Abstract
Purposes
In the surgical treatment of pancreatic cancer, margin-negative status is one of the most important determinants of survival. We conducted this study to explore surgical margin status as well as other factors affecting the survival of borderline-resectable pancreatic cancer (BRPC) patients who received neoadjuvant chemotherapy with gemcitabine and S-1.
Methods
Eighteen BRPC patients were prospectively treated with concurrent gemcitabine and S-1 neoadjuvant chemotherapy (NAC+) and 15 of these patients underwent resection. We evaluated the safety and efficacy of this treatment regimen by comparing the outcomes of these patients with those of 19 BRPC patients who did not receive neoadjuvant chemotherapy (NAC−) during the same period.
Results
Fifteen (83 %) of the NAC+ patients underwent pancreatectomy. The remaining three patients (17 %) had regional tumor progression or liver metastasis. Of the 15 NAC+ patients who underwent resection, 3 (20 %) had margin-positive status, whereas 9 of the 19 (43 %) NAC− patients had margin-positive status (p = 0.002). However, disease-free survival and overall survival were similar in the two groups (MST 21.7 vs. 21.1 months). NAC+ patients with tumors smaller than 30 mm had favorable overall survival (MST 43.9 vs. 23.1 months, p = 0.0321). Most recurrences developed at distant sites rather than locally in both groups.
Conclusions
In the neoadjuvant setting, gemcitabine and S-1 improved the negative surgical margin rate in BRPC patients, but it did not improve survival. Thus, neoadjuvant chemotherapy should be given to BRPC patients at an earlier stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer is the fourth most common cancer in Japan. The fact that its incidence and mortality rates are nearly identical show it to be one of the most lethal malignancies [1]. Several reports, including one on a randomized controlled phase 3 study, have found that negative margin status is the most influential factor determining overall survival (OS) and curability [2–5]. However, even though as many as 93 % of tumor resections achieve negative surgical margins, 29–36 % of pancreatic cancer patients suffer recurrence with regional metastasis [2].
Pancreatic cancer easily invades the common hepatic artery (CHA) and the superior mesenteric arteries (SMA), as well as the portal vein (PV), which are unresectable because of their location. Positive margins are frequently observed around these major vascular structures, and tumor abutment of major vascular structures suggests a high likelihood of residual cancer after tumor resection. Such tumors are defined as borderline-resectable pancreatic cancer (BRPC) according to the National Comprehensive Cancer Network (NCCN) classification [6, 7].
Borderline-resectable status is defined by at least one of the following anatomic characteristics: severe unilateral SMV/PV impingement, circumferential SMA abutment of less than 180 degrees, or encasement of a short segment of the CHA. Since clear margins can be obtained (R0 resection) for SMV/PV impingement with vascular resection and reconstruction without increasing morbidity [8], incomplete resection, especially around the SMA or CHA, is common in patients with BRPC.
Since the early 2000s, Gemcitabine with S-1 has been used widely as standard therapy for advanced pancreatic cancer in Japan. Recently, a Phase III trial of gemcitabine, S-1 and gemcitabine+S-1 for advanced pancreatic cancer (GEST study) was conducted. Although there were no differences among these three groups in overall survival, a higher objective response rate was observed in the gemcitabine+S-1 group than in the gemcitabine group or the S-1 group (29.3 vs. 13.3 and 21.0 %, respectively) [9]. The high objective response rate of gemcitabine+S-1 treatment is favorable for neoadjuvant therapy to shrink the abutment of the tumor.
We designed a prospective Phase II study of concurrent gemcitabine+S-1, administered as neoadjuvant chemotherapy (NAC) to BRPC patients with SMA or CHA abutment, and compared the surgical margin status in these patients with that in those who did not receive NAC. We also investigated whether NAC prolongs the survival of BRPC patients and explored which subsets of BRPC patients benefit from neoadjuvant gemcitabine plus S-1 therapy.
Methods
Among 190 pancreatic cancer patients admitted to Kyoto University Hospital for pancreatectomy between January 2005 and December 2010, BRPC was initially diagnosed in 37. The diagnosis was based on our modified criteria of SMA or CHA abutment and either tumor encasement of a short segment of the hepatic artery or tumor abutment of the SMA involving less than 180° of the vessel circumference. We designed, prospectively, a study of the NAC used to treat these BRPC patients (UMIN000001450). Eighteen patients who received NAC (NAC+ group) were enrolled and compared with 19 BRPC patients who denied enrolling in this clinical study and were treated with upfront resection at Kyoto University Hospital during the same period (NAC− group). All of the patients included in this study had histopathologically diagnosed ductal adenocarcinoma of the pancreas and were followed up after surgery for at least 36 months.
