Abstract
Aims
Diabetic nephropathy (DN) is a chronic inflammatory disease that is accompanied by different degrees of lipid disorders. The present study was conducted to determine whether inflammatory stress exacerbates lipid accumulation in podocytes and to investigate its underlying mechanisms in DN using in vitro and in vivo studies.
Methods
We used IL-1β stimulation in podocytes in vitro and casein injections in db/db mice in vivo to induce inflammatory stress. The plasma levels of serum inflammatory cytokines were determined using an enzyme-linked immunosorbent assay. The renal pathology was evaluated using pathological staining and electron microscopy. Intracellular lipid accumulation was evaluated by Oil Red O staining and a cholesterol quantitative assay. The gene and protein expression levels of extracellular matrix proteins, biomarkers of podocyte injury, and molecules involved in the LDLr pathway were evaluated using immunofluorescence staining, real-time PCR, and western blot analysis.
Results
Increased plasma levels of inflammatory cytokines in the casein-injected db/db mice indicated a successful induction of the inflamed DN model. The kidney morphological changes, podocyte injury, and epithelial mesenchymal transition (EMT) were more significant in casein-injected db/db mice. Moreover, inflammation increased the lipid droplet accumulation in the kidneys of db/db mice, which resulted from the increased protein expression levels of LDLr, sterol regulatory element-binding protein (SREBP) cleavage-activating protein (SCAP), and SREBP-2 in the kidneys of db/db mice. The in vitro studies further demonstrated that inflammation increased the lipid accumulation in the podocytes and induced podocyte EMT, which were correlated with inflammation-mediated increases in the expression levels of LDLr, SCAP, and SREBP-2, and increased translocation of the SCAP/SREBP-2 complex from the endoplasmic reticulum to the Golgi in the podocytes.
Conclusion
Inflammation induced lipid accumulation and the EMT of podocytes through the dysregulation of the LDLr pathway, which contributed to podocyte injury and accelerated the progression of DN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic nephropathy (DN) is the largest single cause of end-stage renal disease (ESRD) and is a medical condition with great impact worldwide [1]. DN results in reduced filtration, albuminuria, and ultimately renal failure [2]. It is shown that the main histological features of the disease are glomerular basement membrane thickening and mesangial extracellular matrix expansion which lead to fibrosis [3]. Although there are some methods to detect early changes in diabetic kidney [4], effective therapeutic interventions and improving outcomes are disappointing. Therefore, it is necessary to understand the mechanism of pathophysiology of DN and research new therapies [5, 6].
Several factors are involved in the pathophysiology of DN, including metabolic and hemodynamic alterations, oxidative stress, and activation of the renin–angiotensin system. Strict glycemic control and renin–angiotensin system inhibition delay the progression of DN but do not prevent ESRD. Recent studies have shown that kidney inflammation is crucial for promoting the development and progression of DN [7]. The expression levels of proinflammatory cytokines, such as C-reactive protein (CRP), tumour necrosis factor (TNF-α), and interleukin (IL-6), are increased in DN and correlate with albuminuria [8]. The activity of these cytokines amplifies the inflammatory response and promotes cell injury and the development of fibrosis [7].
Additionally, abnormal lipid metabolism and the renal accumulation of lipids have also been proposed to play a role in the pathogenesis of DN [9]. In recent years, a number of studies have shown that the histological features of glomerulosclerosis are similar to the changes observed in atherosclerosis (AS), and the term “glomerular atherosclerosis” has been proposed [10, 11]. However, the mechanisms underlying lipid accumulation have not been fully elucidated. The low-density lipoprotein receptor (LDLr) is of primary importance in the binding and internalisation of plasma-derived LDL cholesterol and in regulating plasma LDL concentrations. It is well known that the LDLr pathway is a feedback system that plays important roles in regulating plasma and intracellular cholesterol homoeostasis and is mainly modulated by the intracellular cholesterol concentration and the interaction between sterol regulatory element-binding protein (SREBP) and SREBP cleavage-activating protein (SCAP) [12]. Cholesterol deficiency increases the translocation of SCAP from the endoplasmic reticulum (ER) to the Golgi, where it cleaves SREBP and thus increases LDLr gene expression.
