Abstract
Aims
Clinical and experimental data suggest that early insulin therapy could reduce lipotoxicity in subjects and animal models with type 2 diabetes mellitus. However, the underlying mechanisms need to be clarified. Sterol regulatory element-binding protein 1c (SREBP-1c), which is negatively regulated by AMP-activated protein kinase (AMPK), plays a critical role in lipotoxicity and insulin resistance in skeletal muscle cells. Here, we investigated the effect and molecular mechanism of insulin intervention on the AMPK/SREBP-1c pathway in skeletal muscle cells with chronic exposure to palmitic acid (PA).
Methods
Male C57BL/6 mice were fed with a high-fat diet for 12 weeks and were then treated with insulin, AMPK inhibitor, or metformin. L6 myotubes incubated with palmitic acid (PA) were treated with insulin or metformin. Dominant-negative AMPKα2 (DN-AMPKα2) lentivirus, AMPKα2 siRNA, or Rho-kinase 1 (ROCK1) siRNA were transfected into PA-treated L6 myotubes.
Results
We found that the ability of PA to stimulate SREBP-1c and inhibit AMPK was reversed by insulin in L6 cells. Moreover, DN-AMPKα2 lentivirus and AMPKα2 siRNA were transfected into PA-treated L6 myotubes, and the decrease in SREBP-1c expression caused by insulin was blocked by AMPK inhibition independent of the phosphatidylinositol-4,5-biphosphate-3-kinase (PI3K)/AKT pathway. The serine/threonine kinase Rho-kinase (ROCK) 1, a downstream effector of the small G protein RhoA, was activated by PA. Interestingly, knockdown of ROCK1 by siRNA blocked the downregulation of AMPK phosphorylation under PA-treated L6 myotubes, which indicated that ROCK1 mediated the effect of insulin action on AMPK.
Conclusions
Our study indicated that insulin reduced lipotoxicity via ROCK1 and then improved AMPK/SREBP-1c signaling in skeletal muscle under PA-induced insulin resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipotoxicity, a well-accepted explanation for the development of obesity-associated insulin resistance, is characterized by elevated free fatty acid (FFA) levels in the plasma and excessive lipid accumulation in non-adipose tissue, namely liver and muscle. Clinical evidence has shown that early insulin therapy decreases FFA levels and improves dyslipidemia in patients with type 2 diabetes [1,2,3], and experimental data from diabetic animal models showed that insulin therapy reduces lipid content in the liver and muscle [4, 5]. However, the underlying molecular mechanism has not been completely elucidated.
Sterol regulatory element-binding protein 1c (SREBP-1c) is a key transcription factor that regulates de novo lipogenesis in insulin-sensitive tissues, including liver, muscle, and adipose tissue [6]. Aberrant activation of SREBP-1c leads to lipid accumulation, insulin receptor substrate 1 (IRS-1) suppression, and subsequent muscular insulin resistance [7, 8]. SREBP-1c activity is known to be upregulated by insulin and nutritional status [9] and downregulated by the cellular energy sensor AMP-activated protein kinase (AMPK) [10, 11]. Interestingly, our previous studies showed that insulin inhibits the increases in SREBP-1c expression induced by fatty acid in skeletal muscle in vivo and in vitro [12, 13], results that were similar to those of one study on macrophage cells in diabetes that showed the inhibitory effect of insulin on the increased SREBP-1c expression caused by high glucose [14], indicating that insulin might have different metabolic effects according to intracellular nutritional states.
The serine/threonine kinase Rho-kinase (ROCK), a downstream effector of the small G protein RhoA, exists as two isoforms, ROCK1 and ROCK2. ROCK1 is ubiquitously expressed in human and animal tissues, and ROCK2 expression is prominent in cardiac and brain tissues [15]. RhoA, activated by stimulation of tyrosine kinase and G-protein-coupled receptors (GPCRs), interacts with the Rho-binding domain of ROCK and alters the conformation of ROCK, resulting in ROCK action on downstream molecules. ROCK1 deletion can have a negative or positive effect on insulin signaling in skeletal muscle [16,17,18]. In addition, studies in animals and humans showed that ROCK is activated in metabolic syndrome, while the inhibition of ROCK ameliorates metabolic disorders [19, 20]. Further studies revealed that AMPK is the effector of ROCK in metabolic syndrome and aortic endothelial dysfunction in diabetic animal models [21,22,23], but the effect of insulin action on ROCK is unknown.
