Abstract
Purpose
The effects of laparoscopic colorectal surgery (LAC) on the long-term outcomes of elderly patients remain unclear. This study aimed to assess the short- and long-term outcomes of LAC in elderly colorectal cancer patients and to quantify the effects of LAC on the patient death patterns.
Methods
The clinicopathological data of elderly colorectal cancer patients aged ≥80 years old who were treated between 2006 and 2014 were extracted. The relationships between the clinicopathological factors and overall survival (OS) were assessed using the Cox proportional hazards model and Kaplan–Meier analyses. The risk factors for the types of death were estimated using a competing risk analysis.
Results
A total of 107 patients were included. Fifty-two patients underwent LAC, whereas 55 underwent open surgery (OC). There were no significant differences in the American Society of Anesthesiologists grade or comorbidity rate between the groups. The postoperative complication rate was significantly lower with LAC than OC (p < 0.001). After adjustment for covariates, laparoscopic surgery was not a significant risk factor for any of the types of death.
Conclusions
LAC is an effective and safe technique for elderly patients with colorectal cancer. Furthermore, there was no significant association between the surgical procedure and the pattern of death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparoscopic surgery has gained acceptance for the treatment of colon cancer, and has been widely adopted as a less invasive procedure. Randomized controlled trials have reported that the short- and long-term outcomes of laparoscopic surgery (LAC) for colon cancer are equivalent to those of open surgery (OC) [1–3]. However, most of these previous studies included only young patients and excluded elderly patients. Elderly patients are known to have more comorbidities than younger patients; therefore, they should be considered separately [4, 5]. Although excellent results of colorectal cancer surgery in elderly patients were reported in previous studies [6, 7], the risk of complications associated with surgery for colorectal cancer in elderly patients is expected to be high, and surgeons tend to select OC rather than LAC. As such, it is unclear whether the results from randomized control trials performed in younger patients can be adapted to elderly patients. However, several studies have reported that age is not a risk factor for postoperative complications or the survival outcome following hepatic and biliary tract operations [8–10]. The efficacy of LAC in elderly colorectal cancer patients needs to be evaluated to determine whether it is as safe and effective as the laparoscopic procedures on the hepatic biliary tract.
Several recent studies have reported the safety of LAC and its advantages in terms of the short-term outcomes for elderly patients [11–14]. Mukai et al. [12] reported that LAC for colorectal cancer could be performed safely, with better short-term outcomes than OC, in patients aged ≥85 years old. Furthermore, Nakamura et al. [13] suggested that LAC is suitable for elderly patients (≥85 years old) with colorectal cancer because it is less invasive than OC. However, the short- and long-term outcomes of LAC in elderly patients remain unclear, because large randomized controlled trials have yet to be performed. Furthermore, no studies have analyzed the potential disadvantages of LAC that may contribute to death from comorbidities, such as cardiovascular and pulmonary diseases, in the long-term for patients aged ≥80 years old.
This study was designed to compare the short- and long-term outcomes of elderly patients with colorectal cancer who underwent OC and LAC. The aim was to clarify the safety and efficacy of LAC in elderly patients. Finally, the influence of LAC on the pattern of death following colorectal cancer surgery was assessed using competing risks models.
Materials and methods
Patients and data extraction
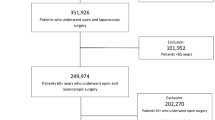
Between January 2006 and June 2014, 974 patients who underwent colorectal cancer surgery were identified in our institution retrospectively. The inclusion criteria for the present study were as follows: (1) elderly patients aged ≥80 years old, and (2) histologically confirmed stage I/II/III colorectal cancer. One hundred and thirty-one elderly patients were identified. Four patients who underwent palliative surgery, one patient who underwent transanal resection, two patients with colon perforation, fifteen patients with bowel obstruction, and two patients with no lymph node dissection were excluded from the study. The remaining 107 individuals met the inclusion criteria and were extracted for the analyses.
Fifty-two patients underwent LAC and 55 patients underwent OC. The patient data, including clinical characteristics [age, gender, weight, height, body mass index (BMI), American Society of Anesthesiologists (ASA) score, comorbidities, tumor location, presence of synchronous metastasis], pathological status [tumor differentiation, depth of invasion, lymphovascular invasion, number of dissected lymph nodes (LN), and number of metastatic LN], surgical outcomes (length of operation, intraoperative blood loss, postoperative hospitalization), postoperative complications and status at the most recent follow-up were collected during a retrospective review of medical records. All data regarding weight and height were obtained at the time of admission. The BMI was calculated using the following formula: patient weight (kg)/the square of their height (m2).