Recent studies have demonstrated that inflammation and dyslipidemia act synergistically in organ injury [9]. Our previous studies demonstrated that inflammation accelerates the progression of AS by disrupting LDLr feedback regulation [13, 14]. The present study was performed to evaluate whether inflammation exacerbates podocyte injury by increasing lipid accumulation and to explore the underlying mechanisms.
Materials and methods
Inflamed diabetic animal model
Male db/db mice in a C57BL/KsJ genetic background obtained from the National Mode Animal Centre of Nanjing University (Nanjing, China) were studied using protocols approved by the Ethical Committee of Southeast University and following the latest version of the Declaration of Helsinki. Eight-week-old male diabetic db/db mice were randomly divided into two groups and subcutaneously injected with either 0.5 ml distilled water (control, n = 10) or 0.5 ml 10 % casein (inflamed group, n = 10) every other day. The mice were maintained under a constant 12-h photoperiod at a temperature between 21 and 23 °C and allowed free access to food and water. Eight-week-old male mice were fed a normal chow diet containing 4 % fat for 8 weeks. Individual mice were placed in metabolic cages for 24-h urine collection. After 8 weeks, blood samples were collected for the biochemical assays, and kidney samples were used for histological evaluations.
Biochemical assays
At the end of the experimental period, the mice were euthanised, and blood samples were collected from the right ventricle for biochemical analysis. Serum concentrations of blood urea nitrogen (BUN), serum creatinine (Scr), triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL), and LDL were determined using automatic analysers (Hitachi, Japan). The quantitative analysis of 24-h urinary protein was conducted using the Lowry assay.
Morphological analysis
Kidneys were either fixed in 10 % buffered formalin and embedded in paraffin (for light microscopy) or fixed in 2.5 % glutaraldehyde and embedded in Lowicryl K4 M resin (for electron microscopy). Other procedures included periodic acid–Schiff (PAS) staining, Masson’s trichrome staining, and transmission electron microscopy.
Immunohistochemical staining
The paraffin-embedded tissue samples were sectioned at a thickness of 3 μm. The sections were then placed in citrate-buffered solution (pH 6.0) and heated for antigen retrieval. The endogenous peroxidase activity was blocked by incubating the tissue with 3 % hydrogen peroxide, and non-specific binding was blocked by incubating the tissue with 10 % normal non-immune serum. Then, the sections were incubated with anti-mouse primary antibodies against LDLr, SREBP-2, and SCAP (Santa Cruz, USA) overnight at 4 °C, followed by incubation with biotinylated secondary antibody. Finally, diaminobenzidine tetrahydrochloride substrate was used to produce the colour reaction. The results of this staining were observed under a light microscope (400× magnification).
Immunofluorescence staining
The kidney sections were fixed with 4 % paraformaldehyde and blocked in PBS containing 10 % BSA (bovine serum albumin) for 60 min. The sections were incubated with rabbit, goat, mouse, or mouse anti-mouse primary antibodies against Wilms tumour 1 (WT-1), nephrin, LDLr, and α-smooth muscle actin (α-SMA) (Santa Cruz, USA) overnight at 4 °C, followed by goat anti-rabbit Fluor 594, donkey anti-rabbit Fluor 488, goat anti-mouse Fluor 488, and donkey anti-goat Fluor 488 secondary antibodies (Invitrogen, USA), respectively. After washing, the slides were examined using laser confocal microscopy (400×).
Cell culture
A mouse conditionally immortalised podocyte cell line (kindly provided by Peter Mundel at Mount Sinai School of Medicine) was cultured and induced to differentiate as previously described [15]. Briefly, cells were cultured on dishes coated with type I collagen (BD, USA) and in RPMI 1640 medium supplemented with 10 % foetal bovine serum, 100 U/ml penicillin, and 100 U/ml streptomycin at 33 °C in the presence of 10 U/ml mouse recombinant interferon-γ (Sigma, USA). To induce differentiation, we grew podocytes under non-permissive conditions at 37 °C in the absence of interferon-γ for 10–14 days as previously described. At 70–80 % confluence, the cells were synchronised with a serum-free culture medium for 24 h and then subsequently incubated with 30 mmol/l glucose (Sigma, USA) and stimulated with 30 µg/ml cholesterol (Sigma, USA), 5 ng/ml IL-1β (R&D, USA), or 30 µg/ml cholesterol plus 5 ng/ml IL-1β.
