Abstract
Purpose
To compare the efficacy of chlorhexidine-gluconate versus povidone iodine in preoperative skin preparation in the prevention of surgical site infections (SSIs) in clean-contaminated upper abdominal surgeries.
Methods
This was a prospective randomized controlled trial conducted on patients undergoing clean-contaminated upper abdominal surgeries. A total of 351 patients 18–70 years old were randomized into two groups; chlorhexidine and povidone iodine skin preparation before surgery.
Results
The incidence of SSIs in the chlorhexidine group was 10.8 %, in comparison to 17.9 % in the povidone iodine group. The odds ratio was 0.6 in favor of chlorhexidine use, but the results were not statistically significant (P = 0.06). In the first postoperative week, SSIs developed in 7 % of patients in the chlorhexidine group and 14.1 % in the povidone iodine group (P = 0.03), and in the second postoperative week, SSIs were present in 4.1 % of the patients in the chlorhexidine group and 4.4 % in the povidone iodine group, which was not statistically significant (P = 0.88).
Conclusions
The incidence of SSIs after clean-contaminated upper abdominal surgeries was lower with the use of chlorhexidine skin preparation than with povidone iodine preparation, although the results were not statistically significant. However, the odds ratio between the two groups favored the use of chlorhexidine over povidone iodine for preventing SSIs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgical site infections (SSIs) are one of the major complications that develop in surgical patients, and are the most common nosocomial infection in patients undergoing surgery, carrying significant morbidity, and mortality rates [1]. SSIs are the third most frequently reported health care-associated infection based on data derived from the Centers for Disease Control and Prevention’s (CDC) National Healthcare Safety Network [1]. There are considerable medical and health care implications associated with SSIs, including increased mortality, longer lengths of hospitalization, higher rates of re-operation and re-admission, and ultimately, higher health care costs [1–3]. A surgical infection is defined as an infection that arises from a surgical or invasive procedure or an infection that requires a surgical intervention to remedy, occurring within 30 days of surgery [4]. The CDC has recognized and differentiated SSIs into superficial incisional (above the fascia), deep incisional (at or below the fascia), and organ space SSIs [4, 5]. There are numerous risk factors associated with the development of SSIs related to the patient, the environment, and the treatment provided [6–8]. It has been recognized that the most important source of pathogens causing SSI is neither the operating surgeon and instruments, nor the environment, but the patient’s own microbial flora [9]. The primary source of contamination and cause of SSIs is patient skin flora and endogenous contamination, particularly when surgery involves the respiratory, urogenital, or the alimentary tract [9].
Strict antisepsis of the surgical site, which is the major source of pathogens, and optimization of preoperative antisepsis may decrease the incidence of postoperative SSIs. The prevention of an SSI is easier and more economical, as well as scientifically more feasible than treating an established SSI. Prophylactic antibiotics are obviously less useful in the setting of a polymicrobial infection, and there is the risk of emergence of resistance associated with the frequent use of broad-spectrum antibiotics in the routine prophylaxis of SSI [10].
Preoperative skin preparation of the surgical site using appropriate antiseptic products is one of the important interventions to prevent SSIs [11]. Several antiseptic agents are available for preoperative preparation of the skin at the incision site, including iodophors and alcohol-containing products like chlorhexidine gluconate (CHG). Povidone iodine (PVI) and CHG are well-known antiseptics which are used for surgical-site skin preparation. An effective preoperative skin antiseptic is an agent that rapidly (i.e., within 10 min of application) reduces the number of transient and resident microorganisms in the surgical field before wound incision and suppresses rebound growth for six hours after application [12–17]. The antimicrobial activity of CHG, as measured by skin surface microbial log reduction, has been found to persist several hours after application compared with PVI.
Several studies have been conducted wherein the preoperative preparation of the patients’ skin with chlorhexidine-alcohol-based solutions has been found to be superior to PVI solutions for preventing SSI [18–25]. These studies were done in a wide variety of surgeries with a heterogeneous group of patients. There have been no studies that have specifically reported a comparison of the efficacy of CHG versus PVI for surgical-site antisepsis in reducing SSI in clean-contaminated upper abdominal surgeries, which are the most common surgeries performed in General Surgery. The present study was conducted to compare the efficacy of CHG versus PVI for the prevention of SSIs in clean-contaminated upper abdominal surgeries.
