Abstract
Purpose
The function of regulatory T cells (Foxp3+CD4+CD25+ T cells: Treg) after surgery remains unspecified. We investigated the potential role of Treg as a new stress marker for various operations.
Methods
Thirty-three patients who underwent various operations at our department were divided into the following three groups based on the invasiveness of their surgery: Group A, those who underwent massively invasive surgery; Group B, those who underwent moderately invasive surgery; and Group C, those who underwent minimally invasive surgery. We measured Treg levels in the peripheral blood by flow cytometry and labeling with anti-CD4, CD25, and Foxp3 antibodies on preoperative day 1 and then on postoperative days (PODs) 1 and 6. Treg subpopulations in each group on the 3 days were compared.
Results
Treg subpopulations were significantly higher on POD 6 than on preoperative day 1 in all patients. In Group B, Treg subpopulations varied according to the operative procedures undertaken. For example, there were marked differences between open and laparoscopic abdominal surgery. In Group A, the Treg subpopulations tended to be increased on POD 6, although those on POD 1 were lower than those on preoperative day 1.
Conclusions
These findings suggest that Treg is an efficient biomarker, indicative of the degree of surgical stress and its impact on immunological status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunosuppressive conditions following surgical stress are often associated with postoperative complications; however, the mechanism of their pathological formation, including immunoreaction, is unclear and there are no useful markers of degrees of stress in a living body.
Regulatory T cells (Treg) comprise a subset of T cells with immunosuppressive function. The Treg subset in both humans and rodents is defined as CD4+ CD25+ cells that express high levels of the transcription factor, Foxp3, which is required for their development in vivo [1, 2]. Treg has been reported to be involved in the pathogenesis of allergic and autoimmune diseases, antigen-specific immune response, and modulation of inflammatory response [3–6]. Recently, Monneret et al. [7] reported increased numbers of CD4+CD25+ T cells in the peripheral blood of septic patients. The role of Treg in various stresses, including surgery, means it could become a useful biomarker indicative of living body defense mechanisms.
This study investigates the changes of Treg in the blood after surgery, in an attempt to clarify its potential as a new biomarker of the degrees of surgical stress.
Methods
Patients
The subjects of this study were 33 patients who underwent surgery at the University of Tokushima Hospital between October 2006 and March 2007. The patients were divided into the following three groups based on the degree of surgical invasiveness. The classification was decided with reference to the physiologic ability and surgical stress (E-PASS) scoring system [8–10]. This system classifies the degree of digestive surgical stress caused by blood loss, operation time, and extent of the skin incision. Group A comprised patients undergoing massively invasive surgery, including pancreatoduodenectomy (n = 3), distal pancreatectomy (n = 2), segmentectomy or more extensive resection of the liver (n = 11), and gastrectomy (n = 3). Group B comprised patients undergoing moderately invasive surgery, including partial resection of the liver (n = 1), open colectomy (n = 3), intra-abdominal tumor extirpation for peritoneal recurrence of pancreatic cancer or gastrointestinal stromal tumor of mesocolon (n = 2), splenectomy (n = 1), and laparoscopic colectomy (n = 3). Group C comprised patients undergoing minimally invasive surgery, including laparoscopic cholecystectomy (n = 2) and inguinal hernioplasty (n = 2).
This study was authorized in advance by the Institutional Review Board of the Tokushima University Graduate School. All patients provided written informed consent to take part in our study.