In the present study, we investigated the effects and molecular mechanisms of insulin intervention on the AMPK/SREBP-1c pathway in skeletal muscle cells with a lipid surplus. Our results demonstrated that insulin increased AMPKα phosphorylation and decreased SREBP-1c expression in palmitate (PA)-treated skeletal muscle cells in vivo and in vitro, which was mediated by the suppression of ROCK1, which indicated that an interaction between the insulin signaling and AMPK appeared to play an important role in early insulin therapy of type 2 diabetes.
Methods
Antibodies
Antibodies against AMPKα1/2, phospho-AMPKα (Thr172), AKT, phospho-AKT (Ser473), acetyl-CoA carboxylase(ACC), phospho-acetyl-CoA carboxylase (Ser79)(p-ACC), and Rho-kinase 1 (ROCK1) were purchased from Cell Signaling Technology (Danvers, MA, USA). The anti-SREBP-1 antibody was obtained from Santa Cruz Biotechnology (Dallas, Texas, USA). Antibodies against IRS-1, phospho-IRS-1 (Tyr608/612), and GAPDH were from EMD Millipore (Billerica, MA, USA).
Animals and intervention
Male C57BL/6 mice at 6 weeks of age were purchased from the Comparative Medicine Centre of Yangzhou University (Yangzhou, China) and were maintained on a 12-h light–dark cycle (temperature 23 ± 1 °C, humidity 45–65%) and fed on a standard chow diet (70% carbohydrates, 10% fat, and 20% protein; CON) or a high-fat diet (20% carbohydrates, 60% fat, and 20% protein; HFD) for 12 weeks. The HFD mice were then randomized into 4 groups (5 per group): (1) HFD control group, injected with phosphate-buffered saline (PBS) as a vehicle control; (2) metformin treatment group (HFD+Met), administered metformin (Sigma-Aldrich, St. Louis, MI, USA) by gavage at a dosage of 250 mg kg−1 day−1; (3) insulin treatment group (HFD+Ins), treated with Glargine (Sanofi-Aventis, France) by subcutaneous injection at a dosage of 0.5 IU; and (4) insulin and compound C treatment group (HFD+Ins+CC), treated with insulin and compound C (Sigma-Aldrich, St Louis, MI, USA) by intraperitoneal injection at a dosage of 20 mg kg−1 day−1. The medication dosages were adjusted to maintain blood glucose (BG) close to that of the CON mice. BG was measured in the tail vein with a glucometer (Accu-Check Active, Roche diagnostic, Germany) at 10 am. After 2 weeks of intervention, mice were deprived of food for 8 h and then killed for tissue collection. All animal procedures were performed according to the National Institutes of Health guidelines and approved by the animal care committee of Drum Tower Hospital affiliated with Nanjing University Medical School (Nanjing, China).
Cell culture
L6 cells were purchased from the Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS, vol./vol.), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were differentiated into myotubes in DMEM with 2% FBS for 5–7 days with medium replacement every day. The cells were then incubated in serum-free medium for 8 h and treated with 0.5 mmol/l PA for 24 h. After incubation with PA, cells were treated with 100 nmol/l insulin for a further 12 h. To determine whether AMPK or AKT was involved in the effect of insulin, cells were pretreated with AMPK inhibitor compound C at 10 μmol/l or with phosphatidylinositol-4,5-biphosphate-3-kinase (PI3K) inhibitor wortmannin at 1 μmol/l (Sigma-Aldrich, St. Louis, MI, USA) for 30 min prior to PA treatment. Cell lysates were collected for western blotting analysis.
Glucose uptake assay
L6 cells were treated with PA for 24 h and 100 nmol/l insulin for a further 12 h. The medium was then replaced with low-glucose and serum-free DMEM containing 100 μmol/l 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-amino]-2-deoxy-d-glucose (2-NBDG, Cayman, Ann Arbor, MI, USA). After incubation for 1 h at 37 °C in the dark, cells were collected, and the fluorescence intensity was measured at an excitation of 485 nm and emission of 520 nm using a FACS Calibur flow cytometer (BD Bioscience).
Recombinant lentivirus infection
A recombinant lentivirus encoding a rat c-Myc-tagged dominant-negative AMPKα2 (DN-AMPKα2; aspartic acid was substituted for alanine159) was purchased from GeneChem (Shanghai, China). L6 cells grown in one well of a 6-well plate were infected with 50 MOI of DN-AMPKα2 lentiviruses or with GFP lentiviruses as a control. The culture medium was changed 6 h later. At 48 h, protein extracts were harvested for detection by the following methods.
