Abstract
Background
Because of the rarity and variety of pancreatic neuroendocrine tumors (PNETs), there have been few reports regarding the indication for lymph node dissection in patients with these tumors. This study aimed to evaluate the risk of lymph node metastasis of PNETs based on the tumor size and hormonal production.
Methods
Data for a total of 66 patients who had PNETs resected at our department between 1987 and 2010 were retrospectively studied. The clinicopathological features, including the disease-specific survival rate, were assessed based on the status of lymph node metastasis at the time of initial surgical resection. Then the cut-off point of tumor size to predict lymph node metastasis was estimated.
Results
There were 12 patients (18%) with lymph node metastasis. The frequency of lymph node metastasis tended to be higher in gastrinomas than that in other tumors (43 vs. 15%; P = 0.08). The size of PNETs with lymph node metastasis was significantly larger than that of the PNETs without metastasis (P = 0.04). The postoperative survival rate in the PNET patients with lymph node metastasis was significantly lower than that in the patients without metastasis (P < 0.0001). Only 2 (8%) of 26 PNETs with a tumor size of <15 mm had lymph node metastasis, and both of these were gastrinomas. On the other hand, 10 (25%) of the remaining 40 PNETs with a tumor size of ≥15 mm had lymph node metastasis. Notably, there were no PNETs with lymph node metastasis in 22 non-gastrinomas with a tumor size of <15 mm.
Conclusions
Non-gastrinomas with a tumor size of ≥15 mm and all gastrinomas would be an indication for pancreatectomy with lymph node dissection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic neuroendocrine tumors (PNETs) are rare, accounting for approximately 3% of all pancreatic neoplasms [1]. Although the natural history of PNETs is not fully understood because of their rarity, PNETs are generally considered to be slow-growing tumors and show a better long-term postoperative survival rate than tumors of the exocrine pancreas [1–4]. However, a standard surgical treatment for PNETs has not been established to date.
Consensus guidelines for the standards of care in gastroenteropancreatic neuroendocrine tumors (GEP-NETs) have been recently proposed by the European Neuroendocrine Tumor Society (ENETS) [5]. In addition to stratification according to histological type, grade, and TMN stage [6], GEP-NETs are categorized into 3 treatment groups: localized tumor, tumor with nodal metastases, and tumor with lymph node and hematogenous metastases. Although a comprehensive treatment strategy for GEP-NETs is shown in the guidelines, recommendation for an appropriate operative procedure in each case is not mentioned. The National Comprehensive Cancer Network (NCCN) guidelines also demonstrate treatment strategies for GEP-NETs [7]. In these guidelines, a tumor size of 20 mm is specified to determine how to treat carcinoid tumors of the appendix, rectum, and stomach; however, there is no mention about the tumor size regarding decisions for the treatment of PNETs.
Regional lymph nodes are the most frequent metastatic sites of PNETs, and the risk of nodal metastasis is one of the important factors to consider when determining the appropriate surgical treatment. Tumor size might be one of the factors to consider when making a decision on the treatment; however, there have been few reports investigating the relationship between the tumor size and lymph node metastasis. Our objectives were to evaluate the risk of lymph node metastasis of PNETs based on the tumor size and hormonal production, and further, to suggest a management strategy to choose an appropriate surgical procedure.
Patients and methods
The medical records of 66 patients who underwent surgical resection of PNETs at the Department of Surgery and Oncology, Kyushu University Hospital, between 1987 and 2010 were retrospectively reviewed. Histopathological findings, including immunohistochemistry, were reviewed by two experienced pathologists (M.F. and S.A.) according to the World Health Organization (WHO) classification [8]. In this study, tumor size was defined as the maximal diameter in the pathological tissue. If PNETs were multiple, the diameter of the largest tumor was regarded as the tumor size. PNETs were considered as non-functioning if the patients had neither evidence of clinical symptoms of hormonal excess nor showed immunohistological staining for specific hormones in the resected specimen. PNETs with immunohistochemical reactivity for specific hormones in the resected specimen but with neither specific symptoms nor abnormal elevation of specific hormone in the peripheral blood were regarded as non-functioning tumors. On the other hand, a functioning tumor with specific symptoms caused by excessive hormonal production was confirmed on the basis of both the elevation of serum hormone levels and immunohistochemical reactivity in the resected specimen. To investigate the relationship between hormonal production and lymph node metastasis, the characteristics of lymph node metastasis of 3 major PNET categories–non-functioning tumor, insulinoma, and gastrinoma–were analyzed. All resected PNETs were classified as gastrinomas and non-gastrinomas, because the biological behavior of gastrinomas is considered to be different from that of other PNETs, with quite high malignant potential and frequent development of multiple tumors in both the pancreas and duodenum [9–12]. Non-gastrinomas included all other functioning tumors and non-functioning tumors.