Methods
This study was a prospective randomized controlled trial conducted in the Department of General Surgery in PGIMER, Chandigarh, India, from January 2011 to June 2012. Clearance from the host Institute Ethical Committee (PGIMER, Chandigarh, India) was obtained before starting the study. Random allocation of patients to one of the two groups was achieved by the sealed envelope method. Patients undergoing clean-contaminated upper abdominal surgeries in the elective setting were explained the details about the study, and written informed consent was obtained. The surgeries included hepatobiliary surgeries, including gall bladder surgeries, pancreatic surgeries, and gastroesophageal surgeries.
Patients
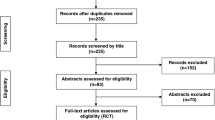
The group A patients included those who were preoperatively painted three times, around the site of the incision, using an applicator containing chlorhexidine-alcohol (0.5 % CHG in 70 % isopropyl alcohol). Group B included those who were preoperatively painted with 5 % PVI solution three times. A total of 351 patients aged 18–70 years old were randomized to group A or group B (Fig. 1). In both groups, when drains were required, they were brought out through separate stab incisions, well away from the surgical, and were covered with sterile gauze. Patients not fitting the inclusion criteria, who did not provide the required consent for surgery or patients lost to follow-up were excluded from the study.
The numbers of subjects were decided depending on the incidence of SSIs in clean-contaminated surgeries, which was considered to be about 22 % in our country [6]. We presumed the use of chlorhexidine-alcohol would decrease the incidence of SSI by about 30 %. For a power of 80 %, we had planned to include at least 350 subjects in each group. However, since our study was time bound, we could study 351 subjects; 163 in the chlorohexidine gluconate group and 188 in the PVI group in 18 months, including a one-month follow-up for all patients.
All of the included patients underwent a preoperative soap and water shower on the day of surgery and had their hair shaved prior to the surgery. Retractors were uniformly used in all cases where indicated. All patients received preoperative antibiotic treatment (Injected cefuroxime 1.5 g i.v. stat) at the time of induction of anesthesia. If the duration of surgery exceeded four hours, the antibiotic treatment was repeated.
All patients undergoing upper abdominal clean-contaminated surgeries in the elective setting, who had given consent for the above-mentioned study, and who uniformly received the preoperative antibiotic during the induction of anesthesia were enrolled in the study. Patients with any one of the following were excluded from the study: (1) No consent given for participation in the trial, (2) a history of allergy to chlorhexidine, alcohol, or iodophors, (3) clinical/microbiological evidence of infection at/adjacent to the surgical site, (4) ongoing systemic sepsis, (5) patients who died intra-operatively or before the completion of the 30-day follow-up period, (6) patients who left the hospital against medical advice or who were lost to follow-up, and (7) those who required a second operation within two weeks of the first operation.
Evaluations
Following surgery, the primary dressing was opened after 24 h and was assessed for any evidence of a SSI. During hospitalization, the wound was assessed daily for any SSI or if the patient was discharged, the patient was called once a week on an outpatient basis and assessed for SSI. The SSIs were diagnosed on the basis of the CDC criteria [4]. When there were doubts, clinically relevant microbiological samples were sent and assessed for evidence of a SSI. If a serous discharge appeared, it was swabbed and cultured, and the wound was classified according to the culture results. The primary endpoint was the occurrence of a SSI within the 30-day follow-up period. The secondary endpoints assessed were the occurrence of individual types of SSIs, the length of hospital stay, and any additional procedure done for SSIs. Both the patients and the clinicians who diagnosed the surgical-site infections on the basis of the criteria developed by the CDC remained blinded to the group assignments.
Statistical analysis
The statistical analysis was carried out using the SPSS software package, version 17 (SPSS Inc., Chicago, IL). Differences between parameters in the different patient groups were evaluated using Fisher’s exact test and Student’s t test where appropriate. Differences between proportions were evaluated using the Chi-square test. We conducted univariate and multivariate analyses to assess whether the risk factors contributed to the occurrence of SSIs. The univariate analysis for categorical factors was performed using Fisher’s exact test. For continuous factors, we used a single-variable logistic regression model. A multivariate logistic regression analysis was performed to assess the factors deemed significant (P ≤ 0.10) by the univariate analysis or considered to be clinically important. The assessed risk factors were prespecified in the protocol, and the statistical methods were preplanned.