RNA interference
AMPKα2 and ROCK1 gene expression were silenced with small interfering RNA (siRNA). The siRNA sequence for targeting AMPKα2 or ROCK1 (GenePharma, Shanghai, China) was 5′-CCACUCUCCUGAUGCAUAUTT-3′ or 5′-GGGUAACUCAUCUGGUAAATT-3′, respectively. The siRNA sequence used as a negative control (siRNA-NULL) was 5′-UUCUCCGAACGUGUCACGUTT-3′. L6 myoblasts were transfected with siRNA (100 nM) via Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. After 24 h of transfection, cells were treated with 0.5 mmol/l PA for an additional 24 h, and then, cells were harvested for western blotting analysis.
Western blotting
The treated cells were lysed in ice-cold cell lysis buffer containing a protease inhibitor cocktail (Roche, Basel, Switzerland). The soleus muscle (approximately 200 mg) was homogenized in ice-cold buffer. Protein concentrations were determined by the bicinchoninic acid (BCA) assay. Protein lysates were boiled for 5 min and dissolved in Laemmli buffer. 20 mg of protein per lane was separated by SDS-PAGE, then transferred onto polyvinylidene fluoride membranes, blocked with 5% nonfat milk (wt./vol.), washed with Tris-buffered saline with 0.1% Tween 20 (TBST) and incubated with the appropriate primary antibodies overnight at 4 °C. After three washes with TBST, membranes were incubated with the appropriate HRP-conjugated secondary antibodies for 2 h at room temperature, followed by detection with enhanced chemiluminescence (EMD, Millipore). Band intensities were quantified with Quantity One (Bio-Rad, Hercules, CA, USA).
Statistical analysis
The data are expressed as the mean ± SE. The differences among groups were analyzed by one-way ANOVA with least significant difference (LSD) or Dunnett T3 post hoc comparison analysis, as appropriate. Values of p < 0.05 were considered statistically significant.
Results
The ability of PA to stimulate SREBP-1c and inhibit AMPK in L6 cells is reversed by insulin
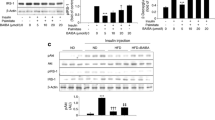
First, the effects of insulin on the AMPK/SREBP-1c pathway and insulin signaling were studied. As shown in Fig. 1a, insulin stimulated 2-NBDG uptake in control cells, and PA decreased 2-NBDG uptake, which was upregulated by insulin. To determine the effect of PA and insulin on insulin signaling and the AMPK/SREBP-1c pathway in vitro, p-IRS-1 (Tyr608/612), p-AKT (Ser473), mTOR, SREBP-1c, and fatty acid synthase (FAS) were measured. In control cells, insulin stimulated the phosphorylation of IRS-1 (Tyr608/612) and AKT (Ser473) and the expression of mTOR (Fig. 1c) and increased the expression of SREBP-1c and its target molecule FAS (Fig. 1b). In cells exposed to PA, p-IRS-1 (Tyr608/612) and p-AKT (Ser473) were reduced (Fig. 1c). Meanwhile, AMPKα2 phosphorylation and phospho-acetyl-CoA carboxylase were decreased (Fig. 1d), and SREBP-1c was significantly increased (Fig. 1b). However, the ability of PA to stimulate SREBP-1c and inhibit AMPK was reversed by insulin, and p-IRS-1 (Tyr608/612) and p-AKT (Ser473) increased in L6 cells after insulin intervention.
Insulin promoted glucose uptake, and the ability of PA to stimulate SREBP-1c and inhibit AMPK was reversed by insulin in L6 cells. a 2-NBDG uptake was measured following PA treatment. b The SREBP-1c and FAS protein expressions in L6 cells were determined by western blotting incubated with PA either in the absence or presence of insulin. c Insulin signaling protein expressions incubated with PA and insulin. d p-AMPKα/AMPKα2 and p-ACC expressions. Data are expressed as the mean ± SE of three independent experiments. The significance between groups is presented as indicated; *p < 0.05 versus control cells. #p < 0.05 versus PA-treated cells
The decrease in SREBP-1c expression caused by insulin is blocked by AMPK inhibition independent of the PI3K/AKT pathway
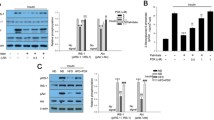
Next, the effects of insulin intervention on SREBP-1c were studied by using the AMPK inhibitor compound C or the PI3K inhibitor wortmannin. As shown in Fig. 2, insulin decreased PA-induced SREBP-1c, and this effect was blocked by AMPK inhibitor but not by PI3K inhibitor, indicating that insulin decreased SREBP-1c expression through AMPK activation independent of the PI3K/AKT pathway in L6 cells with chronic PA elevation. These results suggest that AMPK is involved in insulin’s downregulation of SREBP-1c in skeletal muscle cells incubated with PA.