Lymph node station was shown according to the General rules for the study of pancreatic cancer (6th edition) proposed by the Japan Pancreas Society [13]. The status of lymph node metastasis was determined at the time of initial surgical resection. Enucleated PNETs were regarded as clinical N0 stage because there was no evidence of lymph node involvement during long-term surveillance after the resection.
The cumulative disease-specific survival rate was calculated from the time of pancreatic resection to death due to PNETs. Prognosis was examined in October 2010 and follow-up data of all patients were available. The median observation time for the disease-specific survival rate was 48 months, ranging from 1 to 278 months.
Statistical analyses were performed using JMP statistical software (version 6.0.3; SAS, Cary, NC, USA). Data were analyzed by the Fisher’s exact probability test for categorical variables and the Mann–Whitney U-test for continuous variables. Survival curves were constructed by the Kaplan–Meier product-limit method and compared by Log-rank test. To evaluate the risk of lymph node metastasis of PNETs based on the tumor size, four representative points were established, of 10, 15, 20, and 30 mm. The optimal cut-off points of tumor size to discriminate the presence of lymph node metastasis were sought by constructing receiver operating characteristic (ROC) curves, using the real tumor sizes; the curves were generated by calculating the sensitivities and specificities at all tumor sizes [14]. A difference was considered statistically significant when the P value was less than 0.05.
Results
Demographics and characteristics of resected PNETs
The patient cohort consisted of 30 males and 36 females with a median age of 55 years (range 20–79 years). The median size of the tumors was 17 mm (range 4–90 mm). As shown in Fig. 1, there were 32 functioning tumors (48%) and 34 non-functioning tumors (52%). The functioning tumors consisted of 23 insulinomas (35%), 7 gastrinomas (11%), one glucagonoma (1%), and one somatostatinoma (1%). Multiple tumors were observed in 8 patients (12%), while a solitary tumor was observed in 58 (88%). Five patients (8%) had an association with multiple endocrine neoplasia type-1 (MEN-1). Operative procedures performed for the PNETs are listed in Table 1. Enucleation was performed in 13 patients with insulinomas, and in 2 with non-functioning tumors, with tumor sizes of 8 and 11 mm.
Demographics of all patients with pancreatic neuroendocrine tumors (PNETs) and the status of lymph node metastasis. A total of 66 patients had PNETs resected at our department between 1987 and 2010. There were 34 non-functioning tumors and 32 functioning tumors, including 23 insulinomas, 7 gastrinomas, one glucagonoma, and one somatostatinoma. The numbers of patients with lymph node metastasis at the time of initial surgical resection are shown in parentheses. The incidence of PNETs with lymph node metastasis was 43% (n = 3) in gastrinomas, 18% (n = 6) in non-functioning tumors, and 9% (n = 2) in insulinomas
Comparison of clinicopathological features between PNETs with and without lymph node metastasis at the time of surgical resection
The incidences of lymph node metastasis at the time of surgical resection were 18% (12/66) in all the resected PNETs, 43% (3/7) in the gastrinomas, and 15% (9/59) in non-gastrinomas. The details of patients with lymph node metastasis are listed in Table 2. The median size of the tumors was 35 mm (range 4–80 mm). The range (D0–D3) of lymph nodes retrieved for each PNET is shown in the Table. The average number of retrieved lymph nodes was 3.4 (range 0–43). The clinicopathological features of the 12 patients with lymph node metastasis were compared with those of the 54 patients without lymph node metastasis. The patients with PNETs with lymph node metastasis were significantly younger than those without lymph node metastasis (Table 3, P = 0.02). The number and size of PNETs with lymph node metastasis were significantly greater than the number and size of those without lymph node metastasis (Table 3, number: P = 0.03, size: P = 0.04). The prevalence of lymph node metastasis in patients who had gastrinomas tended to be higher than that in those with non-gastrinomas (43 vs. 15%), but the difference did not reach statistical significance (P = 0.08). There were no significant differences in gender, the presence of MEN-1, or symptoms of hormonal excess between patients with and without lymph node metastasis.
