Abstract
Aim
This study aimed to investigate the effects of aspirin on podocyte injury and its underlying mechanisms in diabetic nephropathy (DN).
Methods
Eight-week-old male Sprague–Dawley rats were divided into three groups: non-diabetic rats (Control), streptozotocin-induced diabetic rats (DM), and diabetic rats treated with aspirin (DM + Aspirin) for 12 weeks. Intracellular lipid accumulation was evaluated by Oil Red O staining and quantitative free cholesterol assays. Podocyte injury and the levels of COX-2, inflammatory cytokines, and low-density lipoprotein receptor (LDLr) pathway-related proteins were evaluated by electron microscopy, immunohistochemical staining, and Western blotting, respectively.
Results
Lipid levels and urinary albumin–creatinine ratios were higher in the DM rats than in the Control rats. Periodic acid-Schiff staining showed glomerular hypertrophy and mild mesangial area widening in the DM rats. Electron microscopy showed that the podocyte foot processes were significantly flattened or absent in the DM rats. The protein expression levels of WT-1 and nephrin in the podocytes of DM rats were reduced. Interestingly, lipid accumulation in the kidneys of DM rats was significantly increased due to increased protein expression levels of LDLr, sterol regulatory element-binding protein (SREBP) cleavage-activating protein (SCAP), SREBP-2, cyclooxygenase-2 (COX-2), and inflammatory cytokines. Confocal immunofluorescent staining showed that COX-2 and WT-1 were co-expressed. Furthermore, COX-2 protein expression levels were positively correlated with LDLr protein expression levels. However, when COX-2 expression was inhibited by aspirin, these changes in the DM rats were significantly attenuated.
Conclusion
Aspirin attenuates podocyte injury in DN, which may be through COX-2-mediated dysregulation of LDLr pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic nephropathy (DN) is one of the most serious complications of diabetes mellitus (DM) and is the leading cause of end-stage renal disease (ESRD) in developed countries [1]. Therefore, it is important to elucidate the pathogenesis of early diabetic nephropathy and develop therapeutic strategies. The pathogenesis and development of DN are highly complex due to the involvement of multiple interlinked mechanisms. Podocyte injury plays a significant role in the early stage of DN; however, the mechanism underlying podocyte injury has not yet been fully elucidated.
Lipid disorders are traditional independent risk factors for the development and progression of DN [2]. It is well-known that lipid disorders are mainly related to the dysregulation of plasma lipid homeostasis and intracellular cholesterol homeostasis. The low-density lipoprotein receptor (LDLr) pathway is one of the vital mechanisms that regulate plasma and intracellular cholesterol homeostasis. When intracellular cholesterol concentrations are too high, sterol regulatory element-binding protein (SREBP), cleavage-activating protein (SCAP), and SREBP-2 form a complex in the endoplasmic reticulum (ER). Then, SREBP-2 cannot be translocated into the Golgi for cleavage activation; this action results in decreased LDLr gene expression levels. Conversely, when intracellular cholesterol levels are low, SCAP escorts SREBP-2 from the ER to the Golgi to increase the production of nuclear SREBP-2; these actions upregulate LDLr transcription. The dysregulation of LDLr expression results in abnormal lipid accumulation in cells and tissues [3].
Cyclooxygenase (COX), also called prostaglandin-endoperoxide synthase, is an enzyme that is responsible for the formation of prostanoids, including thromboxane and prostaglandins. Two isoforms of COX have been identified, COX-1 and COX-2. COX-1 is constitutively expressed in most tissues. In contrast, COX-2 is an inducible enzyme with low or undetectable levels in most tissues, and its expression levels can be markedly increased by a number of stimuli, such as inflammatory cytokines or physiological stimuli. Although it is considered to be an inducible enzyme, COX-2 is constitutively expressed in the macula densa, podocytes, mesangial cells, and endothelial cells [4, 5]. Both COX isoforms metabolize arachidonic acid to generate prostaglandins and thromboxanes. The binding of prostaglandins to their corresponding receptors can generate various proinflammatory factors, such as IL-1β, IL-6, and TNF-α [6,7,8].
