Abstract
Aims
To investigate the function of localized retinal areas in highly selected type 1 diabetes mellitus patients (DM1) with no or mild signs of diabetic retinopathy (NO DR and NPDR, respectively) and its correlation with age, diabetes duration and glycemic control.
Methods
Multifocal electroretinograms (mfERG) were recorded in 35 eyes of 18 NO DR patients and 38 eyes of 19 NPDR patients. Thirty-one eyes of 17 normal subjects were enrolled as controls. N1-P1 response amplitude densities (RADs) and P1 implicit times (ITs) from isolated (R1: 0°–2.5°, R2: 2.5°–5°, R3: 5°–10°) and combined (R1 + R2, R2 + R3 and R1 + R2 + R3) annular rings and from four retinal sectors (nasal, N; temporal, T; superior, S and inferior, I) with increasing eccentricities up to 10° (S1, S2, S3, S1 + S2, S1 + S2 + S3) were measured. The statistical differences between DM1 groups and controls were tested by ANOVA. The electrophysiological data were correlated with age, duration of diabetes and glycated hemoglobin (HbA1c) level using the Pearson’s test.
Results
MfERG RADs, but not ITs, from all isolated and combined rings and sectors up to 10° of foveal eccentricity were statistically different between DM1 groups compared to controls. No significant differences were found between NO DR and NPDR patients. The mfERG abnormalities of the central retinal areas were correlated significantly with age in both DM1 groups and with diabetes duration mainly in NPDR group.
Conclusions
In DM1 patients, localized retinal dysfunction, described by reduced mfERG RAD, can be observed also in the absence of clinical signs of DR and it is related to aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multifocal electroretinogram (mfERG) has been widely used to evaluate the function of the preganglionic elements (photereceptors and collector cells) in selected retinal areas [1].
This method allowed to detect dysfunction of localized retinal regions in patients with diabetes mellitus aiming to understand which areas would be the most susceptible to damage in this chronic metabolic disorder [2,3,4]. Indeed, identifying regions of early dysfunction or structural damage, as it happens using the innovative ultra-widefield fluorescein angiography [5] or the optical coherence tomography angiography (OCT-A) [6,7,8], may help guiding surveillance for diabetic changes in the presence or absence of signs of retinopathy.
In the analysis of mfERG signal, different parameters, such as response amplitude density (RAD), implicit time (IT) and retinal eccentricities, were considered to study functional changes occurring in the diabetic retina before the onset of retinopathy [9]. In particular, it has been reported that IT measurement can be sensitive enough to assess local retinal function in diabetes and therefore the IT changes have been thought to represent an early indicator of dysfunction in diabetic retinopathy (DR) before amplitude is reduced [[9, 10].
From very recent electrophysiological studies performed in a preclinical model used to investigate on the evolution of DR, the C57BL/KsJ-db/db mouse [11], it appears that full-field ERG responses, evaluating synaptic transmission between outer and inner retinal elements, are abnormal at very early age [12]. Time-dependent reduced ERG bioelectrical responses in db/db mice are likely due to microglia activation, pro-inflammatory retinal setting and blood glucose level changes at first, when retinal appearance and visual function are normal [13]. During DR progression, macroglia, in addition to microglia, possibly triggers neuroinflammatory action, and by initiating reactive gliosis, concurs to retinal neuronal apoptosis and so to neurodegeneration [14].
The predictive role of mfERG parameters on the subsequent development of retinopathy provides clinicians a useful screening tool for implementing accurate strategies for early detection of DR [15]. Interestingly, Harrison et al. [16] described that the power of subtle changes of mfERG IT in predicting the risk of developing retinopathy is different in type 2 and type 1 diabetes (DM2 and DM1, respectively).
Minimally reduced mfERG N1-P1 amplitudes with significantly delayed ITs have been found in DM2 [16], meaning that there exists a mixed dysfunction of outer and inner retinal layers [17, 18], probably reflecting an impairment in both vascular and neural retinal components even in the absence of signs of retinopathy [19, 20].
In DM1, mfERG abnormalities have been detected [4] with a differential dysfunction of the outer cellular elements located in the nasal and the temporal retina [21]. Moreover, an early dysfunction of the inner retinal layers (ganglion and pre-ganglion cells), even in the absence of DR, might precede the photoreceptors impairment, as suggested by several studies performed by adequate electrophysiological methods such as oscillatory potentials, pattern ERG or focal ERG [22,23,24].
Although it was reported that in DM1 the functional impairment of the inner neural elements is independent from disease duration and glycemic control [22, 23], the relationship between the function of pre-ganglionic elements and the descriptive parameters of diabetic disease, at this moment is not entirely well defined. In fact, there are several discordant reports regarding the relationship between the duration of the disease, glycemic control and mfERG responses [17, 21, 25]. If positive association exists, it means that worse metabolic control is associated with greater neuroretinal dysfunction and consequently a good glycemic control should induce a reduction of the neuroretinal functional impairment [[21, 26,27,28].