SREBP-1c expression decreased by insulin was blocked by AMPK inhibition independent of the PI3K/AKT pathway. a The SREBP-1c protein expressions in L6 cells were determined by western blotting. b The p-AMPKα, p-AKT/AKT and p-ACC expressions were determined by western blotting. Data are expressed as the mean ± SE of 3 independent experiments. The significance between groups is presented as indicated; *p < 0.05 versus PA-treated cells. #p < 0.05, insulin combined with compound C-treated cells versus insulin-treated cells. &p < 0.05, insulin combined with wortmannin-treated cells versus insulin-treated cells
Insulin, via activation of AMPKα2, alleviates the increase in SREBP-1c induced by fatty acid in skeletal muscle in vivo and in vitro
High-fat diet (HFD)-induced diabetic mice were treated with medical interventions for 2 weeks. Compared to chow diet mice, the HFD mice had a marked increase in random BG (blood glucose) levels. After 2 weeks of insulin administration, their hyperglycemia was restored to near normal. Compared with insulin treatment, insulin combined with compound C had no effect on hyperglycemia or body weight (Fig. 3a, b).
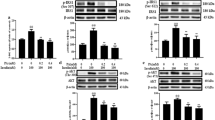
Insulin, via activation of AMPKα2, alleviated the increment of SREBP-1c induced by fatty acid in skeletal muscle in vivo and in vitro. Mice were fed normal chow diet(ND, n = 6), high-fat diet (n = 6), HFD supplemented with metformin (HFD+met, n = 6), HFD supplemented with insulin (HFD+Ins), or HFD supplemented with insulin combined with Compound C(HFD+Ins+CC). Mice were initially fed on a HFD for 12 weeks, followed by HFD plus medical treatment for additional 2 weeks. The body weight (a) and non-fasting BG (b) of all mice were monitored during the intervention study. Values are the mean ± SE (n = 6). c The muscle p-AMPKα, SREBP-1c protein expression was determined by western blotting after insulin, metformin, or insulin combined Compound C treatment in HFD-fed mice. SREBP-1c expression decreased by insulin was blocked by AMPK inhibitor. The significance between groups is presented as indicated; *p < 0.05 versus ND. #p < 0.05 versus HFD. &p < 0.05 versus HFD+Ins. d Downregulation of AMPK activity by AMPKα2 siRNA attenuated the impact of insulin on the inhibition of SREBP-1c in PA-treated L6 myotubes. e DN-AMPKα2 lentivirus blocked the effect of insulin on the inhibition of SREBP-1c. The p-AMPKα, p-AKT/AKT, p-IRS-1/IRS-1, SREBP-1c protein expressions in L6 cells were determined by western blotting. Data are expressed as the mean ± SE of three independent experiments. The significance between groups is presented as indicated; *p < 0.05 versus control cells
As shown in Fig. 3c and Fig. 1S, AMPKα2 and AKT phosphorylation decreased and SREBP-1c and ROCK1 increased in skeletal muscle of HFD mice compared with those in the chow diet mice. By contrast, insulin treatment increased AMPKα2 and AKT phosphorylation and downregulated SREBP-1c compared with those in the HFD mice. Insulin co-treatment with compound C blocked the effects of insulin on increased AMPKα2 phosphorylation and suppressed SREBP-1 expression (Fig. 3c). To further investigate the role of AMPK in insulin-reduced SREBP-1c expression in an independent setting, we used siRNA targeting AMPKα2. As shown in Fig. 3d, compared with transfection with siRNA-NULL, AMPKα2 knockdown mitigated the insulin-induced decrease in SREBP-1c protein under the condition of chronic PA treatment. We used DN-AMPKα2 to downregulate AMPKα2 activation and showed that the downregulation of SREBP-1c by insulin was restored in L6 cells cultured with PA (Fig. 3e). These results indicated that insulin decreased SREBP-1c expression through AMPK activation in L6 cells with chronic PA elevation.