Some studies have indicated that COX-2 overexpression contributes to podocyte injury in DN [9,10,11], whereas other studies have shown that the use of COX-2 inhibitors can ameliorate podocyte lesions [12,13,14]. Our previous studies demonstrated that chronic inflammation and hyperglycaemia disrupted LDLr feedback regulation to induce lipid accumulation and podocyte injury in the kidneys of diabetic mice [15]. Since aspirin is a non-specific inhibitor of COX-2 activation, this study aimed to investigate the role of aspirin in podocyte injury in streptozotocin-induced diabetic rats and its underlying mechanisms in the treatment of DN.
Materials and methods
Animal model
Specific pathogen-free healthy male Sprague–Dawley rats (Shanghai Bikai Animal Company, China) that initially weighed 150–200 g were utilized to generate a diabetes model. The rats were maintained under a constant 12-h photoperiod at a temperature between 21 and 23 °C and allowed free access to food and water. After fasting for 12 h, the rats were given a single intraperitoneal injection of streptozotocin (65 mg/kg body weight) (Sigma, USA). Three days later, the successful induction of the diabetes model was confirmed when tail blood glucose (BG) levels were greater than or equal to 16.7 mmol/L. The rats were then randomly divided into three groups: non-diabetic rats (Control), streptozocin-induced diabetic rats (DM), and diabetic rats treated with aspirin (DM + aspirin) (n = 10). The Control and DM groups were intragastrically administered 5 g/L sodium carboxymethyl cellulose (15 mg/kg/day) (Sigma, USA). The DM + Aspirin group was administered 2.5 g/L aspirin (15 mg/kg/day) (Sigma, USA). All of these treatments were given for 12 weeks. At the end of the 12 weeks, all rats were humanely sacrificed. The blood samples and kidney tissues from the rats were preserved. The procedures for the animal experiments were approved by the Ethical Committee of Southeast University and followed the latest version of the Declaration of Helsinki.
Testing of blood and urine specimens
Blood glucose (BG), triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and serum creatinine (Scr) concentrations were analysed by using automatic analysers (Hitachi, Japan). The rats were placed individually in metabolic cages for 24-h urine collections. The quantitative analyses of 24-h urinary protein levels were performed by using Lowry assays.
Morphological analysis
The rat kidneys were fixed in 10% formalin and embedded in paraffin. The wax blocks were cut into 2-micron slices. After removing the paraffin, the slices were stained with periodic acid-Schiff (PAS) solution. The results were observed under a light microscope (Olympus, Japan).
Immunohistochemical staining
The rat kidneys were fixed in 10% formalin and embedded in paraffin. The wax blocks were cut into 2-micron slices. After removing the paraffin, the sections were placed in a citrate-buffered solution (pH 6.0) and heated for antigen retrieval. Subsequently, the sections were incubated with COX-2 (CST, USA), LDLr, SCAP, and SREBP-2 (Santa Cruz, USA) primary antibodies overnight at 4 °C and then with biotinylated secondary antibodies. The slices were subsequently stained with diaminobenzidine and haematoxylin. The results were observed under a light microscope (Olympus, Japan).
Immunofluorescent staining
The kidney sections were fixed in 4% paraformaldehyde and blocked in phosphate-buffered saline (PBS) containing 10% bovine serum albumin (BSA) for 60 min. The sections were incubated with anti-rat primary antibodies against COX-2 (CST, USA), Wilms’ tumour 1 (WT-1), and nephrin (Santa Cruz, USA) overnight at 4 °C and then with goat anti-rabbit Fluor 488, goat anti-mouse Fluor 594, and donkey anti-goat Fluor 488 fluorescent antibodies (Invitrogen, USA). Additionally, we also evaluated the co-expression of COX-2 and WT-1. After washing, the slides were examined by laser confocal microscopy (Leica, Germany).