In the present study, we assessed in highly selected patients with DM1 either with no or mild signs of non-proliferative diabetic retinopathy (NO DR and NPDR, respectively), the function of selected retinal areas located in the macular region by means of an innovative method of analysis of mfERG responses [29]. Our aim was primarily to investigate which selected retinal areas of the macular region might present a functional involvement due to diabetic injury. Furthermore, we wanted also to contribute for elucidating the interesting topic about the controversial data on the relationship between age, diabetes duration and HbA1c levels and functional retinal change.
Materials and methods
Subjects
All research procedures described in this work adhered to the tenets of Declaration of Helsinki. The study protocol was approved by the local Ethics Committee (Comitato Etico Centrale IRCCS Lazio, Sezione IFO/Fondazione Bietti, Rome, Italy) and upon recruitment, informed consent was obtained from each eligible subject after full explanation of the procedure. Inclusion criteria for DM1 patients were: (1) age > 18 years, and (2) diagnosis of DM1 at least 1 year prior to study enrollment according to ADA criteria [Fasting plasma glucose ≥ 126 mg/dL (7.0 mmol/L)]. Fasting is defined as no caloric intake for at least 8 h; or 2-h plasma glucose ≥ 200 mg/dL (11.1 mmol/L) during oral glucose tolerance test, OGTT. The test should be performed as described by the WHO, using a glucose load containing the equivalent of 75-g anhydrous glucose dissolved in water; or HbA1C ≥ 6.5% (48 mmol/mol). The test should be performed in a laboratory using a method that is NGSP certified and standardized to the Diabetes Control and Complications Trial assay; or in a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose ≥ 200 mg/dL (11.1 mmol/L) [30], (3) treatment with continuous subcutaneous insulin infusion or with multiple daily insulin injections.
Exclusion criteria for DM1 patients were: best corrected visual acuity (BCVA) less than 0.0 logarithm of the minimum angle of resolution (logMAR) evaluated by ETDRS charts, refractive error greater than ± 2.00 diopters (D), the history or presence of maculopathy or positive recording of Amsler test, any other ocular diseases involving cornea and lens or previous eye surgery, detectable spontaneous eye movements (i.e., nystagmus), intraocular pressure > 21 mmHg, drug intake that can interfere with macular function, the presence of diabetic peripheral and autonomic neuropathy and microalbuminuria (urinary albumin/creatinine ratio ≥ 30 mg/g).
In DM1 eyes, the presence or absence of DR was assessed by macular clinical evaluation (slit-lamp and indirect ophthalmoscopy using + 90 D or + 78 D no-contact lens) (Volk Optical, Mentor, OH) and color photographs (TRC-50 DX, Topcon Instr. Corp, Tokyo, Japan) after pupillary dilatation using tropicamide 1% eye drops. According to the ETDRS severity scale [31, 32], the analysis of the grading of fundus and the stereoscopic color photographs were examined by two expert retinal specialists (MP and ML). Mild NPDR was defined as the presence of at least one microaneurysm and/or mild hemorrhages; NO DR was defined as the absence of any of the above signs.
HbA1c was analyzed by high-performance liquid chromatography (VARIANT 2; BioRad Laboratories, Munich, Germany), with intra-and interassay coefficients of variation of 0.46–0.77 and 0.69–0.91%, respectively.
Healthy subjects, recruited in the study as controls, had no history of systemic or ocular diseases and no previous eye surgery, age > 18 years, BCVA of 0.0 logMAR, with manifest refraction between + 2.00 D and − 2.00 D.
MfERG recordings
VERIS Clinic TM 4.9 (Electro-Diagnostic Imaging, San Mateo, CA, USA) was used for mfERG assessment in midriatic state using previous methodology [33,34,35,36].
Ring analysis
MfERG ring analysis was used to differentiate changes of the bioelectrical responses of the central foveal regions with respect to the more eccentric retinal areas in the macular region. We analyzed the averaged response obtained from three concentric annular retinal regions (rings) centered on the fovea. Therefore, we measured responses derived from 0° to 2.5° (ring 1, R1), from 2.5° to 5° (ring 2, R2), from 5° to 10° (ring 3, R3), as shown in Fig. 1. We also analyzed the responses from combined rings enclosing responses derived from the total area from 0° to 5° (R1 + R2), from 2.5° to 10° (R2 + R3) and the whole central macular area from the fovea up to 10° (R1 + R2 + R3), as displayed in Online Resource 1, to assess the retinal function in wide-ranging areas. Therefore, each ring was considered isolated or regrouped between the other rings (R1, R2, R3 or R1 + R2, R2 + R3 or R1 + R2 + R3).