ROCK1 mediates the effect of insulin on AMPK
We determined whether ROCK1 mediated the effect of insulin action on AMPK in PA-treated skeletal muscle cells. As shown in Fig. 4, compared with the control, PA treatment increased ROCK1 expression, which was inhibited by additional insulin treatment. Furthermore, knockdown of ROCK1 by siRNA blocked the PA-induced downregulation of AMPK and decreased the expression of SREBP-1c, AKT, and FAS. Taken together, these results indicate that PA stimulated ROCK1, which led to metabolic disorder by upregulation of fatty acid synthesis, and that the inhibition of ROCK1 by insulin may improve the action of the AMPK/SREBP-1c pathway to downregulate lipid accumulation in PA-treated skeletal muscle cells.
Knockdown of ROCK1 by siRNA blocked the downregulation of PA-induced SREBP-1c and FAS levels. a Protein levels of ROCK1, p-AMPKα, and p-ACC in L6 cells were determined by western blotting. b SREBP-1c and FAS protein expressions were determined by western blotting. Data are expressed as the mean ± SE of three independent experiments. The significance between groups is presented as indicated; *p < 0.05 versus control cells. #p < 0.05 versus PA-treated cells
Discussion
The major finding of the current study is that insulin, via activation of AMPKα2, alleviated the increase in SREBP-1c induced by fatty acids in skeletal muscle in vivo and in vitro. Moreover, the suppression of ROCK1 potentially accounted for the molecular mechanism of insulin regulation of the AMPK/SREBP-1c pathway.
Chronic exposure to excess nutrients, especially saturated fatty acids, leads to insulin resistance in skeletal muscle [24]. SREBP-1c plays a role in the molecular mechanism of insulin resistance by causing lipotoxicity in the case of chronic PA elevation. Our recent data revealed that SREBP-1c could directly suppress insulin receptor substrate 1 (IRS-1) expression and the subsequent insulin signaling pathway in PA-treated skeletal muscle cells [8]. Thus, SREBP-1c plays a critical role in skeletal muscle lipotoxicity.
Insulin’s stimulation of SREBP-1c is PI3K/AKT dependent [25]. Inhibition of PI3K by chemical inhibitors or expression of a dominant-negative AKT inhibits ER-to-Golgi transport of SREBP-1c and proteolytic activation. Consistent with this, insulin stimulates ER-to-Golgi transport of SREBP-1c by promoting its phosphorylation and association with COPII vesicles [26]. The mammalian target of rapamycin (mTOR) kinase is the major PI3K/AKT downstream effector. Rapamycin, the mTOR inhibitor, potently inhibits insulin-stimulated SREBP activation in primary rat hepatocytes [27]. Our data show that insulin stimulated AKT phosphorylation and mTOR expression, which promoted SREBP-1c and its target molecule FAS in control cells. However, a somewhat surprising finding in our study was that insulin inhibited SREBP-1c expression in skeletal muscle cells with lipid surplus, which was different from the SREBP-1c response to insulin in physiological states. Further in vivo and in vitro experiments showed that the decrease in SREBP-1c expression due to insulin was blocked by AMPK inhibition but not by PI3K inhibition, indicating that AMPK was involved in the insulin inhibition of SREBP-1c expression in skeletal muscle cells incubated with PA.
AMPK is a serine-threonine kinase and a key metabolic regulator [19]. In the physiological state, insulin decreases AMPK activity by downregulating phosphorylation on Thr172 of its α-subunit. The mechanism involved in the AMPK inactivation is the upregulation of the AKT/mTOR pathway [28]. Activation of AMPK enhances insulin sensitivity in a variety of tissues, including heart, liver, adipose tissue, and skeletal muscle [29, 30], whereas loss of AMPK contributes to insulin resistance [31]. With regard to fatty acid metabolism in skeletal muscle, AMPK reduces fatty acid synthesis by inhibiting SREBP-1c [11]. Experimental data from diabetic animal models have shown that insulin therapy reduces lipid content in liver and muscle by downregulating SREBP-1c [4, 5]. Our data show that AMPKα2 phosphorylation was decreased by PA treatment and that insulin intervention increased p-AMPKα and decreased SREBP-1c protein expression. Moreover, the decrease in SREBP-1c by insulin was blocked by AMPK inhibition in skeletal muscle cells incubated with PA, indicating that insulin decreased SREBP-1c expression through AMPK activation under lipid surplus conditions, which was different from insulin’s effect on AMPK inhibition in the physiological state. However, the effect and mechanism of insulin treatment on the AMPK pathway in skeletal muscle with PA elevation needs to be explored.