Observation of lipid accumulation
The lipid accumulation in cultured podocytes and the kidneys of db/db mice were evaluated by Oil Red O staining. Briefly, samples were fixed with 4 % paraformaldehyde solution and then stained with Oil Red O for 30 min. Finally, the samples were counterstained with haematoxylin for 5 min. The results were examined by light microscopy.
Quantitative measurement of intracellular free cholesterol/cholesterol ester
Quantitative measurement of intracellular total and free cholesterol in vitro and in vivo was conducted using the method described by Gamble et al. [16]. Briefly, samples were collected, and lipids were extracted by adding 1 ml of chloroform/methanol (2:1). After sonication, the samples were centrifuged, and the lipid phase was collected. The samples were dried in a vacuum and then dissolved in 2-propanol containing 10 % Triton X-100. Cholesterol ester was converted to free cholesterol by cholesterol ester hydrolase (Sigma, USA) to determine the amount of total cholesterol. Concentrations of total and free cholesterol per sample were analysed using a standard curve and normalised to the total cell protein. The concentration of cholesterol ester was calculated by subtracting the amount of free cholesterol from the total cholesterol.
Confocal microscopy
Podocytes cultured in chamber slides were washed, fixed, and permeabilised. The cells were then incubated with primary antibodies against nephrin, α-SMA, fibronectin, collagen I, SCAP, and Golgi (Santa Cruz, USA), followed by incubation with secondary fluorescent antibodies (Invitrogen, USA). After washing, the cells were examined by confocal microscopy. Non-immune immunoglobulin G served as a negative control.
Real-time reverse transcription polymerase chain reaction
Total RNA was isolated from cells using the guanidinium–phenol–chloroform method. Real-time reverse transcription PCR was performed in an ABI7300 Sequence Detection System using SYBR Green dye in accordance with the manufacturer’s protocol. All TaqMan primers were designed using the Primer Express Software V2.0 (Applied Biosystems, UK) (Table 1).
Western blot analysis
Equal amounts of total protein from cell extracts or kidney homogenates from mice were denatured and then subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis. Gel transfer was performed, and the membranes were blocked with blocking buffer for 1 h at room temperature. The membranes were then incubated overnight at 4 °C with anti-mouse antibodies against nephrin, α-SMA, fibronectin, collagen I, LDLr, SCAP, and SREBP-2 (Santa Cruz, USA). This incubation was then followed by incubation with horseradish peroxidase-conjugated secondary antibodies for 1 h at 4 °C. Finally, the signals were detected using the ECL Advance system (Amersham Biosciences, USA).
Data analysis
All of the data are presented as the mean ± standard deviation (SD) and were analysed with SPSS 16.0. In all experiments, data were evaluated for statistical significance using one-way analysis of variance followed by Q test. The correlations between variables were analysed by Spearman’s rank-order correlation. A difference was considered significant if the P value was less than 0.05.
Results
Inflamed db/db mice were made by subcutaneously injecting with 10 % casein every other day according to our previous method [17]. Our result showed that there were significantly increased serum levels of inflammatory cytokines and the protein expression of inflammatory cytokines in the kidneys of casein-injected mice compared with the control mice, indicating that systemic and local inflammation were successfully induced (Fig. 1a–c).