Results
A total of 351 patients were randomly assigned to the study groups; 163 to the chlorhexidine gluconate–alcohol (CHG) group and 188 to the PVI group, and were qualified for the intention-to-treat analysis (Fig. 1). Nine patients were excluded from the study, five from the CHG group (three patients were lost to follow-up, two patients underwent contaminated surgeries rather than clean-contaminated surgeries) and four were excluded from the PVI group (two patients were lost to follow-up, one patient underwent re-operation less than one week after surgery and one patient underwent contaminated surgery). Thus, 342 patients were selected for the study, 158 in the CHG group and 184 in the PVI group. All of the selected patients underwent clean-contaminated upper abdominal surgeries (including hepatopancreaticobiliary and upper gastrointestinal surgeries (Table 1)).
The patients in both groups were comparable with respect to demographic variables (age and sex), body mass index, co-existing illnesses, and other risk factors for SSIs, namely diabetes mellitus, smoking, the use of drains, duration of surgery, and also the type of surgery performed i.e., hepatobiliary surgeries, including gallbladder surgeries and pancreatic surgeries or gastroesophageal surgeries (Table 1). All patients received preoperative antibiotic treatment during induction of anesthesia and received postoperative antibiotic treatment when indicated.
The patients in both the groups were assessed for SSIs after a 30-day follow-up period. The primary endpoint was the incidence of SSIs in both groups, with other secondary endpoints assessed. The rate of SSIs was 10.8 % in the CHG group and 17.9 % in the PVI group. Although the incidence of SSIs was lower in the CHG group compared to the PVI group, the difference was not statistically significant (P = 0.061). Similarly, the rate of superficial SSIs was lower in the CHG group (10.8 %) compared to the PVI group (16.84 %), but this differences was also not significant (P = 0.3) (Fig. 2). No cases of deep SSIs were noted in the CHG group, whereas two cases of deep SSIs occurred in the PVI group (1.3 %). Fisher’s test indicated that the difference in the incidence between the two groups was not significant (P = 0.54). No cases of organ/space SSIs were noted in either of the groups (Table 2). The odds ratio between the CHG group and the PVI group for the occurrence of a SSI was 0.6. The SSIs predominantly occurred during the first week after surgery (74 %), while 36 % occurred in the second week. There were no SSIs that occurred after the second week.
It was noted that the incidence of SSIs in the first postoperative week was significantly lower in the CHG group (7 %) compared to the PVI group (14.1 %) (P = 0.033). However, in the second postoperative week, the incidence of SSIs was similar between the two groups at 4.1 and 4.4 % in the GHG and PVI groups, respectively (P = 0.88). There were no SSIs in the third or fourth weeks in either group (Fig. 2) and (Table 3).
A multivariate logistic regression model was applied to analyze the risk factors for SSIs. The analysis revealed that male sex, the use of drains and the use of PVI contributed significantly to the development of SSIs (Table 4). Since an analysis of risk factors other than the assigned intervention constitutes an exploratory analysis, which involves multiple simultaneous statistical tests, it could inflate the probability of a false positive finding (type II error). There were no adverse events observed during the study in either group. Wound swabs were obtained from the surgical sites in 25 patients. Thirteen cultures revealed Gram-negative organisms, five were Gram-positive organisms, and seven were polymicrobial. E. coli were the predominant Gram-negative organism isolated. Contamination by E. coli may be attributed to minor leaks from the hollow viscus under controlled conditions or from the external atmosphere, including the hands of the medical personnel.
Discussion
The selection of the most appropriate antiseptic product is an essential step in preoperative skin preparation. Povidone iodine (polyvinylpyrrolidone iodine) has been used as a multivalent, local, broad-spectrum antiseptic having bactericidal, fungicidal, sporicidal, protocidal, and viricidal properties [13]. Molecular iodine is a well-established and effective disinfectant, the use of which has not been associated with the development of bacterial resistance [14]. It is the most widely used antiseptic in surgical practice. Povidone iodine has a potent and persistent bactericidal effect toward bacteria on healthy skin [14]. PVI may be less effective in the presence of blood, necrotic tissue, and pus [12].
