Abstract
Purpose
Pancreatic neuroendocrine tumor (PNET) is relatively rare and has a generally better prognosis than does pancreatic cancer. However, as its prognosis in patients with lymph node metastasis (LNM) is unclear, lymph node dissection for PNET is controversial. Our study aimed to clarify the significance of LNM in PNET.
Methods
We retrospectively examined 83 PNET patients who underwent pancreatic resections with lymph node dissection at Kumamoto University Hospital, Saiseikai Kumamoto Hospital, and Kumamoto Regional Medical Center from April 2001 to December 2014. Their clinicopathological parameters were analyzed by the absence or presence of LNM, and with regard to the disease-free survival (DFS) and overall survival (OS). A predictive score of LNM was also made using the age, tumor size, primary tumor location, and tumor function.
Results
Although the 5-year OS was 74.8% for LNM+ and 94.6% for LNM− (P = 0.002), LNM was not an independent risk factor for the OS in a multivariate analysis. However, tumors larger than 1.8 cm were found to be an independent prognostic factor, and the cut-off value for the predictive score was 1.69.
Conclusions
Although LNM was not an independent prognostic factor, lymph node dissection is recommended for patients whose predictive score is larger than 1.69.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic neuroendocrine tumor (PNET) is uncommon, and its prognosis is generally better than that of pancreatic cancer [1, 2]. Although some PNET patients have lymph node metastasis (LNM), its effect on their prognosis is unclear. The prognosis for PNET has been associated with the tumor location and histological differentiation [1].
Parek et al. evaluated the predictive value of lymph node sampling and LNM for PNET [3] and found that race, functional status and metastatic disease were associated with LNM in a univariate analysis, but only distant disease (and not tumor size) predicted LNM in a multivariate analysis. While some investigations of factors that predict the outcomes in PNET have been conducted, lymph node dissection for PNET remains controversial [4–8].
Therefore, in this study, we aimed to clarify the significance of LNM in PNET.
Methods
Patients and diagnoses
All patients underwent pancreatic resection for PNET at Kumamoto University Hospital, Saiseikai Kumamoto Hospital, or Kumamoto Regional Medical Center from April 2001 to December 2014. From a total of 94 patients, we excluded 11 patients who did not undergo lymph node dissection, leaving 83 patients who underwent lymph node dissection in this analysis. The patients underwent imaging studies, such as computed tomography (CT), magnetic resonance imaging (MRI), and endoscopic ultrasound (EUS), for the diagnosis before operation. The final diagnoses were confirmed pathologically using resected specimens. Only a PNET patient with liver metastasis underwent pancreatic resection and liver resection who was clinically diagnosed to have both pancreatic cancer and hepatocellular carcinoma before the operation. Tumors were classified as functional PNET according to the clinical signs and symptoms of hormonal excess and the increased levels of corresponding serum peptides and hormones. Tumors were classified as non-functional if they were not associated with distinct clinical manifestations or hormone alterations [9].
Treatment
Surgical procedures were selected based on each tumor’s location and extent and the patient’s general condition. Pancreatic resection was considered the first-choice treatment for patients with PNET.
Estimated risk factors for the survival and recurrence
Follow-up information was collected from clinical records at each hospital. The interval times of follow-up after surgery were every 3 months. The duration of the overall survival (OS) was calculated from the surgery date to the tumor-specific death or the patient’s last follow-up. The relapse time [disease-free survival (DFS)] was calculated from the surgery date to the date when recurrence was diagnosed. We analyzed seven variables: age, sex, tumor size, primary tumor location, tumor function, regional LNM, and 2010 World Health Organization (WHO) classification. We set the tumor size cutoff at 1.8 cm, based on the median size.
Estimated risk factors for LNM
We analyzed four variables to predict LNM: the age, tumor size, primary tumor location, and tumor function. We calculated the predictive score of LNM using these variables.
Statistical analyses
Discrete and continuous variables were compared using the χ 2 and Student’s t tests, respectively. We analyzed the DFS and OS by the Kaplan–Meier method; differences were analyzed by the log-rank test. To estimate the risk factors for the survival and recurrence using a Cox proportional hazards regression analysis, continuous variables were converted to binary variables. To estimate the risk factors of LNM using a logistic regression analysis, continuous variables were converted to binary variables as well. All analyses were performed using the JMP software program (Release 10.0.2; SAS Institute, Cary, NC, USA). P < 0.05 was considered significant.
Ethical standards
The study protocol was approved as number 1120 by the Institutional Review Board of Kumamoto University Hospital.