Western blot
Equal amounts of total protein from rat kidney homogenates were denatured and subjected to sodium dodecyl sulphate polyacrylamide gel electrophoresis. Gel transfer was performed, and the membranes were blocked with blocking buffer for 1 h at room temperature. The membranes were then incubated overnight at 4 °C with anti-rat antibodies against COX-2 (CST, USA), LDLr, SCAP, SREBP-2, Wilms’ tumour-1 (WT-1), nephrin, IL-1β, IL-6, TNF-α, and MCP-1 (Santa Cruz, USA). The membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies for one hour at 4 °C. Finally, the signals were detected using the ECL Advance system (Amersham Biosciences, USA).
Transmission electron microscopy
Rat renal tissues were fixed in 4% glutaraldehyde and then fixed in 2% osmium acid and embedded in Epon812. The ultra-microstructure of the podocytes and glomerular basement membrane (GBM) were observed by transmission electron microscopy (JEM-1010, Japan).
Observation of lipid accumulation
Rat kidneys were fixed in 4% paraformaldehyde and embedded in optimal cutting temperature (O.C.T.) compound. The kidneys were then cut into 5-micron frozen sections. The frozen sections were fixed in 4% paraformaldehyde for 10 min and incubated in 1,2-propylene glycol for 2 min. The sections were stained with Oil Red O working solution at room temperature for 30 min, followed by haematoxylin staining for 1 min and a distilled water rinse for 5 min. The results were observed by a light microscope (Olympus, Japan).
Quantitative measurement of tissue cholesterol
The kidney tissues were stored in liquid nitrogen and then added to 1 mL of chloroform:methanol (v:v = 2:1). The tissues were homogenized by an ultrasonic homogenizer and centrifuged at 3000 rpm/min (centrifugal radius 13.5 cm) for 10 min. The supernatants were added to Eppendorf tubes, and 500 µL of a 1 mmol/L sodium hydroxide solution was added to the precipitates for 2 h. The protein concentrations were determined by Lowry assays. The supernatant was dried by a vacuum machine and then dissolved in isopropanol containing 10% Triton X-100. A total of 50 µL of each specimen was added to a 96-well plate. Then, 50 µL of the detection solution was added to each well. The mixture was incubated at 37 °C for 60 min. Finally, the samples were analysed by a spectrophotometer at a wavelength of 500 nm.
Statistical analysis
All the data are expressed as the mean ± standard deviation (SD) and were analysed by SPSS 18.0 (IBM, USA). Statistical comparisons among multiple groups were analysed for significance by one-way analysis of variance (ANOVA). Correlations between variables were analysed by Spearman rank-order correlations. P values of less than 0.05 were considered statistically significant.
Results
Aspirin ameliorated podocyte injury in diabetic rats
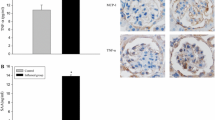
Blood glucose (BG) levels and urinary creatinine and kidney weight-to-body weight ratios were higher in the diabetic rats than in the control rats (Fig. 1a–c). Furthermore, glomerular hypertrophy, mild glomerular mesangial widening, and podocyte foot process effacement were seen in the diabetic rats but not in the other rats (Fig. 1d–e). Immunofluorescent staining and Western blot assays demonstrated that the protein expression levels of nephrin and WT-1, specific biomarkers for podocytes, were increased in the diabetic rats compared with those in the control rats. However, aspirin treatment attenuated podocyte injury and the pathological glomerular changes that occurred in the diabetic rats (Fig. 1f–h). As aspirin is a non-specific inhibitor of cyclooxygenase, these findings suggest that cyclooxygenase may be involved in kidney injury in diabetic rats.