Comparison of disease-specific survival rates between patients with PNETs with and without lymph node metastasis
Recurrence was observed in 9 patients (14%) in this study. None of the patients in the present study had any recurrence of local lymph node metastasis during the follow-up period. Eight of the patients with recurrence had liver metastasis, and the other patient had recurrence in the remnant pancreas, which was considered as metachronous multicentric occurence. During the follow-up period, 5 patients died of PNETs, 4 died of other diseases, and 57 were alive at the end of the period. The 5- and 10-year disease-specific survival rates of all patients were 89.8 and 89.8%, respectively. As shown in Fig. 2, survival analysis revealed a significantly lower postoperative survival rate in patients with PNETs with lymph node metastasis (5- and 10-year survival rates of 46.9 and 46.9%, respectively), compared with those without lymph node metastasis (5- and 10-year survival rates of 100 and 100%, respectively) (P < 0.0001).
Comparison of disease-specific survival rates between patients with PNETs with (n = 12) and without (n = 54) lymph node metastasis at the time of surgical resection. Results are presented as Kaplan–Meier actuarial survival curves. The patients with PNETs with lymph node metastasis (5- and 10-year survival rates of 46.9 and 46.9%, respectively) had a significantly lower postoperative survival rate than those without lymph node metastasis (5- and 10-year survival rates of 100 and 100%, respectively) (P < 0.0001)
Analysis of lymph node metastasis based on tumor size and hormonal production
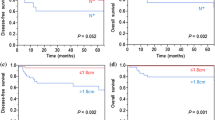
When all PNETs were divided into 2 groups at a tumor size of 20 or 30 mm, the larger-size groups had a significantly higher incidence of lymph node metastasis than the smaller-size groups (Fig. 3, 20 mm; P = 0.04, 30 mm; P = 0.003). When non-gastrinomas were divided into 2 groups at sizes of 15, 20, or 30 mm, the larger-size groups had a significantly higher incidence of lymph node metastasis than the smaller-size groups (Fig. 4, 15 mm: P = 0.01, 20 mm: P = 0.02, 30 mm: P = 0.002). Only 2 (8%) of 26 PNETs with a tumor size of less than 15 mm had lymph node metastasis, and both of them were gastrinomas. On the other hand, 10 (25%) of the other 40 PNETs with a tumor size of 15 mm or larger had lymph node metastasis. Notably, there were no PNETs with lymph node metastasis in the 22 non-gastrinomas with a tumor size of less than 15 mm.
Analysis of lymph node metastasis based on the tumor size in all PNETs. When divided into 2 groups at a size of 20 or 30 mm, the larger-size groups had a significantly higher incidence of lymph node metastasis than the smaller-size groups (20 mm: P = 0.04; 30 mm: P = 0.003). Two (8%) of 26 PNETs with a tumor size of less than 15 mm had lymph node metastasis, and both of these were gastrinomas, while 10 (25%) of the remaining 40 PNETs with a tumor size of 15 mm or larger had nodal metastasis. LN (+), PNETs with lymph node metastasis; LN (−), PNETs without lymph node metastasis
Analysis of lymph node metastasis based on the tumor size in non-gastrinomas. When divided into 2 groups at sizes of 15, 20, or 30 mm, the larger-size groups had a significantly higher incidence of lymph node metastasis than the smaller-size groups (15 mm: P = 0.01, 20 mm: P = 0.02, 30 mm: P = 0.002). Notably, there were no PNETs with lymph node metastasis in 22 non-gastrinomas with a tumor size of less than 15 mm. LN (+), non-gastrinomas with lymph node metastasis; LN (−), non-gastrinomas without lymph node metastasis
The median size of the 23 insulinomas was 13 mm (range 8–60 mm), and 2 (20%) of 10 insulinomas with a tumor size of 15 mm or larger had lymph node metastasis. On the other hand, the median size of the 34 non-functioning tumors was 18 mm (range 8–80 mm), and 6 (24%) of 25 non-functioning tumors with a tumor size of 15 mm or larger had lymph node metastasis. These results indicated that insulinomas with a tumor size of 15 mm or larger had approximately the same incidence of lymph node metastasis as non-functioning tumors (P = 1.00).