Alcohols are broad-spectrum, fast-acting antimicrobials. They are ineffective against bacterial spores, but generally effective against fungal species and some viruses. Although alcoholic antiseptics have excellent immediate antimicrobial action, they have limited persistence and residual effects [12]. The combination of alcohol with chlorhexidine shows an improvement in immediate antimicrobial properties that provides excellent clinical efficacy as a skin antiseptic agent. The chlorhexidine component of this combination results in persistent antimicrobial action [12]. As an antiseptic agent, chlorhexidine exhibits a broad spectrum of antibacterial activity that is effective against both Gram-positive and Gram-negative non-spore forming bacteria [15]. The antiviral activity of CHG also encompasses selected enveloped viruses, including HIV [26]. Its antimicrobial spectrum of activity is similar to that of PVI; however, as an added advantage, CHG is not inactivated by blood or serum protein and exhibits a residual antimicrobial activity on the surface of the skin, suppressing microbial growth for several hours after application [26–28]. In one previous study, preoperative cleansing of the patients’ skin with chlorhexidine-alcohol was found to be superior to PVI for preventing SSIs in patients undergoing clean-contaminated surgery [26]. The application of CHG to the skin surface has been demonstrated to result in a greater microbial log reduction compared with PVI. Furthermore, the antimicrobial activity of CHG, as measured by the skin surface microbial log reduction, has been found to persist several hours after application compared with povidone-iodine [15].
Although several studies have been conducted comparing the efficacies of different skin antiseptic agents, the methods used in these studies have differed, as have the endpoints. Our present study aimed to compare the efficacy of CHG and PVI for preventing SSIs. The unique features of our study are that it was conducted at a single institute, and was limited to only clean-contaminated upper abdominal surgeries in order to avoid the influence of other factors on the results. Multiple risk factors common to both groups were matched.
The overall incidence of SSIs in our study was 14.62 %. In a multicentre study by Darouiche et al. [24], the incidence of SSIs in clean-contaminated upper abdominal surgeries was 10.45 %. Other authors have reported that the incidence of SSIs in clean-contaminated surgery ranged from 22–35 % [6, 7].
The overall incidence of SSI in the CHG group (10.8 %) was lower than that in the PVI group (17.9 %), although the difference was not statistically significant (P = 0.061). In addition, the incidence of superficial SSIs was lower in the CHG group (10.8 %) than in the PVI group (16.8 %), but this difference was not statistically significant. There were no deep SSIs in the CHG group, whereas two cases were reported in the PVI group. There were no organ/space SSIs in either of the groups.
The microbiological analysis of the skin site cultures revealed Gram-negative bacteria outnumbering the Gram-positive bacteria, with E. coli being predominantly isolated. This was in contrast to what was reported in an earlier study, where Gram-positive bacteria were most frequently isolated [24]. Several studies and meta-analyses have been conducted in order to compare the efficacy of CHG and PVI as skin antiseptics in preventing SSIs [22–26]. Although many studies have proven that CHG is more effective than PVI in preventing SSIs, there were also studies which found no significant difference between the two [27, 28]. In addition, the study methodologies and endpoints have been different in these studies, confounding a comparison of the results. The percentage of CHG used has been different in these studies, where 0.5, 2, and 4 % CHG have been used [27, 28].
Darouiche et al. demonstrated that the incidence of SSIs in patients undergoing clean-contaminated surgeries was significantly lower in the CHG group (9.5 %) than in the PVI group (16.1 %) (P = 0.004) [24]. However, in that study, various clean-contaminated non-abdominal surgeries were included, unlike in our study. In addition, 2 % chlorhexidine was used for skin preparation. Although the previous trial was not powered to compare the rates of infection in the sub-categories, the rate of SSIs was significantly lower in the CHG group than in the PVI group for small intestinal surgeries (P = 0.04) and abdominal surgeries as a whole (P = 0.009). However, our study included only patients undergoing upper abdominal clean-contaminated surgeries, including hepatopancreaticobiliary surgeries and gastroesophageal surgeries.