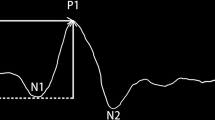
Layouts of multifocal electroretinogram traces from a representative control subject showing on the left the 61 averaged bioelectrical responses from a single recording session. First-order kernel measurements were obtained after automatic rejection of artifacts. Averaged traces are displayed on the right: the ring 1 (R1, 0–2.5 foveal degrees: corresponding to the dashed black area), ring 2 (R2, 2.5–5 foveal degrees: corresponding to the solid black area) and ring 3 (R3, 5–10 foveal degrees: corresponding to the gray area), whose N1–P1 response amplitude density (RAD, nV/deg2) and P1 implicit time (IT, milliseconds) are indicated by arrows
For each obtained averaged response, we evaluated the amplitude densities (RAD, expressed in nV/deg2) between the first negative peak, N1, and the first positive peak, P1, and the implicit time (IT) of the first positive peak (P1).
Sector analysis
MfERG sector analysis was used to differentiate changes of the bioelectrical responses of the central macular region up to 10°sectioning it in four sectors: inferior (I), nasal (N), superior (S) and temporal (T). We considered isolated and combined responses from the foveal center (sector 1, S1 = R1: 0°–2.5°) to external areas up to 5° (sector 2, S2) or to 10° (sector 3, S3), naming those regroups S1 + S2 and S1 + S2 + S3, respectively, as represented in Online Resource 2.
For each obtained averaged response, we evaluated the N1-P1 RADs and the P1 ITs.
Signal-to-noise ratio
MfERG signal-to-noise ratio (SNR) was estimated following the methodology discussed in Hood and Greenstein [37], used in our previous works [35] and displayed in Fig. 1. SNR was defined as the ratio of root mean square (RMS) signal plus noise (measured in the signal temporal window) of a given record to the mean RMS of all noise windows (61 for the mfERG). A SNR of ≥ 3 was accepted for mfERG measurements.
Statistical analysis
The Anderson–Darling and Kolmogorov–Smirnov tests were applied to verify if data were normally distributed. Data were analyzed for identifying outliers and these were discarded if present. In this study, no subject was identified as outlier.
To verify that electrophysiological data (RADs and ITs detected in each isolated and combined rings and sectors) were statistically different, an unpaired two-sample t test from right and left eyes of NO DR, NPDR and Controls was performed. A p value ≥ 0.05 was found in each group. All this allowed us to consider both eyes in the statistical evaluations for each enrolled subject.
The one-way analysis of variance (ANOVA) was used to evaluate differences of electrophysiological measurements between study and control groups. In details, grouping information has been performed by using Dunnett test to take in account of multiple comparisons. Thus, mfERG RADs and ITs from different topographic areas (rings and sectors) have been tested to assess if NO DR and NPDR were different from controls. The statistical relevance was considered for p ≤ 0.05.
ANOVA was first performed to evaluate differences of the rings’ RADs and ITs between groups. Thereafter, the same procedure was applied for measurements from retinal sectors. Data from ring and sector analyses were correlated with age (all groups), duration of diabetes and blood HbA1c level (only NO DR and NPDR groups) using the Pearson’s test.
Minitab 17 (version 1) software was used for statistical analysis.
Results
We enrolled in the study 18 NO DR patients, 19 NPDR patients and 17 controls providing 52 right and 52 left eyes (4 eyes were excluded on the basis of inclusion criteria). Participants’ characteristics were summarized in Table 1.
No differences in age and HbA1c level between NO DR and NPDR patients (unpaired t test; p = 0.182 and p = 0.962, respectively) were found; on the other hand, diabetes duration was statistically different between study groups (p = 0.005).
Ring analysis
Layouts from a representative control eye displaying mfERG traces from R1, R2 and R3 rings are reported in Fig. 1 (right side).
MfERG RADs, but not ITs, from isolated R1 (0°–2.5°), R2 (2.5°–5°), R3 (5°–10°) and combined rings (R1 + R2, R1 + R2 + R3, and R2 + R3) differed significantly between NO DR and controls (p < 0.001), NPDR and controls (p < 0.001). However, no significant differences were found between NO DR and NPDR patients for both RADs and ITs (see Tables 2, 3). Box plot representations of mfERG ring data are available on Online Resource 1.
Sector analysis
Isolated Nasal, Temporal, Superior, Inferior Sectors S2 and S3 and combined S1 + S2 and S1 + S2 + S3 Sectors: mfERG RADs, but not ITs, differed significantly between NO DR and controls (p < 0.001), and between NPDR and controls (p < 0.001, but in the inferior S3 p = 0.004). No significant differences were found between NO DR and NPDR patients for both parameters (see Tables 4, 5). Box plot representations of mfERG sector data are available on Online Resource 2.
Correlation analysis between mfERG and metabolic parameters
Age
In controls, we did not find any correlation between mfERG parameters and age in all examined rings and sectors taken individually or combined together (data not shown).