Informed consent was obtained from all study participants according to institutional policies. This study was approved by the local ethics committee.
NAC study participants
Eighteen BRPC patients received NAC. After the tumor was diagnosed histologically as tubular ductal adenocarcinoma by endoscopic ultrasonographic fine needle aspiration (EUS-FNA), patients received three cycles of gemcitabine (1000 mg/m2) intravenously, on days 1 and 8 of a 21-day cycle, and oral S-1 (80 mg/m2), twice daily at a dose according to body surface area (BSA) (less than 1.25 m2, 60 mg daily; 1.25–1.5 m2, 80 mg daily; over 1.5 m2, 100 mg daily) on days 1–14. The dosage of S-1 given to the NAC+ group was based on the results of a phase II study of gemcitabine+S-1 (GS) [10, 11] in which 1000 mg/m2 of gemcitabine was combined with a daily dose of 100, 80, or 60 mg for S-1. Following chemotherapy, patients had their disease re-staged by thin slice contrast-enhanced CT (CE-CT) of the chest and abdomen and EOB contrast-enhanced MRI. Patients were examined by our multidisciplinary pancreatic cancer treatment team. If locoregional disease was stable or there was less or no distant metastatic disease, they underwent pancreatectomy with standard lymph node dissection within 6 weeks of chemotherapy. The perineural tissue around the major arteries was dissected by half of the circumference to clear the margin. All patients who underwent resection received gemcitabine or S-1 treatment for 6 months, starting within 8 weeks of tumor resection. If tumor progression was apparent after neoadjuvant treatment, the need for additional chemotherapy or chemoradiotherapy was determined on an individual basis. Pathology and operative reports were reviewed to evaluate the margin status and details about the resection. Resection margins were defined as positive (R1) if malignant cells were found within 1 mm of the pancreatic resection margin, the plexus around the SMA or CHA, bile duct, duodenum, or retroperitoneal tissue. If vein resection was performed, the vein margin was examined by the pathologist.
Follow-up data were based on medical records up to May 2014. Patients were evaluated by CE-CT every 3 months. The first site of disease recurrence was defined as follows: the development of a new low-density mass in the region of the pancreatic bed and root of the mesentery was considered locoregional recurrence. A new low-density region in the liver or lung was defined as distant metastasis. New ascites on ultrasonography (US) or CT, subsequently confirmed by cytological examination, was defined as peritoneal dissemination. Disease-free survival (DFS) was calculated as the time from the date of surgery to the date of initial recurrence. Overall survival (OS) was calculated as the time from the date of initial treatment to the date of death. The length of the tumor was estimated based on the CE-CT image before treatment and on the resected specimen.
Statistical analysis
All statistical analyses were performed using JMP version 10 (SAS Institute Inc., Cary, NC, USA). A two-sided significance level of 0.05 was considered to indicate significance. The Chi squared test was used for univariate analysis of categorical variables, and the unpaired t test was used for univariate analysis of continuous variables. DFS and OS curves were constructed using the Kaplan–Meier method and the log-rank test was used to evaluate differences. The impact of factors on prognosis was determined using Cox proportional hazards models.
Results
Feasibility, safety, radiological, and serological responses of patients who received NAC
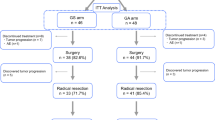
Between March, 2006 and November, 2010, BRPC was diagnosed in 37 patients, based on our modified criteria (see “Methods”). Eighteen patients were treated with NAC, consisting of concurrent gemcitabine and S-1, and 14 of these 18 patients completed three courses of full-dose gemcitabine and S-1 treatment. Four patients had the dose of gemcitabine reduced by 60 % because of hematologic toxicity. The other 19 patients were treated with upfront surgery. After NAC, all 18 patients had their disease restaged using CE-CT. None of the patients showed a complete response (CR), 11 % showed a partial response (PR), 16 % showed progressive disease (PD), and the remaining 73 % were in the stable disease (SD) category (Fig. 1a). One patient with PD had liver metastasis and two had apparent local progression, so these three patients did not undergo surgical resection. The remaining 15 patients (83 %) underwent surgical resection. When NAC response was defined as the percentage of degenerated cancer cells, only 13 % of the NAC group had more than 50 % degenerated cancer cells (Table 2). On the other hand, the levels of the tumor marker CA19-9 decreased in 86 % of the NAC+ patients (Fig. 1b).