The levels of inflammatory cytokines in two groups of mice. Eight-week-old male diabetic db/db mice were randomly divided into two groups; every other day, mice were subcutaneously injected with 0.5 ml of distilled water (control group) or 0.5 ml of 10 % casein (inflamed group) (n = 10). At the end of the experimental period, the levels of SAA and TNF-α in the serum were measured by ELISA (a, b). Values were represented as the mean ± SD *P < 0.01 versus control. The protein expression of TNF-α and MCP-1 in kidneys of db/db mice were measured by immunohistochemical staining (c, brown colour, original magnification ×400)
Inflammation exacerbated the damage to the kidney in db/db mice
There was a significant increase in Scr and a decrease in the creatinine clearance rate (CCr) in the inflamed group compared with the control group (Fig. 2a, b). As shown in Fig. 2c–f, the PAS staining and Masson’s trichrome staining revealed that both the control and inflamed mice developed glomerular damage. Furthermore, the casein injection intensified these changes in the kidneys of the db/db mice, which exhibited significant increases in glomerular hypercellularity, mesangial expansion, and collagen deposition compared with the control mice, which were further confirmed by the quantitative analysis of positive staining using Image J software. The transmission electron microscopy analysis showed that the inflamed db/db mice have a more significantly thickened glomerular basement membrane (GBM) and podocyte foot process fusion compared with the control mice (Fig. 2g). The 24-h urine protein level in the inflamed group was significantly higher and continuously increased compared with the controls as diabetes progressed (Fig. 2h). These findings suggest that chronic inflammation accelerates the progression of DN.
Inflammation exacerbated the damage in the kidneys of db/db mice. Eight-week-old male diabetic db/db mice were randomly divided into two groups; every other day, mice were subcutaneously injected with 0.5 ml of distilled water (control group) or 0.5 ml of 10 % casein (inflamed group) (n = 10). BUN blood urea nitrogen, Scr serum creatinine (a), Ccr creatinine clearance rate (b).The histopathological changes were assessed using PAS staining (c, red colour, original magnification ×400). Masson’s trichrome (d, blue colour, original magnification ×400). The values from the semi-quantitative analysis for the positive areas in PAS staining are expressed as the mean ± SD from the mice in each group (e). *P < 0.05 versus control. The values from the semi-quantitative analysis for the positive areas in Masson staining are expressed as the mean ± SD from mice at each group (f). The change in the glomerular ultramicrostructure was evaluated by electron microscopy (g, original magnification ×1000). The quantitative analysis of 24-h urinary protein was conducted using the Lowry assay (H). *P < 0.05 versus control
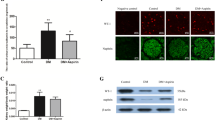
Inflammation increased lipid deposition in the kidney in vivo and in podocytes in vitro
To investigate the potential mechanisms underlying the intensified kidney injury observed under inflammation in the db/db mice, we evaluated the effect of inflammation on lipid metabolism in the kidneys in vivo and in vitro. We first evaluated the effect of inflammation on the serum levels of total cholesterol, triglycerides, LDL, and HDL. The results showed that there were no differences in the serum lipid levels (Fig. 3a). Using Oil Red O staining, we found that inflammation induced by casein injection increased the lipid accumulation in the kidneys of the diabetic mice (Fig. 3b). The cholesterol quantitative assay further demonstrated that there was a significant accumulation of cholesterol ester in the kidneys of the inflamed db/db mice compared with the controls (Fig. 3c). Next, we investigated the effects of inflammation on lipid accumulation in the podocytes in vitro. Oil Red O staining showed that there was an increase in lipid droplets in podocytes in the presence of cholesterol or IL-1β. There was more significant lipid droplet accumulation in the cholesterol plus IL-1β-treated cells compared with the cholesterol alone group (Fig. 3d). Further quantitative analysis of the intracellular cholesterol confirmed the results from the Oil Red O staining (Fig. 3e). These findings suggest that inflammatory stress increases cholesterol accumulation in podocytes.