Aspirin ameliorated podocyte injury in diabetic rats. Eight-week-old male Sprague–Dawley rats were divided into the following groups: non-diabetic rats (control), diabetic rats (DM), and diabetic rats treated with aspirin (DM + aspirin) for 12 weeks (n = 10). Blood glucose levels (A) and urinary micro-albumin creatinine ratios (B). Kidney weight-to-body weight ratios (C). Pathological changes were assessed by PAS staining (D) (original magnification, × 400). Changes in podocytes were evaluated by electron microscopy (E) (original magnification, × 12,000). WT-1 and nephrin protein expression was evaluated by immunofluorescent staining (F) (original magnification, × 400) and Western blot assays (G and H). Values represent the mean ± SD. **P < 0.01 versus the control group, #P < 0.05 versus the DM group
Aspirin decreased lipid accumulation in the kidneys of diabetic rats through cyclooxygenase inhibition
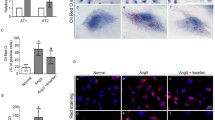
The plasma triglyceride, total cholesterol, HDL, and LDL concentrations were increased considerably in the diabetic rats (Fig. 2a). Interestingly, aspirin treatment decreased the plasma triglyceride and LDL levels. Oil Red O staining and quantitative free cholesterol assays showed that lipid accumulation was prevented in the glomerulus of aspirin-treated diabetic rats compared with diabetic rats (Fig. 2b, c). These data suggest that aspirin override the dysregulation of lipid homeostasis in diabetic rats, which may be through its inhibition of cyclooxygenase activation. Increased COX-2 expression levels in the kidneys of diabetic rats were positively associated with increased LDLr expression levels.
Aspirin decreased lipid accumulation in the kidneys of diabetic rats through cyclooxygenase inhibition. Eight-week-old male Sprague–Dawley rats were divided into the following groups: non-diabetic rats (control), diabetic rats (DM), and diabetic rats treated with aspirin (DM + aspirin) for 12 weeks (n = 10). Plasma lipid profiles (A). Lipid deposition was measured by Oil Red O staining (B) (original magnification, × 400) and quantitative free cholesterol assays (C). Values represent the mean ± SD. **P < 0.01 versus the Control group, #P < 0.05 versus the DM group, ##P < 0.01 versus the DM group
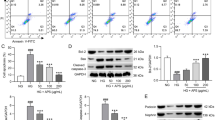
To confirm our hypothesis, we measured the expression levels of COX-2 and LDLr pathway-related proteins in the kidneys of the three groups of rats. The results showed that COX-2 expression levels were higher in the kidneys of the diabetic rats than in the kidneys of the control rats (Fig. 3a, c, d). Immunofluorescence staining demonstrated increased COX-2 expression colocalized with WT-1 expression, suggesting that podocytes were the main location of the increased COX-2 expression levels in the diabetic rats (Fig. 3b). Moreover, the protein expression levels of LDLr, SCAP, and SREBP-2 were higher in the kidneys of the diabetic rats than in the kidneys of the control rats (Fig. 3a, c, d). Interestingly, COX-2 protein expression levels were positively correlated with LDLr protein expression levels (Fig. 3e). Our previous study demonstrated that chronic inflammation disrupted LDLr feedback regulation in the kidneys of diabetic rats [15]. Therefore, we assumed that COX-2 may induce lipid accumulation in the kidneys of diabetic rats through the production of inflammatory cytokines, which may disrupt LDLr feedback regulation.