Results
Clinicopathological characteristics
Among 83 patients, 38 (45.8%) were male, and 45 (54.2%) were female. The median age was 57 years (range: 17–84 years; Table 1). The median tumor size was 1.8 cm (range: 0.5–9.0 cm). At the diagnosis, 66 patients (79.5%) presented with no LNM (N−), and 17 patients (20.5%) presented with LNM (N+). We found that 67.5% of PNETs were located in the distal pancreas and 32.5% in the head. Of the 21 patients (25.3%) who had functional PNET, the most frequent functional PNET entity was insulinoma (19.3%), followed by glucagonoma (2.4%), gastrinoma (1.2%), VIPoma (1.2%), and PPoma (1.2%). Their 2010 WHO classifications were G1: 53.0%; G2: 27.7%; and G3 (neuroendocrine carcinoma): 8.4%.
A comparison of the clinicopathological characteristics between patients with N− disease and those with N+ disease is also summarized in Table 1. Patients with N+ PNET had larger tumors than did the N− PNET group (P = 0.013). Interestingly, N+ PNETs were significantly more likely to be located in the proximal pancreas than were N− PNETs (P = 0.010). The WHO classification was strongly correlated with regional LNM, with N− PNET more likely to be of a lower grade than N+ PNET; N− PNET accounted for 88.7% (39/44) of G1 cases, 69.6% (16/23) of G2 cases, and 28.6% (2/7) of G3 cases (P = 0.003, Pearson’s χ 2 test). Patients with non-functional PNET tended to have N+ disease, although not to a significant degree (P = 0.126).
Surgical procedures
Among the 83 patients, 2 (2.4%) received subtotal pancreatectomies, 50 (60.2%) received distal pancreatectomies, 27 (32.5%) received pancreatoduodenectomies, and 4 (4.8%) received local or partial pancreatectomies. The patients who received distal pancreatectomies and pancreatoduodenectomies were more likely to have LNM than those who underwent other procedures (P = 0.029; Table 1).
Recurrence
Among the 83 patients, a total of 16 (19.3%) recurrences occurred, and their patterns are summarized in Table 1. Liver metastases were observed most often (13.3%), while regional lymph node metastases alone was the least frequently observed pattern (1.2%). The combination of both lymph node and liver metastases accounted for 3.6% of cases. N+ PNETs were significantly more likely to recur than were N− PNETs (P = 0.016). Interestingly, no patients with N− PNETs had lymph node recurrence.
The survival stratified by LNM and tumor size
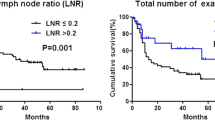
During the follow-up period, eight patients died of recurrence or progression of primary tumor, and two patients died of other causes. The 5-year DFS rates were not significant for node positivity (N+: 60.5% and N−: 83.8%; P = 0.052; Fig. 1a), while the 5-year OS rates were significant for positivity (N+: 74.8% and N−: 94.6%; P = 0.002; Fig. 1b). The 5-year DFS rates were significant for tumor size (>1.8 cm: 63.0% and ≤1.8 cm: 95.2%; P = 0.002; Fig. 1c); the 5-year OS rates were also significant for tumor size (>1.8 cm: 79.5% and ≤1.8 cm: 100%; P = 0.001; Fig. 1d).
Kaplan–Meier survival curves by lymph node metastasis (LNM) and tumor size. a Comparison of the disease-free survival (DFS) between patients with LNM (N+; n = 17) and without LNM (N−; n = 66). The DFS did not significantly differ between N+ patients and N− patients (P = 0.052). b Comparison of the overall survival (OS) between N+ patients (n = 17) and N− patients (n = 66). The N+ patients had a significantly shorter OS than did the N− patients (P = 0.002). c Comparison of the DFS between patients with tumor size >1.8 cm (n = 38) and ≤1.8 cm (n = 45). Patients with tumor size >1.8 cm had a significantly shorter DFS than did those with tumor size ≤1.8 cm (P = 0.002). d Comparison of the OS between patients with tumor size >1.8 cm (n = 38) and ≤1.8 cm (n = 45). Patients with tumor size >1.8 cm had a significantly shorter OS than did those with tumor size ≤1.8 cm (P = 0.001)
Univariate and multivariate analyses of factors affecting the DFS and OS
Factors associated with the DFS were evaluated by univariate and multivariate analyses. A univariate analysis showed that male sex [hazard ratio (HR): 3.17; P = 0.026], tumor size >1.8 cm (HR: 6.12; P = 0.001), and NET G3 (HR: 8.79; P = 0.003) were significant variables influencing a poor DFS (Table 2). However, only tumor size >1.8 cm (HR: 3.86; P = 0.040) remained an independent risk factor for the DFS in a subsequent multivariate analysis (Table 2).
Factors associated with the OS were also evaluated by univariate and multivariate analyses. In the univariate analysis, male sex (HR: 11.5; P = 0.003), tumor size >1.8 cm [HR: not applicable (NA); P < 0.001], non-functional PNET (HR: NA; P = 0.018), regional LNM (HR: 6.89; P = 0.005), and NET G3 (HR: 25.7; P < 0.001) were significantly associated with a poor OS, but only tumor size >1.8 cm (HR: NA; P = 0.015) remained an independent risk factor for the OS in a subsequent multivariate analysis (Table 3).