To verify the accuracy of the analysis of lymph node metastasis based on tumor size and gastrin production, ROC curves were made by application of the real tumor sizes, as shown in Fig. 5. The sensitivity of the tumor size to predict the presence of lymph node metastasis was determined at several specificity levels in all PNETs and in the non-gastrinomas. The optimal cut-off points of tumor size were 30 mm in both cases (all PNETs; sensitivity 58% and specificity 85%, non-gastrinomas; sensitivity 67% and specificity 86%). The area under the curve (AUC) for all PNETs was 0.694 (95% confidence interval 0.568–0.801). On the other hand, the AUC for non-gastrinomas was 0.813 (95% confidence interval 0.691–0.903). These results revealed that the analysis of lymph node metastasis based on tumor size was more accurate in non-gastrinomas than in all PNETs [15].
Accuracy evaluation of the analysis of lymph node metastasis based on tumor size and gastrin production. To verify the accuracy of the analysis of lymph node metastasis based on tumor size and gastrin production, receiver operating characteristic (ROC) curves in all PNETs and non-gastrinomas were constructed by the application of the real tumor sizes. Sensitivity and specificity at the optimal cut-off points of a tumor size of 30 mm were 58 and 85%, respectively, in all PNETs, and 67 and 86%, respectively, in non-gastrinomas. The area under the curve (AUC) for all PNETs was 0.694 (95% confidence interval 0.568–0.801), while that for non-gastrinomas was 0.813 (95% confidence interval 0.691–0.903). These results reveal that the analysis of lymph node metastasis based on tumor size is more accurate in non-gastrinomas than in all PNETs
Discussion
Aggressive surgical resection is currently considered to be the only way to cure PNETs, and it leads to long-term survival even in patients with distant metastasis [16–19]. All functioning tumors, irrespective of the tumor size, are considered to be an indication for surgical resection, to improve the clinical symptoms. However, it is unclear whether lymph node dissection should be performed in all cases of functioning PNETs. On the other hand, non-functioning tumors suspected to have malignant potential are candidates for surgical management; however, the predictive factors for malignancy in the non-functioning tumors are not fully understood, and therefore, the indication for lymph node dissection in non-functioning PNETs is still an issue of controversy [20, 21]. In the present study, we examined the risk of lymph node metastasis of PNETs based on the tumor size and hormonal production, and our results suggested that a tumor size of 15 mm seems to be critical when considering lymph node dissection of non-gastrinoma PNETs (Fig. 6).
Suggested strategy of surgical management for PNETs. Non-gastrinomas with a tumor size of 15 mm or larger and all gastrinomas would be an indication for pancreatectomy with lymph node dissection, while limited pancreatectomy or enucleation without lymph node dissection could be considered in non-gastrinomas with a tumor size of less than 15 mm. However, it is necessary to determine the appropriate resection for each individual case based on the results of preoperative imaging, histological analysis using endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) samples, and intraoperative findings
The detection of small non-functioning tumors has been increasing recently with the advances in imaging modalities. However, how to manage such small PNETs has been an unresolved problem. We previously reported a patient with a non-functioning tumor of 8 mm in size that demonstrated malignant features without metastasis [22]. In addition, Gibril et al. [23], who followed 57 patients with small non-functioning tumors associated with MEN-1 without any treatment until their tumor reached 2–3 cm in size, found hepatic metastasis in 23% and PNET-related death in 5% of the patients during a mean follow-up period of 8 years. Therefore, all PNETs should be regarded as having malignant potential, irrespective of the tumor size, and, in general, rather than carefully observing the patients, we should remove these tumors, although they are not malignant at the time of diagnosis.
As shown in Fig. 6, we suggest that PNETs should be treated based on the differentiation of gastrinomas from non-gastrinomas, because of their biological differences. Gastrinomas are often found to have metastases to the liver and lymph nodes at the time of initial diagnosis, and they are generally considered to have high malignant potential [9, 10]. Actually, in the present study, 2 (50%) of 4 small gastrinomas with a tumor size of less than 15 mm had lymph node metastasis. As previously reported, all gastrinomas should be resected with locoregional lymph node dissection, irrespective of the tumor size [24]. On the other hand, we found that there were no lymph node metastases in 22 non-gastrinoma PNETs with a tumor size of less than 15 mm, and thus, lymph node dissection might be omitted in this group of PNETs.