a Clinical course of the NAC+ and NAC− groups. BRPC borderline resectable pancreatic cancer with abutment of the common hepatic artery (CHA) or superior mesenteric artery (SMA), NAC neoadjuvant chemotherapy, PD progressive disease, SD stable disease, PR partial response, met. metastasis, prog. progression. b Levels of the tumor marker CA19-9 before and after NAC. All but two patients had a reduction in CA19-9 levels after gemcitabine and S-1. Solid line patients with reduced CA19-9 levels, dotted line patients with increased CA19-9 levels. Gray zone normal values
Comparison of preoperative and pathological results in the NAC+ versus NAC− groups
Tables 1 and 2 list the preoperative and tumor pathological factors, respectively, of all the BRPC patients studied. There were no significant differences in preoperative variables such as tumor size or invasion into the portal vein between the groups (Table 1). There were no also differences in blood loss, operative time, morbidity, or postoperative adjuvant chemotherapy. With tumor pathological characteristics, there were no significant differences in tumor size, tumor location, number of lymph nodes with metastasis, or tumor invasiveness between the groups (Table 2). However, the frequency of pathologically curative resection (R0) was significantly higher in the NAC+ group (87 %) than in the NAC− group (53 %, p = 0.002).
Comparison of OS and DFS between the groups
All patients were followed up for at least 36 months with variable adjuvant chemotherapy. Adjuvant chemotherapy consisted of GEM or S1 for 13 of 14 NAC+ patients and 16 of 19 NAC− patients. There were no significant differences in the type of adjuvant chemotherapy. The median follow-up after NAC was 20.5 months (range 3–110 months) for all patients and 45 months (range 43–110 months) for censored patients. There were no treatment-related deaths. Although the NAC+ group had better OS than the upfront surgery group, there were no differences in OS (p = 0.0982) or DFS (p = 0.1162) between the groups. The median OS in the NAC+ and NAC− groups was 21.7 and 21.1 months, respectively (Fig. 2a, b).
In the subgroup of patients with a tumor diameter less than 30 mm, there was a significant difference in the OS curve between the NAC+ and NAC− groups (median OS, 43.9 vs. 23.1 months, p = 0.0321, Fig. 3a) and the NAC improved survival (hazard ratio 0.31; 95 % CI 0.11–0.79). When we analyzed the prognostic factors for overall survival in patients with a tumor less than 3 cm, NAC treatment was a significant improving factor in multivariate analysis (Table 3, p = 0.034), implying NAC treatment affects overall survival in BRPC with tumor diameter less than 3 cm.
Kaplan–Meier survival plots for tumors smaller than 30 mm. a Overall survival and b disease-free survival of the resected NAC+ group (n = 12, solid lines) and the NAC− group (n = 13, broken lines) patients. MST median survival time. All 12 NAC+ patients with a tumor diameter less than 3.0 cm underwent resection after chemotherapy
Although DFS was better in the NAC+ group than in the NAC− group (median DFS, 13.9 vs. 5.6 months), the difference was not significant (p = 0.0801, Fig. 3b). The diameter of the tumor was estimated based on the CE-CT images before treatment. When we measured the tumor diameter in the resected specimen, there was a good correlation between the diameter estimated by image before treatment and that in the resected specimen (R 2 = 0.5758, p < 0.0001, Fig. 4a) and a tumor size less than 30 mm in the resected specimen was again a significant factor for improving the overall survival of the BRPC patients given NAC (p = 0.0314, data not shown). However, the size reduction in the tumor in the resected specimen versus the size estimated by CE-CT before treatment was not significant.
Types of recurrence in the two groups
In the NAC+ group, the major type of recurrence was distant metastasis (9 of 13 patients, 69 %). The remaining 4 patients had local recurrence. In the NAC− group, 8 of 16 patients (50 %) had distant metastasis. Although the NAC+ group had a lower local recurrence rate, this difference was not significant (p = 0.292, Fig. 4b).