In DM1 patients, we found a linear relationship between age and mfERG R1 amplitude values. This correlation was not confirmed in retinal areas outside the 2.5°. The trend of significance of this relationship (RADs and age) was present, however, attenuated, when considering together RADs from R1 + R2 + R3 only in NO DR group. When considering RADs from combined retinal sectors up to 5° (S1 + S2), we found a significant correlation between the parameter of mfERG amplitude and age in both DM1 groups, but in the superior sector of NO DR patients. When incorporating also measurements from S3 (S1 + S2 + S3: 0°–10°), the correlation between RADs and age was present only in NPDR group, but in the temporal combined sector. In the latter sector, the correlation was significant for NO DR group. No correlations were found for the IT parameter with age in all rings and sectors, but in the nasal combined sectors (S1 + S2 + S3: 0°–10°) for the NO DR group (Online Resource 3).
In summary, age correlated with the RADs from the central fovea and from specific retinal sectors in both groups, independently from the presence or absence of diabetic retinopathy. In addition, in all those rings and sectors where we observed that the correlation was significant, we found that the correlation line had a negative slope, meaning that the reduced amplitude was correlated with increased age.
Diabetes duration
In NPDR group, the correlation between diabetes duration and RADs from the central fovea (R1), from combined sectors up to 5° (S1 + S2), but the superior, and from the S1 + S2 + S3 I reached the statistical significance. The NO DR group showed a weak linear correlation only with RADs from the inferior combined sectors up to 5° (S1 + S2) and from the temporal sectors up to 10° (S1 + S2 + S3).
In NPDR group, we found also a linear correlation between ITs and diabetes duration in R2, R3, in the combined R1 + R2 and in the combined superior S1 + S2 and S1 + S2 + S3. By contrast, in NO DR patients, only ITs from combined superior sectors up to 5° (S1 + S2) were significantly related to diabetes duration (Online Resource 3). In all those rings and sectors where a linear correlation between diabetes duration and RADs or ITs was observed, the correlation line had a negative slope for RADs and a positive slope for ITs, meaning that the reduced amplitudes and the increased implicit times were correlated with diabetes duration.
HbA1c level
No correlation between HbA1c and RADs in all rings and combined sectors was found in both diabetic groups. HbA1c was related with low statistical level of significance with ITs from R1 + R2 and from R2 + R3 in NO DR group. This correlation reached the statistical significant level when measured in combined superior sectors (S1 + S2 and S1 + S2 + S3) and in temporal S1 + S2 + S3 sectors in NPDR group (Online Resource 3).
Discussion
In this study, we observed local retinal dysfunction, assessed by mfERG recordings in DM1 patients even with no signs of DR at ophthalmoscopy.
This finding is in agreement with previous works performed in diabetic patients, in which it was suggested that the mfERG is a sensitive method to detect localized retinal areas with functional impairment [3, 10].
The novelty of this study was based on the use of sector analysis to mfERG measurements [29], other than using the traditional ring analysis in highly selected DM1 patients, in good glycemic control and especially not affected by microvascular complications. This detailed selection of the sample allowed to detect very early retinal functional anomalies, in the absence of other confounding factors that could have a significant impact on the results obtained.
Our primary aim was to understand if the local retinal deficits were dependent or not from the applied model of analysis, and we confirmed that local retinal dysfunction truly exists in those examined areas, independently from the paradigm used for analysis.
The mfERG RAD was the parameter constantly reduced in DM1 patients compared to controls, therefore representing the best index of retinal dysfunction even in the absence of ophthalmoscopic changes. Contrary to the previous literature [10] that has identified the IT delay as the main parameter descripting retinal dysfunction in diabetes, in our cohort mfERG IT did not show any significant differences between DM1 patients and controls. We are aware that previous work [10] has shown some delays in the eyes of DM1 and DM2 patients without diabetic retinopathy, however, Fortune et al. [10] did not select functional values from DM1 patients alone, and therefore, we cannot drive any significant conclusion on this discrepancy with our study that focused only on DM1 pure patients with no microvascular complications.
Highly variable outcomes have been reported in previous studies on populations of DM1 patients either on amplitude or implicit time [4, 17, 21, 28, 38,39,40]. We considered the retinal area as the most important factor influencing the mfERG measurements in DM1 patients. For this reason, we wanted to understand if the functional responses could vary by exploring retinal eccentricities with different topographies. Using the ring analysis, we found that RADs measurements remained constantly statistically different in DM1 patients as compared to controls across 10° of foveal eccentricity and that the IT parameter did not show any statistically significance between DM1 patients and controls at any retinal eccentricity. However, the RADs changes were unable to discriminate between eyes with NO DR and NPDR, being amplitude constantly reduced when diabetes was present. Also through the analysis of retinal sectors, we did not find any difference in retinal function between NO DR and NPDR groups in all examined areas, and moreover, we did not identify any functional differences between the nasal and temporal retinal in DM1 patients as previously described [39].