Different from the other PNETs, including non-functioning tumors, the malignant potential of insulinomas is reported to be quite low [25–27], and enucleation of the tumor seems to be the treatment of first choice. However, the present report has demonstrated that the prevalence of lymph node metastasis, based on the tumor size, is similar in insulinomas and non-functioning tumors. Most insulinomas are diagnosed as small tumors because of the specific symptoms, while non-functioning PNETs are found to be large because of their lack of symptoms. However, recent advances in imaging modalities have led to the identification of increased numbers of small non-functioning PNETs, and as a result, the prevalence of malignant non-functioning PNETs with lymph node metastasis has become low. Therefore, the prevalence of malignant insulinomas might be similar to that of non-functioning PNETs from the viewpoint of tumor size.
As shown in Fig. 4, none of 22 non-gastrinomas with a tumor size of less than 15 mm had lymph node metastasis, and thus, local excision such as enucleation might be acceptable in such cases. In contrast, 9 (24%) of 37 non-gastrinomas with a tumor size of 15 mm or larger had lymph node metastasis. Pancreatectomy with regional lymph node dissection might be indicated for non-gastrinomas with a tumor size of 15 mm or larger. However, in our study, 76% of the non-gastrinomas with a tumor size of 15 mm or larger had no lymph node metastasis, and therefore, it is necessary to finally determine the appropriate resection for each individual case based on the results of preoperative imaging, including somatostatin receptor scintigraphy [28], histological analysis using endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) samples [29, 30], and intraoperative findings (Fig. 6).
In fact, it is sometimes difficult to obtain an accurate and detailed preoperative diagnosis for PNETs. Even if EUS-FNA is conducted for the preoperative diagnosis, the precise diagnosis cannot be obtained from a tiny specimen. Moreover, the heterogeneous pathological nature of PNETs prevents us from finding the true malignant potential. However, we can exclude gastrinomas based on the serum gastrin level, the presence of a gastroduodenal ulcer, and the selective arterial secretagogue injection (SASI) test [31]. If gastrinoma is excluded, the indication for lymph node dissection could be determined according to this result in spite of the difficulty of obtaining an accurate preoperative diagnosis.
In conclusion, although the accumulation of further cases is required, our present study suggests that non-gastrinomas with a tumor size of 15 mm or larger and all gastrinomas would be an indication for pancreatectomy with lymph node dissection, while limited pancreatectomy without lymph node dissection could be considered in non-gastrinomas with a tumor size of less than 15 mm.
References
Fesinmeyer MD, Austin MA, Li CI, De Roos AJ, Bowen DJ. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1766–73.
Jarufe NP, Coldham C, Orug T, Mayer AD, Mirza DF, Buckels JA, et al. Neuroendocrine tumours of the pancreas: predictors of survival after surgical treatment. Dig Surg. 2005;22:157–62.
Norton JA. Surgery for primary pancreatic neuroendocrine tumors. J Gastrointest Surg. 2006;10:327–31.
Bilimoria KY, Talamonti MS, Tomlinson JS, Stewart AK, Winchester DP, Ko CY, et al. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: analysis of 3851 patients. Ann Surg. 2008;247:490–500.
Kloppel G, Couvelard A, Perren A, Komminoth P, McNicol AM, Nilsson O, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology. 2009;90:162–6.
Rindi G, Kloppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395–401.
Clark OH, Benson AB, Berlin JD, Choti MA, Doherty GM, Engstrom PF, et al. NCCN Clinical Practice Guidelines in Oncology: neuroendocrine tumors, 3rd ed. J Natl Compr Cancer Netw. 2009;7:712–47.
Klimstra DS, Arnold R, Capella C, Hruban RH, Kloppel G, Komminoth P, et al. Neuroendocrine neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. Lyon: IARC Press; 2010. p. 322–6.
Soga J, Yakuwa Y. The gastrinoma/Zollinger–Ellison syndrome: statistical evaluation of a Japanese series of 359 cases. J Hepatobiliary Pancreat Surg. 1998;5:77–85.
Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–92.