Similarly, in a recent study comparing the efficacy of CHG and PVI skin antisepsis, the rates of SSI were significantly lower in the CHG group (4.5 %) than in the PVI group (14.5 %), P = 0.011 [27]. However, that study was retrospective, and included patients undergoing gynecological procedures. In a meta-analysis of six eligible studies by Noorani et al. in 2010, it was deduced that chlorhexidine reduced the postoperative SSIs compared with povidone–iodine (pooled odds ratio 0.68, 95 % confidence interval 0.50–0.94; P = 0.019) [26]. However, the meta-analysis included studies with differing characteristics, such as those related to the study population, the study methodology, the concentrations of antiseptics used and the types of surgeries included (clean, clean contaminated, contaminated and dirty), with varying rates of SSIs.
In a study by Swenson et al., three different skin preparations were compared for their efficacy in preventing SSIs. Povidone iodine 10 %, chlorhexidine 2 % in 70 % alcohol and iodine povacrylax in alcohol were used in three different phases of the study [28]. Although the final result favored iodine povacrylax in reducing SSIs, the odds ratio between PVI and CHG favored PVI in preventing SSI (OR = 1.06). However, that the study included all classes of surgical patients, and it was not a randomized controlled trial.
There were no cases of adverse events in either of the skin preparation groups in our study (CHG 0.5 % and PVI 5 %), which is in contrast to the study by Darouiche et al., who reported an incidence of 0.7 % with 2 % CHG and 10 % PVI [24]. This may have been because the antiseptics were used at lower concentrations, although this needs further validation. In our study, it was also noted that the incidence of SSIs was highest during the first postoperative week (74 %), with the remaining 36 % of SSIs occurring during the second week after surgery. It was also found in our study that the incidence of SSIs in the first postoperative week was significantly lower in the CHG group (7 %) compared to the PVI group (14.1 %), P = 0.03. However, the incidence of SSIs in the second postoperative week, although marginally lower in the CHG group (4.1 %) than in the PVI group (4.4 %), was not statistically significant. There were no new SSIs beyond two weeks.
Based on these findings, we can conclude that chlorhexidine-alcohol was more effective than PVI in preventing SSIs during the initial postoperative period, probably by virtue of its residual effects. However, during the second week post-surgery, although the rate of SSIs was lower than that during the first week, the efficacy of CHG was similar to that of PVI in preventing SSIs. Since this issue has not been addressed in other studies, it needs to be evaluated in further studies to validate the present results. Although it is logical that a reduction in skin flora might translate into reduced SSI rates, we could find no studies that validated this assertion.
Conclusions
The overall incidence of SSIs in clean-contaminated upper abdominal surgeries was lower with the use of chlorhexidine-based skin preparation than preparation using PVI; the results were not statistically significant. However, the odds ratio between the two groups favored the use of chlorhexidine in preventing SSIs compared to PVI. Moreover, the incidence of SSIs in the initial postoperative period was significantly lower with the use of chlorhexidine than with PVI.
References
Leaper DJ. Risk factors for and epidemiology of surgical site infections. Surg Infect. 2010;11:283–7.
Zhan C, Miller MR. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290:1868–74.
Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725–30.
Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR, The Hospital Infection Control Practices Advisory Committee. Guideline for the prevention of surgical site infection. Infect Control Hosp Epidemil. 1999; 20:247–80.
Kulaylat MN, Dayton MT. Surgical complications. In: Townsend CM, Beauchamp RD, Evers BM, Mattox KL, editors. Sabiston textbook of surgery. 18th ed. Philadelphia: Saunders; 2008. p. 331–4.
Lilani SP, Jangale N, Chowdhary A, Daver GB. Surgical site infection in clean and clean-contaminated cases. Indian J Med Microbiol. 2005;23:249–52.
Kamat US, Fereirra AMA, Kulkarni MS, Motghare DD. A prospective study of surgical site infections in a teaching hospital in Goa. Indian J Surg. 2008;70:120–4.
National Nosocomial Infections Surveillance Systems (NNIS). System report: data summary from January 1992–June 2001. Am J Infect Control. 2001;29:404–21.