About our further investigation on the controversial data on the relationship between retinal function and the descriptive parameters of diabetes, a multivariate model based on mfERG IT analysis, duration of diabetes and blood glucose control has been previously used to validate these factors as the most important predictors of developing recurring retinopathy over a 3-year period [41]. More recently, Harrison et al. [16] found a more sensitive disease progression model for local prediction of DR in adult patients with no signs of retinopathy at baseline, within 1-year window, taking in account mfERG IT after adjusting for diabetes type.
In our study, retinal dysfunction correlated significantly with the aging and more weakly also with diabetes duration, whereas the overall glycemic load, expressed by HbA1c levels, was not related to the early retinal functional impairment.
In details, we found that age correlated with the RAD changes in both NO DR and NPDR groups (RAD values were reduced with aging) independently from the analyzed regions (i.e., ring or sector analysis). Since we did not find any correlation in controls, it is likely that DM1 may be the source of mfERG RAD reduction in patients. IT values were not correlated with age in any study group.
The correlations between diabetes duration and mfERG measurements that reached the statistical relevance were more significant in NPDR than in NO DR patients. NPDR patients showed significant IT delays and RAD reduction across the retina up to 10° from the fovea as significant longer was diabetes condition, as previously reported in DM2 patients [16, 40]. These findings may indicate that functional retinal abnormalities are related with the onset of diabetes also in DM1.
Good metabolic control is crucial to prevent and delay diabetes progression, but whereas some patients escape vision loss even with poor control, others develop vision loss despite good metabolic control [42]. In our study, no correlation was found between gluco-metabolic control and mfERG measurements. The lack of significant correlation between RADs changes and glycated hemoglobin levels, expression of the overall glycemic load, could be explained by the previously reported hypothesis, suggesting that in the early stages of DR, glycemic excursions rather than chronic hyperglycemia are more involved in retinal neuro-dysfunctional processes [43]. Nevertheless, it should be considered also the intricate and multifactorial pathogenesis of the microvascular complications of diabetes recalling hyperglycaemia-induced inflammation, immune response, endothelial function, regulation of angiogenic signaling pathway, oxidative stress and genotype variants [44,45,46]. All this let us to believe that for timely efficacious treatments are required complex therapeutical approaches [5, 47].
In conclusion, our findings indicate that a precocious neuronal signaling impairment involving outer and inner retinal layers occurs in DM1 patients even in the absence of clinical signs of DR. Since mfERG amplitude’s generators are thought to reside into photoreceptors and bipolar cells [1, 48], our findings are in agreement with the concept that retinal neurodegeneration is an early component of DR, which can precede microvascular disease, detectable by OCT-A [8, 49], as well as visible vasculopathy [2, 10, 50,51,52,53,54,55,56,57,58]. The neurodegenerative theory, for which the photoreceptors are involved early in the course of diabetes, has been also supported by our recent in vivo studies using adaptive optics ophthalmoscopy [59,60,61]. The authors have shown early pathological disruption of the parafoveal cone mosaic in DM1 patients even before any sign of diabetic retinopathy was found on fundoscopy.
From all previous reported evidences and from our results, clinicians should be conscious that neuroretinal dysfunction is present much in advance with respect to the clinical onset of DR. Indeed, we are aware that by studying the outer and inner retinal signals of mfERG, it is possible only to address whether neuronal synaptic transmission is impaired and possibly neurodegeneration has already started in diabetic retina.
Our findings could be useful in searching for functional (in association to structural) indicators of DR progression and in the identification of different phenotypes of DR with different risks for the development of vision-threatening complications for understanding diabetic pathologic mechanisms and for pursuing personalized management.
In view of the recent detailed investigations on the retinal vascular microstructure by innovative imaging techniques such as OCT-A, in young DM1 patients [8, 49], the present work provides the base of knowledge for further studies (already started in our institute) that may detect, in a multimodal approach, subtle structural changes of vessels in localized retinal areas, already known to be dysfunctional or vice versa. The following evaluations of the relation between mfERG and OCT-A findings could constitute the next step in searching for valid biomarkers of DR and might uncover the mechanisms of localized retinal dysfunction.Only in such a case, it could be addressed the clinical ability of mfERG, possibly combined with other techniques (i.e. OCT-A), to evaluate DR progression and to be an objective probe for testing the effects of therapeutical approaches.