Discussion
In pancreatic cancer, significant determinants of postoperative survival after upfront surgical resection include negative margin status (R0), tumor size, and lymphnode status [3, 12, 13]. The NCCN defined BRPC as possessing characteristics that make it difficult for R0 status to be achieved; for example, a tumor near adjacent vital vessels such as the CHA or SMA. In this prospective study, we administered three courses of gemcitabine and S-1 concurrently as NAC to BRPC patients and compared them with patients who underwent upfront surgery in terms of R0 status and survival benefit. Although the R0 rate was apparently higher in the NAC+ group, we could not find any survival benefit of NAC. When we examined the subgroup of patients with a tumor size less than 30 mm, the NAC+ group had significantly longer survival than the NAC− group.
In addition to the survival benefit of the NAC+ patients with small tumors, most of the recurrences were as distant metastasis in the NAC+ group, which implies R0 status is not sufficient for cure in BRPC patients. These results suggest that most BRPC patients on admission have disease that is too advanced for curative resection and that NAC is most beneficial for BRPC patients with small tumors, which represents an earlier stage.
As Table 1 shows, operative time, extent of blood loss, morbidity, or the proportion of patients who received adjuvant chemotherapy did not differ significantly between the NAC+ and NAC− groups. In addition, the adverse effects of NAC were manageable. Loss of the opportunity for surgery due to toxicity was not observed and treatment was completed for all of the patients. Therefore, gemcitabine+S-1 treatment in the neoadjuvant setting was acceptably safe compared with other gemcitabine-based therapies [14–16].
In our study, there were no significant pre-treatment differences between the NAC+ and NAC− groups in CA19-9 and CEA levels, or in tumor size or invasion of the adjacent vein. Although a significant reduction in CA19-9 was evident after NAC, there was no improvement in the disease stage. Moreover, two-thirds of the patients did not have tumor regression after neoadjuvant treatment (Evans I, 9 patients; IIa, 4 patients; IIb, 2 patients; III, 0 patients), which is consistent with several studies that reported a response rate to concurrent gemcitabine and S-1 chemotherapy of approximately 29 % [9]. Thus, there is a discrepancy between the significant reduction in CA19-9 levels and tumor regression rates. Recent meta-analyses show that an increased R0 resection rate and local control in advanced pancreatic cancer patients did not translate into significantly improved survival [17, 18]. The MD Anderson Cancer Center group found that 45 % of patients had recurrence in distant organs such as the lung, live, or bone [19], indicating the need for effective systemic therapy. Taken together, their results and our current findings suggest that in addition to improved R0 rates, effective systemic neoadjuvant treatment is also important for improving survival. In this sense, it is possible that neoadjuvant treatment with FOLFIRINOX, which is more potent than gemcitabine, could improve overall oncologic outcomes. In fact, several phase II clinical trials evaluating the potential efficacy of neoadjuvant treatment with FOLFIRINOX in BRPC patients have been launched [20–22].
Our current study showed that NAC with GS therapy improved the survival of BRPC patients with a tumor smaller than 30 mm in diameter. These results were obtained both in the estimation by CE-CT before treatment and that by examination of the tumor specimen. When we compared the diameters by both estimations, we found a good correlation but could not confirm a significant size reduction in the tumor specimen after NAC treatment, implying that GS NAC did not strongly contribute to the shrinkage of the tumor volume. Rather, GS NAC might reduce marginal scattered invasions around major arteries, which could improve the R0 rate versus the NAC− group.
One of the potential drawbacks of NAC is that it may allow disease to progress to an unresectable stage. In our series, three (17 %) patients had radiological evidence of tumor progression after neoadjuvant GS treatment and their treatment was converted to chemo-radiotherapy or chemotherapy after NAC. Similarly, Motoi et al. [23] showed that after neoadjuvant GS in resectable and BRPC patients, the resection rate was 86 %. Our study of BRPC patients shows relatively good results, with a resection rate of 83 % and an R0 resection rate of 72 % (based on intention-to-treat), which are higher than the R0 rates (52 %) in the NAC− group and previous upfront surgery series [24–26]. To improve the resection rate in NAC settings, further study, especially on chemo-resistance [27], is needed.
In our current study, we defined BRPC as limited to SMA or CHA abutment, which is a modification of the NCCN guidelines. Multiple definitions of BRPC have been proposed, which vary, primarily according to the criteria for venous involvement. For example, the MD Anderson Cancer Center includes short-segment occlusion of the SMV, PV, or their confluence in their definition of BRPC [28]. The NCCN adopted venous involvement of the SMV/PV involving tumor abutment with or without impringement and narrowing of the lumen, encasement of the SMV/PV, or short-segment venous occlusion in their definition [6]. More specifically, Chun et al. [29] proposed Ishikawa type II and III vein deformity as the venous involvement criteria for borderline resectability. In contrast, there is more consensus on the definition of arterial involvement. In their analysis of 492 pancreatic cancer patients, Kelly et al. [8] found no significant difference in R0 status according to whether vein resection was performed. Since the main purpose of this study was to examine the R0 resection rate in the NAC+ group, we limited our definition of BRPC to SMA or CHA abutment. The impact of the SMV/PV involvement on BRPC is still being debated, and the impact of NAC on BRPC with SMV/PV involvement should be examined in the future, once consensus is reached.