Cell isolation
Peripheral blood was collected on preoperative day 1 and postoperative days (PODs) 1 and 6 from all patients in Groups A and B; and on preoperative day 1 and POD 1 from those in Group C because of their early discharge from hospital. We calculated the ratio of regulatory T cells to peripheral blood mononuclear cells (PBMCs), not the absolute number of regulatory T cells or the ratios of Treg cells to CD4 positive cells, using flow cytometry, as described previously [11]. The authors of this previous clinical report also calculated Treg subpopulations gating PBMCs using the anti CD4 antibody and the anti CD25 antibody and regating these subpopulations using the anti-Foxp3 antibody. We calculated Treg subpopulations in the same manner. These samples were subjected to flow cytometry for multicolor analysis of T cells and stained according to the manufacturer’s guidelines. A 3-mL peripheral blood sample was taken from each subject and the PBMCs were isolated using 4 mL of Lymphoprep (AXIS-SHIELD, Oslo, Norway), centrifuged at 1800 rpm for 20 min at room temperature, and then resuspended with 4 mL of FACS buffer. After the addition of anti-CD4 (Allophycocyanin (APC)-conjugated antihuman CD4 antibody; e-Bioscience, San Diego, CA, USA) and anti-CD25 (FITC conjugated antihuman CD25 antibody; e-Bioscience) antibodies to the cell pellets, the mixture was centrifuged at 2000 rpm for 5 min at 4 °C, placed on ice for 15 min in the dark, and further incubated at 20 °C overnight. After two washes, a blocking buffer was added and the mixture was incubated in the dark at room temperature for 15 min. Next, an anti-Foxp3 antibody (Phycoerythrin (PE)-conjugated monoclonal antihuman Foxp3 antibody; e-Bioscience) was added to the cell pellet in the dark and incubated at room temperature for 30 min. The cells were then washed twice with FACS buffer, centrifuged at 1800 rpm for 5 min at 4 °C, and analyzed using a FACSCalibur (BD Biosciences, San Jose, CA, USA) (Fig. 1). The entire procedure was completed within 24 h of the blood collection.
Statistical analysis
Statistical analyses were performed using statistical software (JMP 8.0.1., SAS Campus Drive, Cary, 27513 NC, USA). Treg subpopulations are expressed as mean ± SEM and analyzed with the Mann–Whitney U test. A p value of 0.05 or less was considered significant.
Results
Based on our analysis, the Treg subpopulations on POD 6 were significantly higher than those on preoperative day 1 in all patients, regardless of surgical invasiveness (1.4× fold changes on POD 6; Fig. 2). When the Treg subpopulations were analyzed according to surgical invasiveness in Group B, they increased gradually after surgery, with statistical differences between preoperative day 1 and POD 6 (Fig. 3). On the other hand, in Group A, the Treg subpopulations were significantly higher on POD 6, whereas those on POD 1 were lower than those on preoperative day 1. There were no significant differences between Groups A and B on PODs 1 and 6.
Treg subpopulations were analyzed according to surgical invasiveness. In Group B, Treg subpopulations gradually increased after surgical treatment. In Group A, Treg subpopulations were significantly higher on postoperative day (POD) 6, although those on POD 1 were lower than those on preoperative day 1
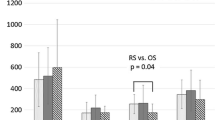
We also evaluated changes in Treg subpopulations within each group. Figure 4 shows the Treg subpopulation changes in Group C. The Treg subpopulations in patients who underwent inguinal hernioplasty tended to increase after surgery; however, the opposite change was seen in those who underwent laparoscopic cholecystectomy. In Group B, the Treg subpopulations varied according to the operative procedures the patients underwent; for example, open versus laparoscopic abdominal surgery. After open colectomy, the Treg subpopulations increased gradually, whereas after laparoscopic colectomy, it normalized quickly even though it increased linearly after open colectomy (Fig. 5). There were no significant differences among preoperative day 1, PODs 1 and 6. Figure 6 shows the Treg subpopulation changes in Group A. The Treg subpopulations tended to be higher on POD 6, although they were lower on POD 1 than on preoperative day 1. For example, with hepatectomy, the Treg subpopulations on POD 1 were significantly lower than those on preoperative day 1 (p < 0.05).