Inflammation increased lipid deposition in the kidney in vivo and in podocytes in vitro. Eight-week-old male diabetic db/db mice were randomly divided into two groups; every other day, mice were subcutaneously injected with 0.5 ml of distilled water (control group) or 0.5 ml of 10 % casein (inflamed group) (n = 10). TG triglyceride, TC total cholesterol, LDL low-density lipoprotein, HDL high-density lipoprotein (a) The tissues were examined for lipid accumulation using Oil Red O staining (b, original magnification ×400). The concentration of cholesterol ester in the livers and kidneys was measured as described in Sect. 2 (c). The results are presented as the mean ± SD. *P < 0.01 versus control. Podocytes were made quiescent using serum-free medium for 24 h and treated with 30 mmol/l glucose (HG) and then incubated with 30 μg/ml cholesterol, 5 ng/ml IL-1β, or 30 μg/ml cholesterol plus 5 ng/ml IL-1β. The cells were examined for lipid accumulation using Oil Red O staining (d, magnification ×400). Free cholesterol and cholesterol ester were assayed as described in Sect. 2 (e). The results are presented as the mean ± SD. *P < 0.05 versus HG, # P < 0.05 versus CHO
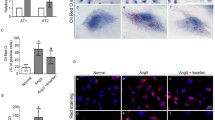
Inflammation disrupted LDLr feedback regulation in podocytes
To explore the mechanisms underlying the lipid accumulation mediated by inflammation in the kidneys of db/db mice, we studied the effects of inflammation on the LDLr pathway, which mediates cholesterol uptake. Western blot analysis showed that there were increases in the protein expression levels of LDLr, SCAP, and SREBP-2 in the inflamed mice compared with the controls (Fig. 4a, b). Co-staining of the LDLr and WT-1, a specific biomarker of podocytes, showed increased co-localisation of the LDLr and WT-1 signals (Fig. 4c). This observation revealed that the LDLr is most abundantly expressed in the podocytes in the diabetic glomeruli. The in vitro results showed that cholesterol significantly inhibited LDLr expression in the podocytes. However, IL-1β increased LDLr, SCAP, and SREBP-2 expression in the absence or presence of cholesterol loading in the podocytes (Fig. 4d–f). Using dual immunofluorescence staining with anti-human antibodies against SCAP and Golgi, we demonstrated that cholesterol inhibited the translocation of the SCAP/SREBP-2 complex from the ER to the Golgi, whereas inflammation negated these changes induced by cholesterol loading (Fig. 4g). These in vitro and in vivo results suggest that inflammation disrupts the feedback regulation of the LDLr pathway and then induces lipid accumulation in the kidneys and podocytes.
Inflammation disrupted LDLr feedback regulation in podocytes. Eight-week-old male diabetic db/db mice were randomly divided into two groups; every other day, mice were subcutaneously injected with 0.5 ml of distilled water (control group) or 0.5 ml of 10 % casein (inflamed group) (n = 10). The protein expression levels of LDLr, SCAP, and SREBP-2 in the kidneys of db/db mice were measured using western blot analysis (a). The histogram represents the mean ± SD of the densitometric scans of the protein bands from mice normalised to β-actin (b). *P < 0.05 versus control. The expression of LDLr in the podocytes of db/db mice was observed by immunofluorescence staining (c, magnification ×400). Podocytes were made quiescent using serum-free medium for 24 h and treated with 30 mmol/l glucose (HG) and then incubated with 30 μg/ml cholesterol, 5 ng/ml IL-1β, or 30 μg/ml cholesterol plus 5 ng/ml IL-1β. Real-time PCR (d) and western blot analysis (e) were conducted to evaluate the LDLr, SCAP, and SREBP-2 expression levels in the podocytes. The histogram represents the mean ± SD of the densitometric scans of the protein bands from the cells normalised to β-actin (f). The translocation of SCAP from the ER to the Golgi in the podocytes was evaluated by confocal microscopy (g, magnification ×400). *P < 0.05 versus HG. The results are presented the mean ± SD. *P < 0.05 versus HG, # P < 0.05 versus HG + CHO
Inflammation accelerated lipid-mediated podocyte injuries
To elucidate the possible synergistic mechanisms involving inflammation and lipid disorder in glomerulosclerosis, we evaluated the effects of inflammation and lipid disorder on the phenotype and function of podocytes. As shown in Fig. 5a, there is a significant decrease in the number of WT-1-positive cells in the glomeruli of inflamed mice compared with the controls. Immunofluorescence staining and western blot analyses demonstrated that inflammation significantly increased the expression of α-SMA but decreased nephrin protein expression in the diabetic mice (Fig. 5a–d). Furthermore, immunofluorescence staining revealed that there was increased α-SMA protein expression in the WT-1-positive cells of the inflamed mice (Fig. 5b). Correlation analysis demonstrated that LDLr protein expression was negatively associated with nephrin expression (r = −0.884, p < 0.01) and positively associated with α-SMA expression (r = 0.882, p < 0.01). Consistent with the results from the in vivo studies, the in vitro studies demonstrated that there were significant increases in the expression levels of fibronectin, collagen I, and α-SMA, but decreased nephrin expression in cholesterol-loaded podocytes in the presence of IL-1β. There was more significant extracellular matrix (ECM) expression in the IL-1β-treated cells in the presence of cholesterol loading compared with the cholesterol-loading or IL-1β alone group, suggesting that inflammation accelerated lipid disorder-mediated podocyte injury (Fig. 5e–h).