Risk model of factors affecting LNM
We developed a risk model of factors associated with LNM. The logistic model is presented in Table 4. A univariate analysis showed that tumor size >1.8 cm [odds ratio (OR): 5.33; P = 0.008] and the primary tumor location (OR: 4.12; P = 0.013) were significant variables influencing LNM. However, only tumor size >1.8 cm (OR: 4.14; P = 0.030) remained an independent risk factor for LNM in a subsequent multivariate analysis. We also presented the predictive score of LNM in Table 4.
To evaluate the model performance, the area under the receiver-operating characteristic curve (AUROC) was calculated for the validation sets. The AUROC for LNM was 0.78. Details of the model performance metrics for LNM are displayed in Fig. 2. We set 1.69 as the cut-off value (sensitivity: 0.88 and specificity: 0.61).
Discussion
Diagnosing LNM in PNET patients before surgery is difficult, unless the metastatic tumors are fairly large. We perform contrast CT, MRI, and EUS for PNET patients at our institution. However, LNM in PNET patients is rarer than liver metastasis. Positron emission tomography (PET)-CT can be used to diagnose malignant tumors, but not all PNETs. Therefore, it is very difficult to detect LNM before surgery. However, not all PNET patients have LNM, and the prognosis of PNET is not very poor. The previous studies have focused on the LNM incidence and/or prognosis based on the tumor size [5, 10–15]. Although LNM has been seen even in patients with tumors <1.0 cm, LNM occurs more often with large tumors than with smaller ones. In our study, LNM was most often seen with pancreatic head tumors, although this finding has not been consistent across studies [6, 16]. Several studies of independent prognostic factors for resected PNET have found that the presence of tumor necrosis and lymphatic or hepatic metastases affects the DFS [17]. Some reports have associated LNM with a shorter DFS or OS [16, 18–21], while other studies have found that the lymph node status did not affect the survival [3, 4, 22, 23]. The age, grade of tumor, and presence of distant metastases predict worse outcomes [24]. As such, the resection status may not affect the survival [25].
Our findings suggest that lymph node dissection should be performed in cases with a predictive score >1.69 but can be omitted in cases with a score ≤1.69.
In this study, we showed that LNM is not an independent risk factor for the DFS or OS. However, this study had several limitations, including its retrospective design, the small number of subjects, and the lack of data on certain pathologic variables (especially the Ki-67 indices and mitotic rates) for all patients. The number of examined lymph nodes was not sufficient, and data on the number of positive lymph nodes were not available for all N+ PNETs. We, therefore, cannot explain the relationship between the lymph node positivity ratio (number positive/examined number) and the survival. Further research regarding advanced PNET is needed [26–28].
In conclusion, we clarified that LNM was not an independent prognostic factor for the DFS or OS. Lymph node dissection is recommended for patients whose predictive score is larger than 1.69.
References
Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72.
Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19:1727–33.
Parekh JR, Wang SC, Bergsland EK, Venook AP, Warren RS, Kim GE, et al. Lymph node sampling rates and predictors of nodal metastasis in pancreatic neuroendocrine tumor resections: the UCSF experience with 149 patients. Pancreas. 2012;41:840–4.
Wong J, Fulp WJ, Strosberg JR, Kvols LK, Centeno BA, Hodul PJ. Predictors of lymph node metastases and impact on survival in resected pancreatic neuroendocrine tumors: a single-center experience. Am J Surg. 2014;208:775–80.
Tsutsumi K, Ohtsuka T, Mori Y, Fujino M, Yasui T, Aishima S, et al. Analysis of lymph node metastasis in pancreatic neuroendocrine tumors (PNETs) based on the tumor size and hormonal production. J Gastroenterol. 2012;47:678–85.
Hashim YM, Trinkaus KM, Linehan DC, Strasberg SS, Fields RC, Cao D, et al. Regional lymphadenectomy is indicated in the surgical treatment of pancreatic neuroendocrine tumors (PNETs). Ann Surg. 2014;259:197–203.
Krampitz GW, Norton JA, Poultsides GA, Visser BC, Sun L, Jensen RT. Lymph nodes and survival in pancreatic neuroendocrine tumors. Arch Surg. 2012;147:820–7.
Yoo YJ, Yang SJ, Hwang HK, Kang CM, Kim H, Lee WJ. Overestimated Oncologic Significance of Lymph Node Metastasis in G1 Nonfunctioning Neuroendocrine Tumor in the Left Side of the Pancreas. Medicine (Baltimore). 2015;94:e1404.