Increased COX-2 expression levels in the kidneys of diabetic rats were positively associated with increased LDLr expression levels. Eight-week-old male Sprague–Dawley rats were divided into the following groups: non-diabetic rats (control), diabetic rats (DM), and diabetic rats treated with aspirin (DM + aspirin) for 12 weeks (n = 10). COX-2 and LDLr pathway-related protein expression levels were evaluated by immunohistochemical staining (A) (original magnification, × 400), immunofluorescent staining (B) (original magnification, × 400), and Western blot assays (C). The histogram represents the mean ± SD of the densitometric scans of the protein bands normalized to β-actin (D). Correlation analysis of COX-2 and LDLr protein expression levels (E). Values represent the mean ± SD. **P < 0.01 versus the Control group, #P < 0.05 versus the DM group, ##P < 0.01 versus the DM group
Increased COX-2 expression levels induced inflammatory cytokine production in the kidneys of diabetic rats
As COX-2 is a rate-limiting enzyme for the downstream production of inflammatory cytokines, we measured the expression levels of inflammatory cytokines in the kidneys of diabetic rats. As shown in Fig. 4a, b, the inflammatory cytokine levels were higher in the kidneys of the diabetic rats than in the kidneys of the control rats. However, aspirin significantly decreased the production of inflammatory cytokines in diabetic rats. These findings suggest that COX-2 is a key modulator of inflammatory signals in the kidneys of diabetic rats. Aspirin may play key roles in DN through inhibition of COX-2-mediated upregulation of inflammatory signals, which disrupts the dysregulation of the LDLr pathway.
Increased COX-2 expression levels induced inflammatory cytokine production in the kidneys of diabetic rats. Eight-week-old male Sprague–Dawley rats were divided into the following groups: non-diabetic rats (control), diabetic rats (DM), and diabetic rats treated with aspirin (DM + aspirin) for 12 weeks (n = 10). The protein expression levels of inflammatory cytokines were measured by Western blotting (A). The histograms represent the mean ± SD of the densitometric scans of the protein bands normalized to β-actin (B). **P < 0.01 versus the Control group, #P < 0.05 versus the DM group, ##P < 0.01 versus the DM group
Discussion
Recent studies have shown that diabetic nephropathy is not only a metabolic disease but also a chronic inflammatory disease. Inflammation plays an important role in the progression and development of diabetic nephropathy [16, 17]. Inflammatory stimuli are mainly caused by inflammatory cytokines released by inflammatory cells or resident kidney cells. Amplified inflammatory signalling leads to decreased glomerular filtration rates, increased endothelial permeability, proteinuria, and mesangial proliferation [18, 19].
In addition to abnormal glucose metabolism, diabetes is accompanied by obvious lipid metabolic abnormalities [20, 21]. The effects of lipid disorders on renal damage have been investigated. In diabetic patients, there are significant increases in LDL cholesterol levels [22]. Joles et al. [23] found that there was significant kidney damage in rats with hypercholesterolemia and hypertriglyceridemia. Chen et al. [2] found that diabetic patients had high levels of plasma LDL, VLDL, and triglycerides. The use of lipid-lowering drugs has protective effects on proteinuria and glomerular sclerosis. Moreover, chronic inflammation exacerbates lipid-mediated renal injury [24]. Our previous studies have shown that inflammatory stress disrupts negative LDLr feedback regulation and results in lipid deposition in the kidneys and podocyte injury, thus accelerating the development of diabetic nephropathy [15, 25].
COX-2 is an enzyme that plays a significant role in the process of inflammation. The expression levels of COX-2 in the kidneys are increased during the onset of inflammation, as well as the prostaglandin levels. Prostaglandins, such as prostaglandin E2, can bind with their receptors and then produce IL-1β and IL-6 to further promote the inflammatory reaction [26, 27]. Non-steroidal anti-inflammatory drugs, such as aspirin, have effective anti-inflammatory effects and ameliorate kidney injury [28].