Inflammation accelerated lipid-mediated podocyte injuries. Eight-week-old male diabetic db/db mice were randomly divided into two groups; every other day, mice were subcutaneously injected with 0.5 ml of distilled water (control group) or 0.5 ml of 10 % casein (inflamed group) (n = 10). The protein expression levels of WT-1 (red), nephrin (green), and α-SMA (green) were evaluated by immunofluorescence staining (a, b, original magnification ×400) and western blot analysis (c). The histogram represents the mean ± SD of the densitometric scans of the protein bands from mice normalised to β-actin (d). *P < 0.05 versus Control. Podocytes were made quiescent by serum-free medium for 24 h and treated with 30 mmol/l glucose (HG) and then incubated with 30 μg/ml cholesterol, 5 ng/ml IL-1β, or 30 μg/ml cholesterol plus 5 ng/ml IL-1β. Immunofluorescence staining (e), real-time PCR (f), and western blot analysis (g) were conducted to evaluate the nephrin, fibronectin, collagen I, and α-SMA expression levels in podocytes. The histogram represents the mean ± SD of the densitometric scans of the protein bands from the cells normalised to β-actin (h). The results are presented as the mean ± SD. *P < 0.05 versus HG, # P < 0.05 versus CHO
Discussion
Inflammation is a critical event in the progression of DN and may exacerbate podocyte injury [17]. Dyslipidemia is a major factor responsible for coronary heart disease, and its reduction decreases coronary risk in patients with diabetes mellitus [18]. Our previous studies demonstrated that inflammation disrupts LDLr-negative feedback regulation to accelerate foam cell formation among the vascular smooth muscle cells and mesangial cells [19, 20]. This study was conducted to investigate the possible synergistic effects of inflammation with lipid disorder on podocyte injury in DN.
First, we established an inflamed animal model of DN using db/db mice subcutaneously injected with 10 % casein based on our previous study [14]. Results showed that serum levels of inflammatory cytokines and their protein expression in the kidneys were significantly increased [17]. Interestingly, inflammation exacerbated renal dysfunction, proteinuria, and glomerular structural abnormalities, specifically, podocyte foot process fusion, GBM thickening, and endothelial cell detachment.
Recent studies showed that inflammation is an aggravating factor in lipid-mediated peripheral cell injury, such as atherogenesis. Interestingly, glomerulosclerosis has many similarities to atherosclerosis [21, 22]. To investigate the potential connection between inflammation and lipid disorder in the glomerulosclerosis of DN mice, we evaluated lipid metabolism in the kidney. Oil Red O staining revealed massive lipid accumulation in the kidneys of the casein-injected mice compared with the controls. The quantitative assay confirmed that there was a significant increase for cholesterol ester in the kidneys of inflamed db/db mice. These results suggest that there is a strong association between enhanced lipid accumulation in the kidney and inflammatory stress.
Podocyte dysfunction is a critical event associated with proteinuria and glomerulosclerosis in DN [23]. In our study, Oil Red O staining showed that inflammatory cytokines significantly increased the intracellular lipid accumulation in podocytes. This finding may be correlated with the imbalance in cholesterol homoeostasis mediated by inflammation in podocytes. It is well known that LDLr is of primary importance in the binding and internalisation of plasma-derived LDL cholesterol and in regulating the plasma LDL concentration [24]. Changes in LDLr activity alter the rate of LDL uptake by the kidney with a corresponding increase or decrease in the amount of lipids in the cells. Using cell culture experiments, we demonstrated that cholesterol significantly inhibited LDLr expression in podocytes. However, IL-1β increased LDLr expression in the absence or presence of cholesterol loading in podocytes. Furthermore, IL-1β increased the SCAP and SREBP-2 mRNA and protein expression levels in the absence or presence of cholesterol loading. Using dual immunofluorescence staining, we investigated SCAP/SREBP-2 complex translocation from the ER to the Golgi in podocytes. Confocal microscopy analysis showed that cholesterol loading decreased SCAP/SREBP-2 complex accumulation in the Golgi. Interestingly, IL-1β stimulation increased the translocation of the SCAP/SREBP-2 complex from the ER to the Golgi even in the presence of cholesterol loading. These in vitro results were further supported by the results from the in vivo studies, suggesting that inflammation disrupted LDLr-negative feedback regulation at the transcriptional level.