Malangoni MA, Cheadle WG, Dodson TF, et al. Roundtable discussion: new opportunities for reducing risk of surgical site infection. Surg Infect. 2006;7:23–9.
US Food and Drug Administration. Tentative final monograph for healthcare antiseptic drug products: proposed rules. 21 CRF Parts 333 and 369. Fed Regist Part III. 1994;59:31401–2.
Alexander WJ, Solomkin J, Edwards M. Updated Recommendations for Control of Surgical Site Infections. Ann Surg. 2011;253:1082–93.
Digison MB, BSN RN. A review of anti-septic agents for pre-operative skin preparation. Plast Surg Nurs. 2007; 27:185–189.
Schenck H, Simak P, Haedicke E. Structure of polyvinylpyrrolidone-lodine (Povidone-lodine). J Pharm Sci. 1979;68:1505–9.
Lacey RW. Antibacterial activity of povidone iodine towards non-sporing bacteria. J Appl Bacteriol. 1979;46:443–9.
Edmiston CE, Okoli O, Graham MB, Sinski S, Seabrook GR. Evidence for using chlorhexidine gluconate preoperative cleansing to reduce the risk of surgical site infection. AORN J. 2010;92:509–18.
Cecilio RL, Pine FJ, Palileo E, Rasco B, Isaac C, Siasoco RE. Microbiological efficacy of chlorhexidine: an in vitro study on 400 bacterial isolates from four Metro Manila hospitals. Phil J Microbiol Infect Dis. 1983;12:7–13.
Mulberrry G, Snyder AT, Heilman J, Pyrek J, Stahl J. Evaluation of a waterless, scrubless chlorhexidine gluconate/ethanol surgical scrub for antimicrobial efficacy. Am J Infect Control. 2001;29:377–82.
Holder C, Zellinger M. Daily bathing with chlorhexidine in the ICU to prevent central line-associated infections. J Clin Outcome Manag. 2009;16:509–13.
Hranjec T, Swenson BR, Sawyer RG. Surgical Site Infection Prevention: how we do it. Surg Infect. 2010;11:289–93.
Hayek U, Emerson JM, Gardner AM. A placebo-controlled trial of the effect of two preoperative baths or showers with chlorhexidine detergent on postoperative wound infection rates. J Hosp Infect. 1987;10:165–72.
Mimoz O, Karim A, Mercat A, et al. Chlorhexidine compared with povidone-iodine as skin preparation before blood culture: a randomized controlled trial. Ann Intern Med. 1999;131:834–7.
Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: expanding the armamentarium for infection control and prevention. Clin Infect Dis. 2008;46:274–81.
Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface prior to surgical admission? J Am Coll Surg. 2008;207:233–9.
Darouiche RO, Wall MJ, Itani KMF, et al. Chlorhexidine-alcohol versus povidine-iodine for surgical-site antisepsis. N Engl J Med. 2010;362:18–26.
Culligan PJ, Kubik K, Murphy M, Blackwell L, Snyder J. A randomized trial that compared povione iodine and chiorhexidine as antiseptics for vaginal hysterectomy. Am J Obstet Gynecol. 2005;192:422–5.
Noorani A, Rabey N, Walsh SR, Davies RJ. Systematic review and meta-analysis of preoperative antisepsis with chlorhexidine versus povidone-iodine in clean- contaminated surgery. Br J Surg. 2010;97:1614–20.
Levin I, Amer-Alshiek J, Avni A, Lessing BJ, Satel A, Almog B. Chlorhexidine and alcohol versus povidone-iodine for antisepsis in gynecological surgery. J Women Health. 2011;20:321–4.
Swenson BR, Hedrick TL, Metzger R, et al. Effects of preoperative skin preparation on postoperative wound infection rates: a prospective study of 3 skin preparation protocols. Infect Control Hosp Epidemiol. 2009;30:964–71.
Acknowledgments
There were no funding resources for this study.
Conflict of interest
There are no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srinivas, A., Kaman, L., Raj, P. et al. Comparison of the efficacy of chlorhexidine gluconate versus povidone iodine as preoperative skin preparation for the prevention of surgical site infections in clean-contaminated upper abdominal surgeries. Surg Today 45, 1378–1384 (2015). https://doi.org/10.1007/s00595-014-1078-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-014-1078-y