Discussion
To the best of our knowledge, this is the first report on Treg subpopulations in peripheral blood during the perioperative period. We reported previously that Treg cells are regulators and suppressors of T cells, which can induce a tolerant state in transplantation [12]. Moreover, Treg cells were found to be good markers for detecting metastasis in patients with pancreatic cancer, especially at advanced stages of the disease [13]. In the present study, we investigated the role of Treg in evaluating surgical stress. We found that Treg subpopulations on POD 6 were higher than the preoperative levels and that in Group A, the Treg subpopulations on POD 1 were lower than those on preoperative day 1. Several measures of surgical stress response have been proposed including serum concentrations of IL-6, C-reactive protein (CRP), lactic acid, and cortisol [14–16]. It is thought that the concentration of a pro-inflammatory cytokine in the blood, such as IL-1b, TNF-a, IL-6 or IL-8 should predict the degree of surgical stress; however, the measurement of these cytokines is labor intensive and requires a long time for analysis. Furthermore, it is not common to measure pro-inflammatory cytokines during the perioperative period. On the contrary, Treg subpopulations in peripheral blood can be measured easily at a low cost. We did not have to culture the blood overnight for the intranuclear staining of Foxp3. Thus, our method is faster than other surgical stress markers.
Increased percentages of Treg in the blood and spleen during the early stage of experimental and human sepsis have been reported [17, 18]. Furthermore Nascimento et al. [19] proposed that the persistent expansion of Treg in sepsis-surviving mice was correlated with a long-term adaptive immune dysfunction and susceptibility to secondary infection. We attributed the tendency of the Treg subpopulations on POD 6 to be higher than those on preoperative day 1 in all patients to be related to immunosuppression, which was modified by anti-inflammatory cytokines such as IL-10 and TGF-β. If homeostasis causes surgical stress, it leads to a form of SIRS, and is controlled by anti-inflammatory cytokines with antagonistic actions [20]. On the other hand, there is evidence that the synergistic effects of IL-2 and TGF-β can induce naive CD4+ cells to become CD25+Foxp3+ suppressor cells that express the characteristic markers of natural Treg cells [21].
Interestingly, after major surgery such as hepatectomy, the Treg subpopulations were lower on POD 1 than on preoperative day 1 (p < 0.05). Jiao et al. [22] reported an accumulation of Treg in inflammatory tissues and found significantly higher numbers of Treg cells in synovial fluid than in paired peripheral blood from patients with rheumatoid arthritis. Thus, the decreased Treg subpopulations we observed in peripheral blood on POD 1 might reflect variation in the body distribution of Treg. There were no significant differences between Groups A and B on PODs 1 and 6. It is likely that the Treg subpopulations in Group A were lower than those in Group B. Further study on a larger number of patients is required to find a way to classify the surgically invasive groups.
Regarding the changes of Treg subpopulations in Group C, we observed higher Treg subpopulations after inguinal hernioplasty; however, the opposite was observed after laparoscopic cholecystectomy. Because of the small number of patients and the lack of Treg subpopulations on POD 6, we could not explain why the changes of Treg subpopulations after inguinal hernioplasty differed so much from those after laparoscopic cholecystectomy. Moreover, the Treg subpopulations had normalized by POD 6 after laparoscopic colectomy even though they remained elevated in the open colectomy group. Conversely, the Treg subpopulations were significantly decreased on POD 1 and increased on POD 6. Taken together, we considered that Treg subpopulations in peripheral blood decreased after surgery depending on the invasiveness of the procedure, increased with recovery, and normalized after the effects of surgical stress had diminished. Further investigations are required to prove this theory, but if it is shown to be accurate, it is possible that the length of hospital stay and complication risk could be predicted from the perspective of Treg subpopulation changes after surgery.
In summary, our findings suggest that Treg subpopulations in peripheral blood vary, distinguishing this marker from existing stress markers. It is possible that Treg will become a new surgical stress marker based on immunological status.
Abbreviations
- Treg :
-
Regulatory T cell
- IL:
-
Interleukin
- SIRS:
-
Systemic inflammatory response syndrome
- POD:
-
Postoperative day
References
Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4CD25 regulatory T cells. Nat Immunol. 2003;4:330–6.
Hori S, Sakaguchi S. Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes Infect. 2004;6:745–51.