Ma et al. [25] reported that lipid loading promoted cell growth and phenotypic alterations in human renal mesangial cells with increased accumulation of collagen I, α-SMA, and fibronectin. Recent evidence suggests that podocytes may also undergo a phenotypic conversion after stimulation, which is characterised by a loss of its epithelial features, such as nephrin, P-cadherin, and ZO-1 expression, while acquiring mesenchymal markers, such as desmin, fibroblast-specific protein-1, matrix metalloproteinase-9, type I collagen, and fibronectin [26–28]. Our results demonstrated that inflammation upregulated the protein expression of α-SMA, but decreased the expression of nephrin and WT-1 in the kidneys of db/db mice. The correlation analysis showed that LDLr protein expression was positively correlated with α-SMA protein expression and negatively correlated with nephrin expression. Using in vitro studies, we demonstrated that lipid loading promoted the expression of collagen I, α-SMA, and fibronectin and decreased the expression of nephrin in podocytes. Interestingly, IL-1β aggravated the phenotypic conversion of podocytes mediated by lipid loading.
This study is the first to demonstrate that inflammation induces lipid accumulation by disrupting the LDLr pathway and induced phenotypic change and dysfunction in podocytes. The relationship between inflammation and lipid disorder accelerates the progression of glomerulosclerosis, suggesting that the management of cholesterol by statins should not be the only component of the treatment regimen. Inhibition of both inflammatory cytokines and their activators/regulators may provide more comprehensive treatment for DN.
References
Dronavalli S, Duka I, Bakris GL (2008) The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab 4(8):444–452
Graves DT, Kayal RA (2008) Diabetic complications and dysregulated innate immunity. Front Biosci 13:1227–1239
Fiorentino L, Cavalera M, Mavilio M, Conserva F, Menghini R, Gesualdo L et al (2013) Regulation of TIMP3 in diabetic nephropathy: a role for microRNAs. Acta Diabetol 50(6):965–969
Fiorina P, Bassi R, Gremizzi C, Vergani A, Caldara R, Mello A et al (2012) 31P-magnetic resonance spectroscopy (31P-MRS) detects early changes in kidney high-energy phosphate metabolism during a 6-month Valsartan treatment in diabetic and non-diabetic kidney-transplanted patients. Acta Diabetol 49(Suppl 1):S133–S139
Atkins RC, Zimmet P (2010) Diabetic kidney disease: act now or pay later. Acta Diabetol 47(1):1–4
D’Addio F, Trevisani A, Ben NM, Bassi R, El EB, Abdi R et al (2014) Harnessing the immunological properties of stem cells as a therapeutic option for diabetic nephropathy. Acta Diabetol 51(6):897–904
Lim AK, Tesch GH (2012) Inflammation in diabetic nephropathy. Mediators Inflamm 2012:146154
Navarro-Gonzalez JF, Mora-Fernandez C (2008) The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 19:433–442
Keane WF (2000) The role of lipids in renal disease: future challenges. Kidney Int Suppl 75:S27–S31
Coritsidis G, Rifici V, Gupta S, Rie J, Shan ZH, Neugarten J et al (1991) Preferential binding of oxidized LDL to rat glomeruli in vivo and cultured mesangial cells in vitro. Kidney Int 39(5):858–866
Wang Z, Jiang T, Li J, Proctor G, McManaman JL, Lucia S et al (2005) Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes 54(8):2328–2335
Goldstein JL, Brown MS (1985) The LDL receptor and the regulation of cellular cholesterol metabolism. J Cell Sci 3:131–137
Ma KL, Ruan XZ, Powis SH, Moorhead JF, Varghese Z (2007) Anti-atherosclerotic effects of sirolimus on human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 292(6):H2721–H2728