Our results showed that increased COX-2 expression levels promoted the release of inflammatory cytokines in diabetic nephropathy, which amplified the inflammatory reaction and dysregulated the LDLr pathway in podocytes; these actions resulted in lipid accumulation in podocytes and podocyte injury and accelerated the progression of diabetic nephropathy, whereas the treatment of aspirin attenuated these changes. These results in diabetic rats suggest that activation of COX-2 may mediate the development of DN through the disruption of LDLr pathway although there are differences in lipid profiles between rats and humans. At present, research studies about the utilization of aspirin and its efficacy in DN therapy are still relatively insufficient. Donadio et al. [29] reported that aspirin treatment stabilizes kidney function via reducing platelet hypersensitivity and the production of thromboxanes, which in turn decreases the constrictor activity in the glomerular vessels and improves the renal microcirculation. Khajehdehi et al. [30] found that treatment with aspirin daily for 2 months significantly reduces proteinuria of patients with type-2 DN. However, the potential mechanisms for aspirin in kidney protection are not fully elucidated.
In summary, our these findings demonstrated that aspirin may provide a protective role in DN for the COX-2-mediated disruption of the LDLr pathway, which may provide a basis for future studies concerning the potential therapeutic implications in preventing the development of DN.
References
Chen J, Cui W, Zhang Q, Jia Y, Sun Y, Weng L et al (2015) Low molecular weight fucoidan ameliorates diabetic nephropathy via inhibiting epithelial-mesenchymal transition and fibrotic processes. Am J Transl Res 7(9):1553–1563
Chen HC, Guh JY, Chang JM, Hsieh MC, Shin SJ, Lai YH (2005) Role of lipid control in diabetic nephropathy. Kidney Int 67(Suppl 94):S60–S62
Zhang Y, Ma KL, Ruan XZ, Liu BC (2016) Dysregulation of the low-density lipoprotein receptor pathway is involved in lipid disorder-mediated organ injury. Int J Biol Sci 12(5):569–579
Hao CM, Breyer MD (2007) Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int 71(11):1105–1115
Komers R, Lindsley JN, Oyama TT, Schutzer WE, Reed JF, Mader SL et al (2001) Immunohistochemical and functional correlations of renal cyclooxygenase-2 in experimental diabetes. J Clin Invest 107(7):889–898
Jia Z, Zhang Y, Ding G, Heiney KM, Huang S, Zhang A (2015) Role of COX-2/mPGES-1/prostaglandin E2 cascade in kidney injury. Mediators Inflamm. https://doi.org/10.1155/2015/147894
Park YG, Kang SK, Noh SH, Park KK, Chang YC, Lee YC et al (2004) PGE2 induces IL-1beta gene expression in mouse osteoblasts through a cAMP-PKA signaling pathway. Int Immunopharmacol 4(6):779–789
Sugimoto Y, Inazumi T, Tsuchiya S (2015) Roles of prostaglandin receptors in female reproduction. J Biochem 157(2):73–80
Cheng H, Wang S, Jo YI, Hao CM, Zhang M, Fan X et al (2007) Overexpression of cyclooxygenase-2 predisposes to podocyte injury. J Am Soc Nephrol 18(2):551–559
Cheng H, Fan X, Guan Y, Moeckel GW, Zent R, Harris RC (2009) Distinct roles for basal and induced COX-2 in podocyte injury. J Am Soc Nephrol 20(9):1953–1962
Cheng H, Fan X, Moeckel GW, Harris RC (2011) Podocyte COX-2 exacerbates diabetic nephropathy by increasing podocyte (pro)renin receptor expression. J Am Soc Nephrol 22(7):1240–1251
Cheng HF, Wang CJ, Moeckel GW, Zhang MZ, McKanna JA, Harris RC (2002) Cyclooxygenase-2 inhibitor blocks expression of mediators of renal injury in a model of diabetes and hypertension. Kidney Int 62(3):929–939