Environmental factors regulate intracellular signal transduction through Rho-kinase, leading to cell function and metabolic changes [32]. Studies in animals and humans have shown that ROCK is activated in metabolic syndrome, while the inhibition of ROCK ameliorates metabolic disorders by activation of the AMPK pathway [19, 20]. Lipid-induced insulin resistance in muscle occurs through activation of the RhoA/Rho-kinase signaling pathway [33], implying a crucial role of the RhoA/Rho-kinase pathway in skeletal muscle, in which it mediates lipid-induced insulin resistance in obese mice. Consistent with these studies, we also found that ROCK1 expression was increased in L6 myotubes under PA-induced insulin-resistant conditions. Furthermore, knockdown of ROCK1 by siRNA increased AMPK activity and blocked the PA-induced upregulation of fatty acid synthesis-related protein SREBP-1c and FAS. The ROCK inhibitor fasudil improves IRS-1-dependent insulin signaling in skeletal muscle cells of hyperinsulinemic Zucker rats [34]. Rho-kinase inhibits AMPK, with a resultant reduction in whole-body energy consumption and dyslipidemia [21]. Rho-kinase inhibition increases AMPK phosphorylation and increases energy consumption, resulting in the amelioration of lipid profiles in n/i/eNOSs−/− mice [22]. These results indicate that the lipid-lowering effects of Rho-kinase inhibition are mediated by AMPK phosphorylation, suggesting that Rho-kinase is also an upstream effector of AMPK and an important therapeutic target of metabolic disorders.
Insulin enhances the vasodilator capacity of VSMCs by inactivating the small GTPase RhoA and its target ROCK [35]. Unlike the other effects of insulin, which are mediated by the PI3K/AKT signaling pathway [36], insulin’s effect on Rho-kinase inhibition does not appear to involve PI3K, as wortmannin does not prevent insulin-mediated Rho-kinase inactivation even though it completely abolishes insulin-mediated PI3K/AKT activation [35]. Furthermore, treatment with sodium nitroprusside (SNP), a nitric oxide (NO) donor, mimics the effect of insulin on Rho-kinase inactivation, indicating that insulin inhibits Rho-kinase activity via nitric oxide synthase (NOS) signaling [35, 37]. Our results seem to suggest that the decrease in SREBP-1c expression caused by insulin was blocked by AMPK inhibition independent of the PI3K/AKT pathway, and insulin attenuated the PA-induced upregulation of fatty acid synthesis-related protein SREBP-1c and FAS by inactivating ROCK1 expression. Why wortmannin failed to block insulin’s effect on SREBP-1c in skeletal muscle in a PA-induced insulin-resistant state is not clear [35]. And the activation of AMPK promoted GLUT4 location to the cell membrane to increase glucose uptake [29]. Thus, the present study indicated that activation of AMPK partly mediated such effects in addition to PI3K/ATK pathway in PA-induced insulin resistance. Further studies are needed to clearly establish the mechanism by which insulin inactivates Rho-kinase and how this inactivation leads to the downregulation of SREBP-1c. Nonetheless, these results, though indirect, support our hypothesis: Suppression of ROCK1 was responsible for insulin-dependent regulation of the AMPK/SREBP-1c pathway in skeletal muscle cells exposed to PA (Fig. 5).
In conclusion, our study revealed that insulin reduced lipotoxicity via ROCK1 and then improved AMPK/SREBP-1c signaling in skeletal muscle under PA-induced insulin resistance. An interaction between the insulin signaling and AMPK appears to play a role in early insulin therapy of type 2 diabetes, which implies that insulin combined with an AMPK activator could serve as a more effective treatment for achieving complete remission of type 2 diabetes.