Surgical procedures
Expert colorectal surgeons or surgical residents under the supervision of experts performed all LAC and OC procedures. In addition, LAC was performed with consultant colorectal surgeons who were officially qualified by the Japan Society of Endoscopic Surgery (JSES). LAC was selected more frequently for colorectal cancer cases without advanced disease and previous laparotomy.
Endpoints
The primary endpoint was the overall survival (OS), defined as the time between the day of the operation and the date of death or last follow-up. Deaths were classified as cancer-related (death caused by colorectal cancer), cardiac or pulmonary (death caused by cardiac or pulmonary dysfunction), or other causes. The secondary endpoint was the quality of the oncological surgery, including the length of the operation time, estimated blood loss, the number of resected LN, the length of postoperative hospital stay, and the occurrence of postoperative complications.
Oncological management
Adjuvant chemotherapy was suggested for patients with stage 2 or 3 tumors. Follow-up involved a combination of physical examinations, ultrasound studies, computed tomography scans at 6-month intervals, and colonoscopies annually. Recurrence was evaluated comprehensively on the basis of the results of these imaging studies and histopathological examinations.
Statistical analysis
All statistical analyses were performed using the Stata software program, version 11.0 (Stat Corp, College Station, TX, USA). Patients were grouped according to whether they underwent OC or LAC. Continuous variables were presented as medians with interquartile ranges (IQR). The relationships between the two groups and among clinicopathological variables were evaluated using Chi squared tests or Mann–Whitney U tests. p values <0.05 were considered to be statistically significant. The survival distributions were estimated using the adjusted Kaplan–Meier method and compared using a log-rank test.
Univariate and multivariate Cox proportional hazards models were used to quantify the prognostic impact of individual covariates on the OS, and the hazard ratios (HRs) and their 95 % confidence intervals (CIs) were calculated. Covariates with values of p < 0.10 in the univariate Cox models were included in further multivariate analyses. Unadjusted and covariate-adjusted models were used in these analyses.
To analyze the death patterns, the cumulative incidences were estimated using a competing risk analysis. Cardiovascular or pulmonary-related and other deaths were treated as competing events. The cumulative incidence of death was compared for the types of procedures (LAC vs. OC). A competing risks regression model, as defined by Fine and Gray [15], was then applied.
Results
Patient characteristics
In total, data from 107 patients (54/110 males, 49 %; 56/110 females, 51 %) were included in this analysis. The median age at diagnosis was 83 years old (IQR 81–86 years). The tumors of 95 patients were located in the colon and the other 12 were located in the rectum. Twenty-six patients had stage I disease, 47 had stage II, and 34 had stage III disease. The median OS was 29 months (IQR 13–48).
The patients were divided into two groups according to the surgical procedure: LAC (n = 52) and OC (n = 55). The demographic and oncological characteristics of the patient populations are shown in Table 1. There were no significant differences in the age, gender, BMI, or ASA scores between the LAC and OC groups. The patients had various comorbidities, including hypertension, heart disease, pulmonary functional disorder, diabetes mellitus, central nervous system disease, and liver dysfunction. The overall presence of comorbidities was 29 % in the LAC group compared with 40 % in the OC group; however, there was no significant difference between the two groups.
The oncological outcomes were also compared between the two groups. No differences were found with regard to the tumor location, tumor differentiation, the number of dissected LN, vascular invasion, or the administration of adjuvant chemotherapy. In contrast, significant differences between LAC and OC were identified in terms of the indicators of advanced disease, including the depth of tumor invasion (p = 0.014), LN metastasis (p = 0.024) and lymphatic invasion (p = 0.050), suggesting that LAC was applied more often for less-advanced disease compared with OC. In addition, the TNM cancer stage was significantly higher in the OC group than in the LAC group (p = 0.032).
Postoperative outcomes
Short-term outcome
The surgical background data are summarized in Table 2. There were no significant differences in the length of the operation (p = 0.099) or the surgical procedure (high ligation resection, p = 0.220). However, there was a significant reduction in blood loss in the LAC group compared with the OC group (p < 0.001). No patients required conversion to open surgery in the LAC group.