References
Hood DC, Odel JG, Chen CS, Winn BJ (2003) The multifocal electroretinogram. J Neuroophthalmol 23:225–235
Bearse MA Jr, Ozawa GY (2014) Multifocal electroretinography in diabetic retinopathy and diabetic macular edema. Curr Diab Rep 14:526
Reis A, Mateus C, Melo P, Figueira J, Cunha-Vaz J, Castelo-Branco M (2014) Neuroretinal dysfunction with intact blood-retinal barrier and absent vasculopathy in type 1 diabetes. Diabetes 63:3926–3937
Tan W, Wright T, Dupuis A, Lakhani E, Westall C (2014) Localizing functional damage in the neural retina of adolescents and young adults with type 1 diabetes. Invest Ophthalmol Vis Sci 55:2432–2441
Querques L, Parravano M, Sacconi R, Rabiolo A, Bandello F, Querques G (2017) Ischemic index changes in diabetic retinopathy after intravitreal dexamethasone implant using ultra-widefield fluorescein angiography: a pilot study. Acta Diabetol 54:769–773
Simonett JM, Scarinci F, Picconi F, Giorno P, De Geronimo D, Di Renzo A, Varano M, Frontoni S, Parravano M (2017) Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol 95:e751–e755
Cao D, Yang D, Huang Z, Zeng Y, Wang J, Hu Y, Zhang L (2018) Optical coherence tomography angiography discerns preclinical diabetic retinopathy in eyes of patients with type 2 diabetes without clinical diabetic Retinopathy. Acta Diabetol 55:469–477
Scarinci F, Picconi F, Giorno P, Boccassini B, De Geronimo D, Varano M, Frontoni S, Parravano M (2018) Deep capillary plexus impairment in patients with type 1 diabetes mellitus with no signs of diabetic retinopathy revealed using optical coherence tomography angiography. Acta Ophthalmol 96:e264–e265
Wright T, Cortese F, Nilsson J, Westall C (2012) Analysis of multifocal electroretinograms from a population with type 1 diabetes using partial least squares reveals spatial and temporal distribution of changes to retinal function. Doc Ophthalmol 125:31–42
Fortune B, Schneck ME, Adams AJ (1999) Multifocal electroretinogram delays reveal local retinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci 40:2638–2651
Robinson R, Barathi VA, Chaurasia SS, Wong TY, Kern TS (2012) Update on animal models of diabetic retinopathy: from molecular approaches to mice and higher mammals. Dis Model Mech 5:444–456
Arroba AI, Alcalde-Estevez E, García-Ramírez M, Cazzoni D, de la Villa P, Sánchez-Fernández EM, Mellet CO, García Fernández JM, Hernández C, Simó R, Valverde ÁM (2016) Modulation of microglia polarization dynamics during diabetic retinopathy in db/db mice. Biochim Biophys Acta 1862:1663–1674
Arroba AI, Valverde ÁM (2017) Modulation of microglia in the retina: new insights into diabetic retinopathy. Acta Diabetol 54:527–533
Bogdanov P, Corraliza L, Villena JA, Carvalho AR, Garcia-Arumí J, Ramos D, Ruberte J, Simó R, Hernández C (2014) The db/db mouse: a useful model for the study of diabetic retinal neurodegeneration. PLoS One 9:e97302
Bearse MA Jr, Adams AJ, Han Y, Schneck ME, Ng J, Bronson-Castain K, Barez S (2006) A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res 25:425–448
Harrison WW, Bearse MA Jr, Ng JS, Jewell NP, Barez S, Burger D, Schneck ME, Adams AJ (2011) Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Invest Ophthalmol Vis Sci 52:772–777
Adhikari P, Marasini S, Sah RP, Joshi SN, Shrestha JK (2014) Multifocal electroretinogram responses in Nepalese diabetic patients without retinopathy. Doc Ophthalmol 129:39–46 (ErratumDoc Ophthalmol 129:47)
Hare WA, Ton H (2002) Effects of APB, PDA, and TTX on ERG responses recorded using both multifocal and conventional methods in monkey. Doc Ophthalmol 105:189–222
Tyrberg M, Ponjavic V, Lövestam-Adrian M (2005) Multifocal electroretinography (mfERG) in insulin dependent diabetics with and without clinically apparent retinopathy. Doc Ophthalmol 110:137–143
Santos AR, Ribeiro L, Bandello F, Lattanzio R, Egan C, Frydkjaer-Olsen U, García-Arumí J, Gibson J, Grauslund J, Harding SP, Lang GE, Massin P, Midena E, Scanlon P, Aldington SJ, Simão S, Schwartz C, Ponsati B, Porta M, Costa M, Hernández C, Cunha-Vaz J, Simó R, European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR) (2017) Functional and structural findings of neurodegeneration in early stages of diabetic retinopathy: cross-sectional analyses of baseline data of the EUROCONDOR project. Diabetes 66:2503–2510
Laron M, Bearse MA Jr, Bronson-Castain K, Jonasdottir S, King-Hooper B, Barez S, Schneck ME, Adams AJ (2012) Association between local neuroretinal function and control of adolescent type 1 diabetes. Invest Ophthalmol Vis Sci 53:7071–7076