One of the limitations of this study is the small number of subjects. During this study, we found good R0 rates, but OS and DFS for BRPC patients overall was not satisfactory. Therefore, we switched the NAC regimen for BRPC patients to neoadjuvant chemo-radiotherapy (NACRT) after 2011. Several promising results have been reported recently for BRPC patients with NACRT [30, 31]. For patients with potentially resectable pancreatic cancer, we are currently participating in a multi-institutional phase II/III GS-NAC study.
In conclusion, NAC consisting of concurrent gemcitabine and S-1, which can be safely achieved, improves the R0 resection rate. Specifically, it prolongs OS in BRPC patients with tumors smaller than 30 mm in size. The findings of this study suggest that GS-NAC should be used in the treatment of pancreatic cancer patients with earlier stage disease, such as those with potentially resectable tumors.
References
Ando M, Shimizu Y, Sano T, Senda Y, Nimura Y, Yamao K, et al. Poor prognosis of common-type invasive ductal carcinomas that originate in the branching pancreatic duct. Surg Today. 2015;45(10):1291–8.
Nimura Y, Nagino M, Takao S, Takada T, Miyazaki K, Kawarada Y, et al. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: long-term results of a Japanese multicenter randomized controlled trial. J Hepato-Biliary-Pancreat Sci. 2012;19(3):230–41.
Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4(6):567–79 (PubMed PMID: 11307091).
Christein JD, Kendrick ML, Iqbal CW, Nagorney DM, Farnell MB. Distal pancreatectomy for resectable adenocarcinoma of the body and tail of the pancreas. J Gastrointest Surg. 2005;9(7):922–7 (PubMed PMID: 16137585).
Bilimoria KY, Talamonti MS, Sener SF, Bilimoria MM, Stewart AK, Winchester DP, et al. Effect of hospital volume on margin status after pancreaticoduodenectomy for cancer. J Am Coll Surg. 2008;207(4):510–9.
Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16(7):1727–33.
(NCCN) NCCN. NCCN updates pancreatic adenocarcinoma guidelines. 2011. http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
Kelly KJ, Winslow E, Kooby D, Lad NL, Parikh AA, Scoggins CR, et al. Vein involvement during pancreaticoduodenectomy: is there a need for redefinition of “borderline resectable disease”? J Gastrointest Surg. 2013;17(7):1209–17 (discussion 17, PubMed PMID: 23620151).
Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol. 2013;31(13):1640–8.
Nakamura K, Yamaguchi T, Ishihara T, Sudo K, Kato H, Saisho H. Phase II trial of oral S-1 combined with gemcitabine in metastatic pancreatic cancer. Br J Cancer. 2006;94(11):1575–9 (PubMed PMID: 16721372, Pubmed Central PMCID: 2361295).
Ueno H, Okusaka T, Furuse J, Yamao K, Funakoshi A, Boku N, et al. Multicenter phase II study of gemcitabine and S-1 combination therapy (GS therapy) in patients with metastatic pancreatic cancer. Jpn J Clin Oncol. 2011;41(8):953–8.
Benassai G, Mastrorilli M, Quarto G, Cappiello A, Giani U, Forestieri P, et al. Factors influencing survival after resection for ductal adenocarcinoma of the head of the pancreas. J Surg Oncol. 2000;73(4):212–8.
Konstantinidis IT, Deshpande V, Zheng H, Wargo JA, Fernandez-del Castillo C, Thayer SP, et al. Does the mechanism of lymph node invasion affect survival in patients with pancreatic ductal adenocarcinoma? J Gastrointest Surg. 2010;14(2):261–7 (PubMed PMID: 19937477, Pubmed Central PMCID: 3135335).
Palmer DH, Stocken DD, Hewitt H, Markham CE, Hassan AB, Johnson PJ, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol. 2007;14(7):2088–96.