Imamura M, Kanda M, Takahashi K, Shimada Y, Miyahara T, Wagata T, et al. Clinicopathological characteristics of duodenal microgastrinomas. World J Surg. 1992;16:703–9.
Pipeleers-Marichal M, Donow C, Heitz PU, Kloppel G. Pathologic aspects of gastrinomas in patients with Zollinger–Ellison syndrome with and without multiple endocrine neoplasia type I. World J Surg. 1993;17:481–8.
Japan Pancreas Society. General Rules for the Study of Pancreatic Cancer. 6th ed. Kanehara: Tokyo; 2009.
Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–77.
Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr. 2007;96:644–7.
Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29–37.
Elias D, Lasser P, Ducreux M, Duvillard P, Ouellet JF, Dromain C, et al. Liver resection (and associated extrahepatic resections) for metastatic well-differentiated endocrine tumors: a 15-year single center prospective study. Surgery. 2003;133:375–82.
Norton JA, Warren RS, Kelly MG, Zuraek MB, Jensen RT. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery. 2003;134:1057–63.
Touzios JG, Kiely JM, Pitt SC, Rilling WS, Quebbeman EJ, Wilson SD, et al. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg. 2005;241:776–83.
Bettini R, Boninsegna L, Mantovani W, Capelli P, Bassi C, Pederzoli P, et al. Prognostic factors at diagnosis and value of WHO classification in a mono-institutional series of 180 non-functioning pancreatic endocrine tumours. Ann Oncol. 2008;19:903–8.
Nomura N, Fujii T, Kanazumi N, Takeda S, Nomoto S, Kasuya H, et al. Nonfunctioning neuroendocrine pancreatic tumors: our experience and management. J Hepatobiliary Pancreat Surg. 2009;16:639–47.
Ikenaga N, Yamaguchi K, Konomi H, Fujii K, Sugitani A, Tanaka M. A minute nonfunctioning islet cell tumor demonstrating malignant features. J Hepatobiliary Pancreat Surg. 2005;12:84–7.
Gibril F, Venzon DJ, Ojeaburu JV, Bashir S, Jensen RT. Prospective study of the natural history of gastrinoma in patients with MEN1: definition of an aggressive and a nonaggressive form. J Clin Endocrinol Metab. 2001;86:5282–93.
Imamura M, Komoto I, Ota S. Changing treatment strategy for gastrinoma in patients with Zollinger–Ellison syndrome. World J Surg. 2006;30:1–11.
de Herder WW, Niederle B, Scoazec JY, Pauwels S, Kloppel G, Falconi M, et al. Well-differentiated pancreatic tumor/carcinoma: insulinoma. Neuroendocrinology. 2006;84:183–8.
Nikfarjam M, Warshaw AL, Axelrod L, Deshpande V, Thayer SP, Ferrone CR, et al. Improved contemporary surgical management of insulinomas: a 25-year experience at the Massachusetts General Hospital. Ann Surg. 2008;247:165–72.
Ito T, Sasano H, Tanaka M, Osamura RY, Sasaki I, Kimura W, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45:234–43.
Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WA, Kooij PP, Oei HY, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20:716–31.
Chang F, Vu C, Chandra A, Meenan J, Herbert A. Endoscopic ultrasound-guided fine needle aspiration cytology of pancreatic neuroendocrine tumours: cytomorphological and immunocytochemical evaluation. Cytopathology. 2006;17:10–7.
Chatzipantelis P, Konstantinou P, Kaklamanos M, Apostolou G, Salla C. The role of cytomorphology and proliferative activity in predicting biologic behavior of pancreatic neuroendocrine tumors: a study by endoscopic ultrasound-guided fine-needle aspiration cytology. Cancer Cytopathol. 2009;117:211–6.
Imamura M, Takahashi K, Adachi H, Minematsu S, Shimada Y, Naito M, et al. Usefulness of selective arterial secretin injection test for localization of gastrinoma in the Zollinger–Ellison syndrome. Ann Surg. 1987;205:230–9.
Conflict of interest
No competing financial interests exist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsutsumi, K., Ohtsuka, T., Mori, Y. et al. Analysis of lymph node metastasis in pancreatic neuroendocrine tumors (PNETs) based on the tumor size and hormonal production. J Gastroenterol 47, 678–685 (2012). https://doi.org/10.1007/s00535-012-0540-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-012-0540-0