Parisi V, Uccioli L, Parisi L, Colacino G, Manni G, Menzinger G, Bucci MG (1998) Neural conduction in visual pathways in newly-diagnosed IDDM patients. Electroencephalogr Clin Neurophysiol 108:490–496
Parisi V, Uccioli L (2001) Visual electrophysiological responses in persons with type 1 diabetes. Diabetes Metab Res Rev 17:12–18
Di Leo MA, Caputo S, Falsini B, Porciatti V, Greco AV, Ghirlanda G (1994) Presence and further development of retinal dysfunction after 3-year follow up in IDDM patients without angiographically documented vasculopathy. Diabetologia 37:911–916
Kim SJ, Song SJ, Yu HG (2007) Multifocal electroretinogram responses of the clinically normal retinal areas in diabetes. Ophthalmic Res 39:282–288
Klemp K, Larsen M, Sander B, Vaag A, Brockhoff PB, Lund-Andersen H (2004) Effect of short-term hyperglycemia on multifocal electroretinogram in diabetic patients without retinopathy. Invest Ophthalmol Vis Sci 45:3812–3819
Klemp K, Sander B, Brockhoff PB, Vaag A, Lund-Andersen H, Larsen M (2005) The multifocal ERG in diabetic patients without retinopathy during euglycemic clamping. Invest Ophthalmol Vis Sci 46:2620–2626
Bronson-Castain KW, Bearse MA Jr, Neuville J, Jonasdottir S, King-Hooper B, Barez S, Schneck ME, Adams AJ (2012) Early neural and vascular changes in the adolescent type 1 and type 2 diabetic retina. Retina 32:92–102
Cascavilla ML, Parisi V, Triolo G, Ziccardi L, Borrelli E, Di Renzo A, Balducci N, Lamperti C, Bianchi Marzoli S, Darvizeh F, Sadun AA, Carelli V, Bandello F, Barboni P (2018) Retinal dysfunction characterizes subtypes of dominant optic atrophy. Acta Ophthalmol 96:e156–e163
American Diabetes Association (2018) Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care 41:S13–S27
Early Treatment Diabetic Retinopathy Study research group (1985) Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 103:1796–1806
Early Treatment Diabetic Retinopathy Study Research Group (1991) Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology 98:786–806
Parisi V, Perillo L, Tedeschi M, Scassa C, Gallinaro G, Capaldo N, Varano M (2007) Macular function in eyes with early age-related macular degeneration with or without contralateral late age-related macular degeneration. Retina 27:879–890
Parisi V, Tedeschi M, Gallinaro G, Varano M, Saviano S, Piermarocchi S, CARMIS Study Group (2008) Carotenoids and antioxidants in age-related maculopathy Italian study: multifocal electroretinogram modifications after 1 year. Ophthalmology 115:324–333
Parisi V, Ziccardi L, Stifano G, Montrone L, Gallinaro G, Falsini B (2010) Impact of regional retinal responses on cortical visually evoked responses: multifocal ERGs and VEPs in the retinitis pigmentosa model. Clin Neurophysiol 121:380–385
Parisi V, Ziccardi L, Centofanti M, Tanga L, Gallinaro G, Falsini B, Bucci MG (2012) Macular function in eyes with open-angle glaucoma evaluated by multifocal electroretinogram. Invest Ophthalmol Vis Sci 53:6973–6980
Hood DC, Greenstein VC (2003) Multifocal VEP and ganglion cell damage: applications and limitations for the study of glaucoma. Prog Retin Eye Res 22:201–251
Kurtenbach A, Langrova H, Zrenner E (2000) Multifocal oscillatory potentials in type 1 diabetes without retinopathy. Invest Ophthalmol Vis Sci 41:3234–3241
Laron M, Bearse MA Jr, Bronson-Castain K, Jonasdottir S, King-Hooper B, Barez S, Schneck ME, Adams AJ (2012) Interocular symmetry of abnormal multifocal electroretinograms in adolescents with diabetes and no retinopathy. Invest Ophthalmol Vis Sci 53:316–321
Bronson-Castain KW, Bearse MA Jr, Neuville J, Jonasdottir S, King-Hooper B, Barez S, Schneck ME, Adams AJ (2009) Adolescents with Type 2 diabetes: early indications of focal retinal neuropathy, retinal thinning, and venular dilation. Retina 29:618–626
Ng JS, Bearse MA Jr, Schneck ME, Barez S, Adams AJ (2008) Local diabetic retinopathy prediction by multifocal ERG delays over 3 years. Invest Ophthalmol Vis Sci 49:1622–1628
Cunha-Vaz J, Ribeiro L, Lobo C (2014) Phenotypes and biomarkers of diabetic retinopathy. Prog Retin Eye Res 41:90–111
Picconi F, Parravano M, Ylli D, Pasqualetti P, Coluzzi S, Giordani I, Malandrucco I, Lauro D, Scarinci F, Giorno P, Varano M, Frontoni S (2017) Retinal neurodegeneration in patients with type 1 diabetes mellitus: the role of glycemic variability. Acta Diabetol 54:489–497
