito E, Hiroharu N et al (2013) Gastric remnant necrosis following splenic infarction after distal gastrectomy in a gastric cancer patient. Int J Surg Case Rep 4:583–586
Sakran N, Ilivitzki A, Zeina AR et al (2012) Splenic abscess after sleeve gastrectomy: a report of two cases. Obes Facts 5:635–639
Schiavo L, Scalera G, De Sena G et al (2015) Nonsurgical management of multiple splenic abscesses in an obese patient that underwent laparoscopic sleeve gastrectomy: case report and review of literature. Clin Case Rep 3:870–874
Yazici P, Kaya C, Isil G et al (2015) Splenic infarction—a rare cause of acute abdominal pain following gastric surgery: a case series. Int J Surg Case Rep 10:88–90
Symeonidis A, Papakonstantinou C, Seimeni U et al (2001) Non hypoxia-related splenic infarct in a patient with sickle cell trait and infectious mononucleosis. Acta Haematol 105:53–56
Tan DC, Low AH, Ong HS et al (2006) Unusual abdominal manifestations of catastrophic antiphospholipid syndrome. Br J Haematol 132:538
van Hal S, Senanayake S, Hardiman R (2005) Splenic infarction due to transient antiphospholipid antibodies induced by acute Epstein-Barr virus infection. J Clin Virol 32:245–247
O’Keefe JH Jr, Holmes DR Jr, Schaff HV et al (1986) Thromboembolic splenic infarction. Mayo Clin Proc 61:967–972
Michels NA (1942) The variational anatomy of the spleen and splenic artery. Am J Anat 70:21–72
Goerg C, Schwerk WB, Goerg K (1991) Splenic lesions: sonographic patterns, follow-up, differential diagnosis. Eur J Radiol 13:59–66
Hwang SH, Park DJ, Jee YS et al (2009) Actual 3-year survival after laparoscopy-assisted gastrectomy for gastric cancer. Arch Surg 144:559–564 (discussion 565)
Nores M, Phillips EH, Morgenstern L et al (1998) The clinical spectrum of splenic infarction. Am Surg 64:182–188
Acknowledgements
The authors are grateful for the help of Hyun Kyo Kim for the date collection and organization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jung, Y.J., Seo, H.S., Lee, H.H. et al. Splenic Infarction as a Delayed Febrile Complication Following Radical Gastrectomy for Gastric Cancer Patients: Computed Tomography-Based Analysis. World J Surg 42, 1826–1832 (2018). https://doi.org/10.1007/s00268-017-4401-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-4401-0