Heinrich S, Pestalozzi BC, Schafer M, Weber A, Bauerfeind P, Knuth A, et al. Prospective phase II trial of neoadjuvant chemotherapy with gemcitabine and cisplatin for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(15):2526–31.
Sahora K, Kuehrer I, Eisenhut A, Akan B, Koellblinger C, Goetzinger P, et al. NeoGemOx: gemcitabine and oxaliplatin as neoadjuvant treatment for locally advanced, nonmetastasized pancreatic cancer. Surgery. 2011;149(3):311–20.
Andriulli A, Festa V, Botteri E, Valvano MR, Koch M, Bassi C, et al. Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analysis of prospective studies. Ann Surg Oncol. 2012;19(5):1644–62.
Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7(4):e1000267 (PubMed PMID: 20422030, Pubmed Central PMCID: 2857873).
Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206(5):833–46 (discussion 46-8, PubMed PMID: 18471707).
UMCC 2011.007: a phase II study of neoadjuvant FOLFIRINOX and FDR-gemcitabine with concurrent IMRT in patients with borderline resectable pancreatic cancer [Internet]. 2011. https://www.umms.med.umich.edu/umclinialstudies/detail_pub_study.do?show=YES&id=6574&TYPE=October.
Marsh WR. A pilot study of pre- and post-surgery chemotherapy with mFOLFIRIOX in localized, resectable panreatic adenocatcinoma [Internet]. 2012. http://cllinicaltrialsfeeds.org/clinical-trials/show/NCT01660711.
Varadhachary G. A phase II study of preoperative systemic chemotherapy (modified FOLFIRINOX) followed by radiation therapy for patients with high risk resectable and borderline resectable adenocarcinoma of the pancreas [Internet]. 2012. http://clinicaltrials.gov/ct2/show/NCT01560949.
Motoi F, Ishida K, Fujishima F, Ottomo S, Oikawa M, Okada T, et al. Neoadjuvant chemotherapy with gemcitabine and S-1 for resectable and borderline pancreatic ductal adenocarcinoma: results from a prospective multi-institutional phase 2 trial. Ann Surg Oncol. 2013;20(12):3794–801.
Piperdi M, McDade TP, Shim JK, Piperdi B, Kadish SP, Sullivan ME, et al. A neoadjuvant strategy for pancreatic adenocarcinoma increases the likelihood of receiving all components of care: lessons from a single-institution database. HPB (Oxford). 2010;12(3):204–10 (PubMed PMID: 20590888, Pubmed Central PMCID: 2889273).
Le Scodan R, Mornex F, Girard N, Mercier C, Valette PJ, Ychou M, et al. Preoperative chemoradiation in potentially resectable pancreatic adenocarcinoma: feasibility, treatment effect evaluation and prognostic factors, analysis of the SFRO-FFCD 9704 trial and literature review. Ann Oncol. 2009;20(8):1387–96.
Raut CP, Tseng JF, Sun CC, Wang H, Wolff RA, Crane CH, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246(1):52–60 (PubMed PMID: 17592291, Pubmed Central PMCID: 1899216).
Uwagawa T, Yanaga K. Effect of NF-kappaB inhibition on chemoresistance in biliary-pancreatic cancer. Surg Today. 2015;45(12):1481–8.
Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13(8):1035–46.
Chun YS, Milestone BN, Watson JC, Cohen SJ, Burtness B, Engstrom PF, et al. Defining venous involvement in borderline resectable pancreatic cancer. Ann Surg Oncol. 2010;17(11):2832–8.
Satoi S, Yanagimoto H, Toyokawa H, Takahashi K, Matsui Y, Kitade H, et al. Surgical results after preoperative chemoradiation therapy for patients with pancreatic cancer. Pancreas. 2009;38(3):282–8.
Murata Y, Mizuno S, Kishiwada M, Hamada T, Usui M, Sakurai H, et al. Impact of histological response after neoadjuvant chemoradiotherapy on recurrence-free survival in UICC-T3 pancreatic adenocarcinoma but not in UICC-T4. Pancreas. 2012;41(1):130–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that there is no conflict of interest with any financial organization regarding this manuscript.
Rights and permissions
About this article
Cite this article
Masui, T., Doi, R., Kawaguchi, Y. et al. Concurrent gemcitabine+S-1 neoadjuvant chemotherapy contributes to the improved survival of patients with small borderline-resectable pancreatic cancer tumors. Surg Today 46, 1282–1289 (2016). https://doi.org/10.1007/s00595-016-1310-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-016-1310-z