Ruan XZ, Moorhead JF, Tao JL, Ma KL, Wheeler DC, Powis SH et al (2006) Mechanisms of dysregulation of low-density lipoprotein receptor expression in vascular smooth muscle cells by inflammatory cytokines. Arterioscler Thromb Vasc Biol 26:1150–1155
Mundel P, Reiser J, Kriz W (1997) Induction of differentiation in cultured rat and human podocytes. J Am Soc Nephrol 8(5):697–705
Gamble W, Vaughan M, Kruth HS, Avigan J (1978) Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res 19(8):1068–1070
Ma KL, Zhang Y, Liu J, Wu Y, Hu ZB, Ruan XZ et al (2014) Establishment of an inflamed animal model of diabetic nephropathy. Int J Biol Sci 10(2):149–159
Krauss RM (2004) Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care 27(6):1496–1504
Chen Y, Ruan XZ, Li Q, Huang A, Moorhead JF, Powis SH et al (2007) Inflammatory cytokines disrupt LDL-receptor feedback regulation and cause statin resistance: a comparative study in human hepatic cells and mesangial cells. Am J Physiol Renal Physiol 293:F680–F687
Ma KL, Ruan XZ, Powis SH, Chen Y, Moorhead JF, Varghese Z (2008) Inflammatory stress exacerbates lipid accumulation in hepatic cells and fatty livers of apolipoprotein E knockout mice. Hepatology 48(3):770–781
Diamond JR, Karnovsky MJ (1988) Focal and segmental glomerulosclerosis: analogies to atherosclerosis. Kidney Int 33(5):917–924
Moorhead JF, Chan MK, El-Nahas M, Varghese Z (1982) Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet 2(8311):1309–1311
Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S et al (2011) mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest 121(6):2181–2196
Ma KL, Ruan XZ, Powis SH, Chen Y, Moorhead JF, Varghese Z (2007) Sirolimus modifies cholesterol homeostasis in hepatic cells: a potential molecular mechanism for sirolimus-associated dyslipidemia. Transplantation 84(8):1029–1036
Ma KL, Ni J, Wang CX, Liu J, Zhang Y, Wu Y et al (2013) Interaction of RAS activation and lipid disorders accelerates the progression of glomerulosclerosis. Int J Med Sci 10(12):1615–1624
Li Y, Kang YS, Dai C, Kiss LP, Wen X, Liu Y (2008) Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol 172(2):299–308
Reidy K, Susztak K (2009) Epithelial-mesenchymal transition and podocyte loss in diabetic kidney disease. Am J Kidney Dis 54(4):590–593
Yamaguchi Y, Iwano M, Suzuki D, Nakatani K, Kimura K, Harada K et al (2009) Epithelial-mesenchymal transition as a potential explanation for podocyte depletion in diabetic nephropathy. Am J Kidney Dis 54(4):653–664
Acknowledgments
We would like to thank Professor Ai Hua Zhang from Nanjing Medical University for his kind support of the experiments. This work was supported by the National Natural Science Foundation of China (Grants 81070571, 81170792, and 81470957), the Natural Science Foundation of Jiangsu Province (BK20141343), and the Clinical Medical Science Technology Special Project of Jiangsu Province (BL2014080).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
This study was carried out in accordance with the principles of the Declaration of Helsinki of 1975, as revised in 2008.
Human and animal rights
This article does not contain any studies with human subjects performed by the any of the authors.
Informed consent
This study does not involve human subjects. No informed consent needs to be obtained.
Author information
Authors and Affiliations
Corresponding author
Additional information
Managed by Antonio Secchi.
Rights and permissions
About this article
Cite this article
Zhang, Y., Ma, K.L., Liu, J. et al. Inflammatory stress exacerbates lipid accumulation and podocyte injuries in diabetic nephropathy. Acta Diabetol 52, 1045–1056 (2015). https://doi.org/10.1007/s00592-015-0753-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-015-0753-9