A faster return of postoperative bowel function was observed in the LAC group than in the OC group. The median times to liquid intake (1 day, IQR 1–2 vs. 2 days, IQR 1–3; p < 0.001) and to dietary intake (3 days, IQR 3–5 vs. 4 days, IQR 3–5; p = 0.020) were both significantly shorter in the LAC group than in the OC group. Furthermore, the length of the postoperative hospital stay was significantly shorter in the LAC group than in the OC group (9 days, IQR 8–12 vs. 13 days, IQR 10–23, respectively; p < 0.001).
There was a significant difference in the overall postoperative complication rates between the LAC and OC groups (8 vs. 35 %, respectively; p = 0.001). Wound infections tended to occur more frequently in the OC group than in the LAC group. There was no mortality in either group.
Long-term outcomes
There was no significant difference in the follow-up periods between the LAC and OC groups (30.5 months, IQR 12–48 vs. 27 months, IQR 14–52, respectively; p = 0.861). Recurrence was identified in 17 patients (16 %). Although the difference was not statistically significant, the three-year OS rate (LAC 90 % vs. OC 76 %, p = 0.053) was higher in the LAC group. However, there was no significant difference in the cancer-specific mortality rate between the two surgical procedures (LAC 6 % vs. OC 13 %, p = 0.217).
According to a univariate analysis, the ASA score (HR 2.70, 95 % CI 1.01–7.20; p = 0.047), depth of tumor invasion (HR 2.95; 95 % CI 1.30–6.69; p = 0.010), and the number of metastatic LN (HR 1.31; 95 % CI 1.09–1.57; p = 0.003) were significantly associated with the OS (Table 3). The BMI (HR 0.89, 95 % CI 0.79–1.02; p = 0.087), LAC (HR 0.43; 95 % CI 0.18–1.02; p = 0.055), and the amount of blood loss (HR 1.00; 95 % CI 0.99–1.00; p = 0.063) were the factors that seemed to have a relationship with the OS. Furthermore, a multivariate analysis revealed that the ASA score was the only significant factor that affected the OS (HR 5.04; 95 % CI 1.69–15.03; p = 0.004); while the procedure did not (HR 0.60; 95 % CI 0.23–1.62; p = 0.316). There was no significant difference in the covariate-adjusted OS (adjusted for the depth of tumor invasion and the number of metastatic LN) between the LAC and OC groups (log-rank test, p = 0.108). The Kaplan–Meier survival curves are shown in Fig. 1.
Competing risk regression analysis
A total of 25 patients died during the long-term follow-up, which comprised 12 colorectal cancer-related deaths, eight cardiac or pulmonary deaths, and five other deaths (four other cancer-related deaths and one suicide). The 5-year cumulative incidence of cancer-related death in the covariate-adjusted model was estimated to be 11.0 % in the OC group and 8.5 % in the LAC group, whereas the 5-year cumulative incidence of cardiac- or pulmonary-related death was estimated to be 10.1 % in the OC group and 3.9 % in the LAC group (Fig. 2a, b). The effects of the surgical procedure on the three types of death are shown in Table 4. Although it was not statistically significant, the covariate-adjusted model using preoperative and pathological factors demonstrated that the depth of tumor invasion (HR 3.45, 95 % CI 0.91–13.45; p = 0.068) tended to be associated with cancer-related deaths. Cardiac- or pulmonary-related deaths were significantly associated with the ASA score (HR 10.78, 95 % CI 2.24–51.75; p = 0.003). However, there was no significant association between the surgical procedure and the three types of death.
Discussion
This study compared the short-term outcomes and cumulative incidence of cause-specific deaths between patients who underwent LAC and OC. The data revealed that the use of LAC in elderly patients aged ≥80 years old could lead to less blood loss, faster gastrointestinal recovery, and a shorter hospital stay compared with OC. Furthermore, the incidence of postoperative complications was significantly lower after LAC than OC. These results suggest that LAC is a safe procedure for elderly as well as younger patients.
The comparisons revealed large differences in the characteristics of patients in the LAC and OC groups. This was likely because oncological and technical factors strongly affected patient selection. Although there was a significant difference in the patient background, multivariate analyses revealed that the number of metastatic LN was the only prognostic factor for the OS in elderly colorectal cancer patients. Furthermore, no previous studies reported the cumulative incidence of cause-specific deaths; therefore, the covariate-adjusted model in the current study demonstrated for the first time that LAC was not associated with increased or decreased cause-specific death rates in elderly patients, even in those with a high comorbidity rate.