References
Vaverková H, Chlup R, Ficker L, Novotny D, Bartek J (1997) Complementary insulin therapy improves blood glucose and serum lipid parameters in type 2 (non-insulin-dependent) diabetic patients. II. Effects on serum lipids, lipoproteins and apoproteins. Exp Clin Endocrinol Diabetes 105(Suppl 2):74–77
Hayashi T, Hirano T, Yamamoto T, Ito Y, Adachi M (2006) Intensive insulin therapy reduces small dense low-density lipoprotein particles in patients with type 2 diabetes mellitus: relationship to triglyceride-rich lipoprotein subspecies. Metabolism 55(7):879–884
Juurinen L, Tiikkainen M, Häkkinen AM, Hakkarainen A, Yki-Järvinen H (2007) Effects of insulin therapy on liver fat content and hepatic insulin sensitivity in patients with type 2 diabetes. Am J Physiol Endocrinol Metab 292(3):E829–E835
Bi Y, Sun WP, Chen X et al (2008) Effect of early insulin therapy on nuclear factor kappaB and cytokine gene expressions in the liver and skeletal muscle of high-fat diet, streptozotocin-treated diabetic rats. Acta Diabetol 45(3):167–178
Sun W, Bi Y, Liang H et al (2011) Inhibition of obesity-induced hepatic ER stress by early insulin therapy in obese diabetic rats. Endocrine 39(3):235–241
Ferré P, Foufelle F (2007) SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res 68(2):72–82
Mingrone G, Rosa G, Greco AV et al (2003) Intramyocitic lipid accumulation and SREBP-1c expression are related to insulin resistance and cardiovascular risk in morbid obesity. Atherosclerosis 170(1):155–161
Bi Y, Wu W, Shi J et al (2014) Role for sterol regulatory element binding protein-1c activation in mediating skeletal muscle insulin resistance via repression of rat insulin receptor substrate-1 transcription. Diabetologia 57(3):592–602
Commerford SR, Peng L, Dubé JJ, O’Doherty RM (2004) In vivo regulation of SREBP-1c in skeletal muscle: effects of nutritional status, glucose, insulin, and leptin. Am J Physiol Regul Integr Comp Physiol 287(1):R218–R227
Wu W, Tang S, Shi J et al (2015) Metformin attenuates palmitic acid-induced insulin resistance in L6 cells through the AMP-activated protein kinase/sterol regulatory element-binding protein-1c pathway. Int J Mol Med 35(6):1734–1740
Li Y, Xu S, Mihaylova MM et al (2011) AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 13(4):376–388
Chen X, Yu QQ, Zhu YH et al (2010) Insulin therapy stimulates lipid synthesis and improves endocrine functions of adipocytes in dietary obese C57BL/6 mice. Acta Pharmacol Sin 31(3):341–346
Bi Y, Cai M, Liang H et al (2009) Increased carnitine palmitoyl transferase 1 expression and decreased sterol regulatory element-binding protein 1c expression are associated with reduced intramuscular triglyceride accumulation after insulin therapy in high-fat-diet and streptozotocin-induced diabetic rats. Metabolism 58(6):779–786
Kaplan M, Aviram M, Hayek T (2012) Oxidative stress and macrophage foam cell formation during diabetes mellitus-induced atherogenesis: role of insulin therapy. Pharmacol Ther 136(2):175–185
Zhou H, Li YJ (2012) Rho kinase inhibitors: potential treatments for diabetes and diabetic complications. Curr Pharm Des 18(20):2964–2973
Kanda T, Wakino S, Homma K et al (2006) Rho-kinase as a molecular target for insulin resistance and hypertension. FASEB J 20(1):169–171
Lee SH, Huang H, Choi K et al (2014) ROCK1 isoform-specific deletion reveals a role for diet-induced insulin resistance. Am J Physiol Endocrinol Metab 306(3):E332–E343
Furukawa N, Ongusaha P, Jahng WJ et al (2005) Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab 2(2):119–129
Kikuchi Y, Yamada M, Imakiire T et al (2007) A Rho-kinase inhibitor, fasudil, prevents development of diabetes and nephropathy in insulin-resistant diabetic rats. J Endocrinol 192(3):595–603
Liu PY, Chen JH, Lin LJ, Liao JK (2007) Increased Rho kinase activity in a Taiwanese population with metabolic syndrome. J Am Coll Cardiol 49(15):1619–1624
Noda K, Nakajima S, Godo S et al (2014) Rho-kinase inhibition ameliorates metabolic disorders through activation of AMPK pathway in mice. PLoS ONE 9(11):e110446
Noda K, Godo S, Saito H, Tsutsui M, Shimokawa H (2015) Opposing roles of nitric oxide and rho-kinase in lipid metabolism in mice. Tohoku J Exp Med 235(3):171–183
Tang ST, Zhang Q, Tang HQ, et al (2016) Effects of glucagon-like peptide-1 on advanced glycation endproduct-induced aortic endothelial dysfunction in streptozotocin-induced diabetic rats: possible roles of Rho kinase- and AMP kinase-mediated nuclear factor κB signaling pathways. Endocrine 53(1):107–116
Rachek LI (2014) Free fatty acids and skeletal muscle insulin resistance. Prog Mol Biol Transl Sci 121:267–292