Kaidonis G, Gillies MC, Abhary S, Liu E, Essex RW, Chang JH, Pal B, Sivaprasad S, Pefkianaki M, Daniell M, Lake S, Petrovsky N, Hewitt AW, Jenkins A, Lamoureux EL, Gleadle JM, Craig JE, Burdon KP (2016) A single-nucleotide polymorphism in the MicroRNA-146a gene is associated with diabetic nephropathy and sight-threatening diabetic retinopathy in Caucasian patients. Acta Diabetol 53:643–650
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820
Kowluru RA, Koppolu P, Chakrabarti S, Chen S (2003) Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic Res 37:1169–1180
Iglicki M, Zur D, Busch C, Okada M, Loewenstein A (2018) Progression of diabetic retinopathy severity after treatment with dexamethasone implant: a 24-month cohort study the ‘DR-Pro-DEX Study’. Acta Diabetol 55:541–547
Hood DC, Frishman LJ, Saszik S, Viswanathan S (2002) Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci 43:1673–1685
Carnevali A, Sacconi R, Corbelli E, Tomasso L, Querques L, Zerbini G, Scorcia V, Bandello F, Querques G (2017) Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol 54:695–702
Jonsson KB, Frydkjaer-Olsen U, Grauslund J (2016) Vascular changes and neurodegeneration in the early stages of diabetic retinopathy: which comes first? Ophthalmic Res 56:1–9
Scarinci F, Picconi F, Virgili G, Giorno P, Di Renzo A, Varano M, Frontoni S, Parravano M (2017) Single retinal layer evaluation in patients with Type 1 diabetes with no or early signs of diabetic retinopathy: the first hint of neurovascular crosstalk damage between neurons and capillaries? Ophthalmologica 237:223–231
Vujosevic S, Midena E (2013) Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Müller cells alterations. J Diabetes Res 2013:905058
Palmowski AM, Sutter EE, Bearse MA Jr, Fung W (1997) Mapping of retinal function in diabetic retinopathy using the multifocal electroretinogram. Invest Ophthalmol Vis Sci 38:2586–2596
Cao J, McLeod S, Merges CA, Lutty GA (1998) Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Arch Ophthalmol 116:589–597
Linsenmeier RA, Braun RD, McRipley MA, Padnick LB, Ahmed J, Hatchell DL, McLeod DS, Lutty GA (1998) Retinal hypoxia in long-term diabetic cats. Invest Ophthalmol Vis Sci 39:1647–1657
Natarajan R, Bai W, Lanting L, Gonzales N, Nadler J (1997) Effects of high glucose on vascular endothelial growth factor expression in vascular smooth muscle cells. Am J Physiol 273:H2224–H2231
Williams B, Gallacher B, Patel H, Orme C (1997) Glucose-induced protein kinase C activation regulates vascular permeability factor mRNA expression and peptide production by human vascular smooth muscle cells in vitro. Diabetes 46:1497–1503
Sone H, Kawakami Y, Okuda Y, Kondo S, Hanatani M, Suzuki H, Yamashita K (1996) Vascular endothelial growth factor is induced by long-term high glucose concentration and up-regulated by acute glucose deprivation in cultured bovine retinal pigmented epithelial cells. Biochem Biophys Res Commun 221:193–198
Lombardo M, Parravano M, Serrao S, Ducoli P, Stirpe M, Lombardo G (2013) Analysis of retinal capillaries in patients with type 1 diabetes and non-proliferative diabetic retinopathy using adaptive optics imaging. Retina 33:1630–1639
Lombardo M, Parravano MC, Serrao S, Bocassini B, Varano M, Stirpe M, Lombardo G (2014) Adaptive optics imaging of parafoveal cones in type 1 diabetes. Retina 34:546–557
Lombardo M, Parravano M, Serrao S, Ziccardi L, Giannini D, Lombardo G (2016) Investigation of adaptive optics imaging biomarkers for detecting pathological changes of the cone mosaic in patients with Type 1 diabetes mellitus. PLoS One 11:e015138
Acknowledgements
The authors thank Dr. Valter Valli Fiore for technical help in executing electrophysiological recordings and for collecting data. The contribution by Fondazione Bietti in this paper was supported by the Ministry of Health and Fondazione Roma.
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements, proprietary interest), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national, local ethics committee: Comitato Etico Centrale IRCCS Lazio, Sezione IFO/Fondazione Bietti, Rome, Italy) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Managed by Antonio Secchi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ziccardi, L., Parisi, V., Picconi, F. et al. Early and localized retinal dysfunction in patients with type 1 diabetes mellitus studied by multifocal electroretinogram. Acta Diabetol 55, 1191–1200 (2018). https://doi.org/10.1007/s00592-018-1209-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-018-1209-9