Han X, Xu X, Jin D, Wang D, Ji Y, Lou W. Clinicopathological characteristics and prognosis-related factors of resectable pancreatic neuroendocrine tumors: a retrospective study of 104 cases in a single Chinese center. Pancreas. 2014;43:526–31.
Nomura N, Fujii T, Kanazumi N, Takeda S, Nomoto S, Kasuya H, et al. Nonfunctioning neuroendocrine pancreatic tumors: our experience and management. J Hepatobiliary Pancreat Surg. 2009;16:639–47.
Kim MJ, Choi DW, Choi SH, Heo JS, Park HJ, Choi KK, et al. Surgical strategies for non-functioning pancreatic neuroendocrine tumours. Br J Surg. 2012;99:1562–8.
Kuo EJ, Salem RR. Population-level analysis of pancreatic neuroendocrine tumors 2 cm or less in size. Ann Surg Oncol. 2013;20:2815–21.
Kishi Y, Shimada K, Nara S, Esaki M, Hiraoka N, Kosuge T. Basing treatment strategy for non-functional pancreatic neuroendocrine tumors on tumor size. Ann Surg Oncol. 2014;21:2882–8.
Conrad C, Kutlu OC, Dasari A, Chan JA, Vauthey JN, Adams DB, et al. Prognostic Value of Lymph Node Status and Extent of Lymphadenectomy in Pancreatic Neuroendocrine Tumors Confined To and Extending Beyond the Pancreas. J Gastrointest Surg. 2016;20:1966–74.
Jiang Y, Jin JB, Zhan Q, Deng XX, Shen BY. Impact and Clinical Predictors of Lymph Node Metastases in Nonfunctional Pancreatic Neuroendocrine Tumors. Chin Med J (Engl). 2015;128:3335–44.
Postlewait LM, Ethun CG, Baptiste GG, Le N, McInnis MR, Cardona K, et al. Pancreatic neuroendocrine tumors: Preoperative factors that predict lymph node metastases to guide operative strategy. J Surg Oncol. 2016;114:440–5.
Hochwald SN, Zee S, Conlon KC, Colleoni R, Louie O, Brennan MF, et al. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20:2633–42.
Fendrich V, Langer P, Celik I, Bartsch DK, Zielke A, Ramaswamy A, et al. An aggressive surgical approach leads to long-term survival in patients with pancreatic endocrine tumors. Ann Surg. 2006;244:845–51. (discussion 52-3).
Ballian N, Loeffler AG, Rajamanickam V, Norstedt PA, Weber SM, Cho CS. A simplified prognostic system for resected pancreatic neuroendocrine neoplasms. HPB (Oxford). 2009;11:422–8.
Brunner SM, Weber F, Werner JM, Agha A, Farkas SA, Schlitt HJ, et al. Neuroendocrine tumors of the pancreas: a retrospective single-center analysis using the ENETS TNM-classification and immunohistochemical markers for risk stratification. BMC Surg. 2015;15:49.
Fischer L, Bergmann F, Schimmack S, Hinz U, Priess S, Muller-Stich BP, et al. Outcome of surgery for pancreatic neuroendocrine neoplasms. Br J Surg. 2014;101:1405–12.
Gratian L, Pura J, Dinan M, Roman S, Reed S, Sosa JA. Impact of extent of surgery on survival in patients with small nonfunctional pancreatic neuroendocrine tumors in the United States. Ann Surg Oncol. 2014;21:3515–21.
Kazanjian KK, Reber HA, Hines OJ. Resection of pancreatic neuroendocrine tumors: results of 70 cases. Arch Surg. 2006;141:765–9. (discussion 9–70).
Bilimoria KY, Talamonti MS, Tomlinson JS, Stewart AK, Winchester DP, Ko CY, et al. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: analysis of 3851 patients. Ann Surg. 2008;247:490–500.
Fischer L, Kleeff J, Esposito I, Hinz U, Zimmermann A, Friess H, et al. Clinical outcome and long-term survival in 118 consecutive patients with neuroendocrine tumours of the pancreas. Br J Surg. 2008;95:627–35.
Arima K, Hashimoto D, Okabe H, Inoue R, Kaida T, Higashi T, et al. Intraoperative blood loss is not a predictor of prognosis for pancreatic cancer. Surg Today. 2016;46:792–7.
Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. 2016;46:668–85.
Mizuguchi T, Torigoe T, Satomi F, Shima H, Kutomi G, Ota S, et al. Trials of vaccines for pancreatic ductal adenocarcinoma: Is there any hope of an improved prognosis? Surg Today. 2016;46:139–48.
Acknowledgements
The authors declare no conflicts of interest. No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taki, K., Hashimoto, D., Nakagawa, S. et al. Significance of lymph node metastasis in pancreatic neuroendocrine tumor. Surg Today 47, 1104–1110 (2017). https://doi.org/10.1007/s00595-017-1485-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-017-1485-y