Provoost S, Maes T, van Durme YM, Gevaert P, Bachert C, Schmidt-Weber CB, et al. Decreased FOXP3 protein expression in patients with asthma. Allergy. 2009;64:1539–46.
Wheeler KM, Samy ET, Tung KS. Cutting edge: normal regional lymph node enrichment of antigen-specific regulatory T cells with autoimmune disease-suppressive capacity. J Immunol. 2009;183:7635–8.
Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, Lyerly HK, et al. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood. 2008;112:610–8.
Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, Strom TB, et al. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res. 2009;69:5490–7.
Monneret G, Debard AL, Venet F, Bohe J, Hequet O, Bienvenu J, et al. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med. 2003;31:2068–71.
Haga Y, Ikei S, Ogawa M. Estimation of physiologic ability and surgical stress (E-PASS) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today. 1999;29:219–25.
Oka Y, Nishijima J, Oku K, Azuma T, Inada K, Miyazaki S, et al. Usefulness of an estimation of physiologic ability and surgical stress (E-PASS) scoring system to predict the incidence of postoperative complications in gastrointestinal surgery. World J Surg. 2005;29:1029–33.
Kaneko H, Takagi S, Otsuka Y, Tsuchiya M, Tamura A, Katagiri T, et al. Laparoscopic liver resection of hepatocellular carcinoma. Am J Surg. 2005;189:190–4.
Nakagiri T, Warnecke G, Avsar M, Thissen S, Kruse B, Kühn C, et al. Lung function early after lung transplantation is correlated with the frequency of regulatory T cells. Surg Today. 2012;42:250–8.
Ikemoto T, Tashiro S, Yasutomo K, Kishihara K, Kurita N, Miyake H. Donor-specific tolerance induced by simultaneous allogeneic islet transplantation with CD4 + CD25 + T-cell into hepatic parenchyma in mice. J Med Invest. 2004;51:178–85.
Ikemoto T, Yamaguchi T, Morine Y, Imura S, Soejima Y, Fujii M, et al. Clinical roles of increased populations of Foxp3 + CD4 + T cells in peripheral blood from advanced pancreatic cancer patients. Pancreas. 2006;33:386–90.
Sakamoto K, Arakawa H, Mita S, et al. Elevation of circulating interleukin 6 after surgery: factors influencing the serum level. Cytokine. 1994;6:181–6.
Oka Y, Murata A, Nishijima J, et al. Circulating interleukin 6 as a useful marker for predicting postoperative complications. Cytokine. 1992;4:298–304.
Rahr HB, Bendix J, Ahlburg P, et al. Coagulation, inflammatory and stress responses in a randomized comparison of open and laparoscopic repair of recurrent inguinal hernia. Surg Endosc. 2006;20:468–72.
Monneret G, Debard AL, Venet F, et al. Marked elevation of human irculating CD4+CD25+ regulatory T cells in sepsisinduced immunoparalysis. Crit Care Med. 2003;31:2068–71.
Scumpia PO, Delano MJ, Kelly KM, et al. Increased natural CD4+CD25+ regulatory T cells and their suppressor activity do not contribute to mortality in murine polymicrobial sepsis. J Immunol. 2006;177:7943–9.
Nascimento DC, Alves-Filho JC, Sônego F, Fukada SY, Pereira MS, Benjamim C, et al. Role of regulatory T cells in long-term immune dysfunction associated with severe sepsis. Crit Care Med. 2010;38:1718–25.
Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. 1996;24:1125–8.
Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-β to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–27.
Jiao Z, Wang W, Jia R, Li J, You H, Chen L, et al. Accumulation of FoxP3-expressing CD4+CD25+ T cells with distinct chemokine receptors in synovial fluid of patients with active rheumatoid arthritis. Scand J Rheumatol. 2007;36:428–33.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saito, Y., Shimada, M., Utsunomiya, T. et al. Regulatory T cells in the blood: a new marker of surgical stress. Surg Today 43, 608–612 (2013). https://doi.org/10.1007/s00595-013-0517-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-013-0517-5