Several previous studies reported that laparoscopic surgery is beneficial and less invasive for the treatment of gastrointestinal disease, particularly colorectal cancer [1, 16, 16–18]. However, these previous randomized control studies included younger and excluded elderly patients. Several studies have recently reported the safety and benefits of LAC in elderly patients [11, 19]. For example, Hatakeyama et al. compared the short-term outcomes of LAC and OC in elderly patients aged ≥80 years old, and concluded that LAC improved the short-term outcome 20. Another previous study suggested that the use of LAC for colorectal cancer in patients aged ≥85 years old could be performed safely, and with better short-term outcomes than open surgery [12]. These results are similar to those of the current study, suggesting that the design and results of our study were reliable.
Elderly patients have a high mortality rate due to cardiovascular and pulmonary diseases [4, 5]. Therefore, cardiopulmonary complications can be a significant problem in such cohorts. Gerges et al. reported that laparoscopy using carbon dioxide for intra-abdominal insufflation could worsen preexisting cardiopulmonary complications [21]. In contrast, some other studies reported that intra-abdominal insufflation for LAC did not affect the patient outcome [22, 23]. In the present study, there were no cardiopulmonary complications in the LAC group, whereas two patients experienced pneumonia in the OC group. This suggests that LAC may be safe in elderly patients with cardiopulmonary diseases, who are generally recognized as high risk.
A competing risk analysis generalizes standard survival analyses to evaluate patients who are exposed to more than one cause of failure. This has advantages over the Kaplan–Meier method, because it calculates the cumulative incidence of an event of interest by considering competing risk events 15. Recently, this method was used to assess the effects of LAC on the pattern of recurrence and to assess the relationship between obesity and the recurrence of rectal cancer [24, 25]. To the best of our knowledge, this is the first report in which the patterns of death between LAC and OC were compared using a competing risk analysis, which is very informative for treatment selection in elderly patients.
Several previous studies have reported the advantages of LAC over OC in the short-term outcome of elderly patients, including reduced intraoperative blood loss, a shorter hospitalization, and better postoperative complications. However, the long-term outcomes were unknown. Nakamura et al. [13] reported the disease-free and OS rates in each tumor stage; however, there was no statistical assessment regarding whether LAC was superior to OC in the long-term outcomes. Dekker et al. [26] focused on the cause of death in the first year after curative colorectal cancer surgery, and demonstrated that there was an excess one-year mortality rate, indicating a prolonged impact of the surgery, particularly in elderly patients.
These previous studies suggest that there is a need to assess the effects of LAC on the long-term survival of elderly patients, because comorbidities may be affected in the long term. The present study demonstrated that the number of metastatic LN significantly affected the cancer-related deaths. However, the different surgical procedures (LAC vs. OC) did not affect the overall pattern of deaths. Furthermore, there was no difference between the Kaplan–Meier survival curves in the LAC and OC groups after adjustment for covariates. Although further large-scale studies of elderly patients are required to establish the benefits and risks of LAC, the results of the present study suggest that LAC may be safe and effective for elderly patients in terms of both the short- and long-term outcomes.
The present study is associated with several limitations that must be discussed. First, the number of patients in the current study was too low to yield conclusive results. Second, the follow-up period was relatively short. Some patients decided not to return to the hospital for additional visits, and some are still being treated. A large prospective study assessing postoperative recurrence and the OS should be performed to validate the effects of LAC on the pattern of deaths in elderly colorectal cancer patients. However, given the lack of evidence for the risks of LAC in elderly colorectal cancer patients, the results of this study may be useful to encourage future research and prompt the selection of less invasive treatments. Finally, the present study was performed at a single institution and was conducted retrospectively, thereby limiting the value of the results.
Our present findings indicate that LAC is an effective and safe technique for elderly patients with colorectal cancer, as well as for younger patients. There was no significant association between the type of surgical procedure and the pattern of deaths.
References
Fleshman J, Sargent DJ, Green E, et al. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST study group trial. Ann Surg. 2007;246:655–62 (discussion 662–654).
Colon Cancer Laparoscopic or Open Resection Study G, Buunen M, Veldkamp R, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52.