Quilley J, Santos M, Pedraza P (2011) Renal protective effect of chronic inhibition of COX-2 with SC-58236 in streptozotocin-diabetic rats. Am J Physiol Heart Circ Physiol 300(6):H2316–H2322
Wang JL, Cheng HF, Shappell S, Harris RC (2000) A selective cyclooxygenase-2 inhibitor decreases proteinuria and retards progressive renal injury in rats. Kidney Int 57(6):2334–2342
Zhang Y, Ma KL, Liu J, Wu Y, Hu ZB, Liu L et al (2015) Dysregulation of low-density lipoprotein receptor contributes to podocyte injuries in diabetic nephropathy. Am J Physiol Endocrinol Metab 308(12):E1140–E1148
Duran-Salgado MB, Rubio-Guerra AF (2014) Diabetic nephropathy and inflammation. World J Diabetes 5(3):393–398
Lim AK, Tesch GH (2012) Inflammation in diabetic nephropathy. Mediators Inflamm. https://doi.org/10.1155/2012/146154
Kanasaki K, Taduri G, Koya D (2013) Diabetic nephropathy: the role of inflammation in fibroblast activation and kidney fibrosis. Front Endocrinol (Lausanne) 4:7
Wada J, Makino H (2013) Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) 124(3):139–152
Srivastava SP, Shi S, Koya D, Kanasaki K (2014) Lipid mediators in diabetic nephropathy. Fibrogenesis Tissue Repair 7:12
Taskinen MR (2005) Type 2 diabetes as a lipid disorder. Curr Mol Med 5(3):297–308
Hirano T (2014) Abnormal lipoprotein metabolism in diabetic nephropathy. Clin Exp Nephrol 18(2):206–209
Joles JA, Kunter U, Janssen U, Kriz W, Rabelink TJ, Koomans HA et al (2000) Early mechanisms of renal injury in hypercholesterolemic or hypertriglyceridemic rats. J Am Soc Nephrol 11(4):669–683
Ruan XZ, Varghese Z, Moorhead JF (2003) Inflammation modifies lipid-mediated renal injury. Nephrol Dial Transpl 18(1):27–32
Zhang Y, Ma KL, Liu J, Wu Y, Hu ZB, Liu L et al (2015) Inflammatory stress exacerbates lipid accumulation and podocyte injuries in diabetic nephropathy. Acta Diabetol 52(6):1045–1056
Alhouayek M, Muccioli GG (2014) COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol Sci 35(6):284–292
Rajakariar R, Yaqoob MM, Gilroy DW (2006) COX-2 in inflammation and resolution. Mol Interv 6(4):199–207
Goldstein SL, Leung JC, Silverstein DM (2006) Pro- and anti-inflammatory cytokines in chronic pediatric dialysis patients: effect of aspirin. Clin J Am Soc Nephrol 1(5):979–986
Donadio JV Jr, Ilstrup DM, Holley KE, Romero JC (1988) Platelet-inhibitor treatment of diabetic nephropathy: a 10-year prospective study. Mayo Clin Proc 63(1):3–15
Khajehdehi P, Roozbeh J, Mostafavi H (2002) A comparative randomized and placebo-controlled short-term trial of aspirin and dipyridamole for overt type-2 diabetic nephropathy. Scand J Urol Nephrol 36:145–148
Funding
This work was supported by the National Natural Science Foundation of China (Grant 81470957), the Jiangsu Province Social Development Project (BE2018744), the Project for Jiangsu Provincial Medical Talent (ZDRCA2016077), the Jiangsu Province Six Talent Peaks Project (2015-WSN-002), the Fundamental Research Funds for the Central Universities (KYCX18-0182, KYCX17-0169, KYZZ15-0061), the Jiangsu Province Ordinary University Graduate Research Innovation Project (SJZZ16-004), and the Project of Nanjing Municipal Committee for Health and Family Planning (YKK17280).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ma, K.L., Liu, L., Zhang, Y. et al. Aspirin attenuates podocyte injury in diabetic rats through overriding cyclooxygenase-2-mediated dysregulation of LDL receptor pathway. Int Urol Nephrol 51, 551–558 (2019). https://doi.org/10.1007/s11255-018-2059-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-018-2059-7