Shao W, Espenshade PJ (2012) Expanding roles for SREBP in metabolism. Cell Metab 16(4):414–419
Krycer JR, Sharpe LJ, Luu W, Brown AJ (2010) The AKT-SREBP nexus: cell signaling meets lipid metabolism. Trends Endocrinol Metab 21(5):268–276
Li Y, Xu S, Mihaylova MM et al (2011) AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 13(4):376–388
Dagon Y, Hur E, Zheng B, Wellenstein K, Cantley LC, Kahn BB (2012) p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin’s effect on food intake. Cell Metab 16:104–112
Fujii N, Jessen N, Goodyear LJ (2006) AMP-activated protein kinase and the regulation of glucose transport. Am J Physiol Endocrinol Metab 291:E867–E877
Hegarty BD, Turner N, Cooney GJ, Kraegen EW (2009) Insulin resistance and fuel homeostasis: the role of AMP-activated protein kinase. Acta Physiol (Oxford, England) 196:129–145
Fujii N et al (2008) Ablation of AMP-activated protein kinase alpha2 activity exacerbates insulin resistance induced by high-fat feeding of mice. Diabetes 57:2958–2966
Sun J, Yang T, Wang P et al (2014) Activation of cold-sensing transient receptor potential melastatin subtype 8 antagonizes vasoconstriction and hypertension through attenuating RhoA/Rho kinase pathway. Hypertension 63(6):1354–1363
Tao W et al (2015) Lipid-induced Muscle Insulin Resistance Is Mediated by GGPPS via Modulation of the RhoA/Rho Kinase Signaling Pathway. J Biol Chem 290:20086–20097
Kanda T, Wakino S, Homma K et al (2006) Rho-kinase as a molecular target for insulin resistance and hypertension. FASEB J 20(1):169–171
Begum N, Duddy N, Sandu O, Reinzie J, Ragolia L (2000) Regulation of myosin-bound protein phosphatase by insulin in vascular smooth muscle cells: evaluation of the role of Rho kinase and phosphatidylinositol-3-kinase-dependent signaling pathways. Mol Endocrinol 14(9):1365–1376
Manning BD (2010) Insulin signaling: inositol phosphates get into the AKT. Cell 143:861–863
Begum N, Ragolia L, Rienzie J, McCarthy M, Duddy N (1998) Regulation of mitogen-activated protein kinase phosphatase-1 induction by insulin in vascular smooth muscle cells. Evaluation of the role of the nitric oxide signaling pathway and potential defects in hypertension. J Biol Chem 273:25164–25170
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China Grant Award (81570736, 81270906, 81570737, 81370947, 81500612, 81500630, 81400832, 81300651, 81600632, 81600637), the Project of National Key Clinical Division, the China Postdoctoral Science Foundation (2012M521050), Jiangsu Province’s Key Discipline of Medicine (XK201105), Jiangsu Province’s Key Provincial Talents Program (RC2011011), Jiangsu Key Laboratory for Molecular Medicine (BM2007208), Jiangsu Province’s Project of Standardized Diagnosis and Treatment of Key Diseases (2015604), Jiangsu Postdoctoral Science Foundation, the Key Project of Nanjing Clinical Medical Science, Nanjing Outstanding Youth Fund Projects (JQX13010), Nanjing Science and Technology Development Projects (2013ZD005), and Medical and Health Research Projects of Nanjing Health Bureau (YKK14055, YKK11092), 2016 China Diabetes Young Scientific Talent Research Project.
Author contributions
Yan Bi and Dalong Zhu contributed to the study design, data interpretation, and final approval of the version to be published. Sunyinyan Tang and Wenjun Wu wrote the main manuscript text and contributed to the acquisition of data and approval of the final version. Wenjuan Tang, Zhijuan Ge, and Ting Hong prepared Figures 1–4 and contributed to acquisition of data. All authors reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Sunyinyan Tang, Wenjun Wu, Wenjuan Tang, Zhijuan Ge, Ting Hong, Dalong Zhu, and Yan Bi declare that they have no competing financial interests.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Statement of human and animal rights
All animal procedures were performed according to the National Institutes of Health guidelines and approved by the animal care committee of Drum Tower Hospital affiliated with Nanjing University Medical School (Nanjing, China).
Informed consent
Our study is not clinical trial, so there is no informed consent obtained from individual participants included in the study.
Additional information
Managed by Massimo Porta.
The authors Sunyinyan Tang and Wenjun Wu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, S., Wu, W., Tang, W. et al. Suppression of Rho-kinase 1 is responsible for insulin regulation of the AMPK/SREBP-1c pathway in skeletal muscle cells exposed to palmitate. Acta Diabetol 54, 635–644 (2017). https://doi.org/10.1007/s00592-017-0976-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-017-0976-z