Jayne DG, Thorpe HC, Copeland J, et al. Five-year follow-up of the medical research council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638–45.
Duron JJ, Duron E, Dugue T, et al. Risk factors for mortality in major digestive surgery in the elderly: a multicenter prospective study. Ann Surg. 2011;254:375–82.
Cullen DJ, Apolone G, Greenfield S, et al. ASA physical status and age predict morbidity after three surgical procedures. Ann Surg. 1994;220:3–9.
Ong ES, Alassas M, Dunn KB, et al. Colorectal cancer surgery in the elderly: acceptable morbidity? Am J Surg. 2008;195:344–8 (discussion 348 ).
Tan KY, Kawamura Y, Mizokami K, et al. Colorectal surgery in octogenarian patients—outcomes and predictors of morbidity. Int J Colorectal Dis. 2009;24:185–9.
Ueno M, Hayami S, Tani M, et al. Recent trends in hepatectomy for elderly patients with hepatocellular carcinoma. Surg Today. 2014;44:1651–9.
Nozawa A, Kubo S, Takemura S, et al. Hepatic resection for hepatocellular carcinoma in super-elderly patients aged 80 years and older in the first decade of the 21st century. Surg Today. 2014.
Suzuki S, Kaji S, Koike N, et al. Pancreaticoduodenectomy can be safely performed in the elderly. Surg Today. 2013;43:620–4.
Roscio F, Bertoglio C, De Luca A, et al. Outcomes of laparoscopic surgery for colorectal cancer in elderly patients. JSLS J Soc Laparoendosc Surg/Soc Laparoendosc Surg. 2011;15:315–21.
Mukai T, Akiyoshi T, Ueno M, et al. Outcomes of laparoscopic surgery for colorectal cancer in oldest-old patients. Surg Laparosc Endosc Percutaneous Tech. 2014.
Nakamura T, Sato T, Miura H, et al. Feasibility and outcomes of surgical therapy in very elderly patients with colorectal cancer. Surg Laparosc Endosc Percutaneous Tech. 2014;24:85–8.
Miyasaka Y, Mochidome N, Kobayashi K, et al. Efficacy of laparoscopic resection in elderly patients with colorectal cancer. Surg Today. 2014;44:1834–40.
Fine J, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–26.
Lacy AM, Garcia-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–9.
Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–84.
Akiyoshi T, Kuroyanagi H, Oya M, et al. Short-term outcomes of laparoscopic rectal surgery for primary rectal cancer in elderly patients: is it safe and beneficial? J Gastrointest Surg Off J Soc Surg Aliment Tract. 2009;13:1614–8.
Hatakeyama T, Nakanishi M, Murayama Y, et al. Laparoscopic resection for colorectal cancer improves short-term outcomes in very elderly colorectal cancer patients. Surg Laparosc Endosc Percutaneous Tech. 2013;23:532–5.
Gerges FJ, Kanazi GE. Jabbour-Khoury SI Anesthesia for laparoscopy: a review. J Clin Anesth. 2006;18:67–78.
Gutt CN, Oniu T, Mehrabi A, et al. Circulatory and respiratory complications of carbon dioxide insufflation. Dig Surg. 2004;21:95–105.
Rauh R, Hemmerling TM, Rist M, et al. Influence of pneumoperitoneum and patient positioning on respiratory system compliance. J Clin Anesth. 2001;13:361–5.
Hasegawa H, Okabayashi K, Watanabe M, et al. What is the effect of laparoscopic colectomy on pattern of colon cancer recurrence? A propensity score and competing risk analysis compared with open colectomy. Ann Surg Oncol. 2014.
Seishima R, Okabayashi K, Hasegawa H, et al. Obesity was associated with a decreased postoperative recurrence of rectal cancer in a Japanese population. Surg Today. 2014.
Dekker JW, Gooiker GA, Bastiaannet E, et al. Cause of death the first year after curative colorectal cancer surgery; a prolonged impact of the surgery in elderly colorectal cancer patients. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2014.
Conflict of interest
The authors have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shigeta, K., Baba, H., Yamafuji, K. et al. Effects of laparoscopic surgery on the patterns of death in elderly colorectal cancer patients: competing risk analysis compared with open surgery. Surg Today 46, 422–429 (2016). https://doi.org/10.1007/s00595-015-1171-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-015-1171-x