Abstract
Aims
Nucleoside diphosphate kinase B (NDPKB) is capable of maintaining the cellular nucleotide triphosphate pools. It might therefore supply UTP for the formation of UDP-GlcNAc from glucose. As NDPKB contributes to vascular dysfunction, we speculate that NDPKB might play a role in microangiopathies, such as diabetic retinopathy (DR). Therefore, we investigated the impact of NDPKB on retinal vascular damage using NDPKB−/− mice during development of DR and its possible mechanisms.
Methods
Pericyte loss and acellular capillary (AC) formation were assessed in streptozotocin-induced diabetic NDPKB−/− and wild-type (WT) mice. Expression of angiopoietin-2 (Ang2) and protein N-acetylglucosamine modification (GlcNAcylation) were assessed by western blot and/or immunofluorescence in the diabetic retinas as well as in endothelial cells depleted of NDPKB by siRNA and stimulated with high glucose.
Results
Similar to diabetic WT retinas, non-diabetic NDPKB−/− retinas showed a significant decrease in pericyte coverage in comparison with non-diabetic WT retinas. Hyperglycemia further aggravates pericyte loss in diabetic NDPKB−/− retinas. AC formation was detected in the diabetic NDPKB−/− retinas. Similar to hyperglycemia, NDPKB deficiency induced Ang2 expression and protein GlcNAcylation that were not further altered in the diabetic retinas. In cultured endothelial cells, stimulation with high glucose and NDPKB depletion comparably increased Ang2 expression and protein GlcNAcylation.
Conclusions
Our data identify NDPKB as a protective factor in the retina, which controls Ang2 expression and the hexosamine pathway. NDPKB-deficient mice are a suitable model for studying mechanisms underlying diabetic retinal vascular damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nucleoside diphosphate kinases (NDPK) supply the cells with nucleoside triphosphate including UTP [1–3]. Thus, NDPKs are pivotal to a variety of cellular activities including cell proliferation, differentiation, adhesion, molecular transport and apoptosis [4–9]. The isoform B (NDPKB) is, besides NDPKA, one of the two major isoforms of this enzyme family. It catalyzes the formation of UTP from UDP and ATP [1, 2].
Chronic hyperglycemia leads to an enhanced influx through the hexosamine pathway. In this pathway, glucose is metabolized into UDP-N-acetylglucosamine (UDP-GlcNAc) [10]. As a consequence, O-linked modifications of nuclear and cytoplasmic proteins by addition of the amino sugar N-acetylglucosamine (O-GlcNAc) from UDP-GlcNAc are increased in diabetic endothelial cells (EC) [11]. The synthesis of UDP-GlcNAc requires UTP in a 1:1 ratio to glucose, resulting in enhanced UTP consumption in the diabetic endothelium [11]. Although NDPKB activity is indirectly linked to the formation of UDP-GlcNAc in glucose metabolism, a role of this enzyme in the development of diabetic complications has not been examined.
Our previous data showed that depletion of NDPKB attenuates sprouting angiogenesis and thus leads to vascular dysfunction by modulating vascular integrity [12]. Diabetic retinopathy (DR), the earliest vascular complication in diabetes, is characterized by pericyte loss and subsequent vasoregression [13]. Several lines of evidences suggest that angiopoietin-2 (Ang2) is crucial for initiating vasoregression in DR [14–17]. In the retina, Ang2 is dominantly expressed in endothelial and glial cells. Elevated Ang2 levels in early DR and intravitreal injection of recombinant Ang2 induced pericyte loss in the normal retina [16]. Recently, Yao et al. demonstrated that hyperglycemia induced the elevation of Ang2 in EC by N-acetylglucosamine modification of the transcription factor Sp3. The increased GlcNAcylation of Sp3 facilitates Sp1 binding to the Ang2 promoter, thus activating Ang2 transcription [18]. We hypothesize that NDPKB plays a role in DR. We thus investigated in this study the role of NDPKB in vascular damage during development of DR and the underlying mechanisms by analyzing diabetic NDPKB−/− mice and NDPKB-depleted EC.
Materials and methods
Animals
The use of mice in the study was approved by the local ethics committee (Regierungspräsidium Karlsruhe, Germany). The care and experimental use of animals were in accordance with institutional guidelines and in compliance with the Association for Research in Vision and Ophthalmology statement. The generation of NDPKB−/− mice was previously described [19]. Diabetes was induced in 2-month-old male mice with streptozotocin (STZ, i.p. 145 mg/kg body weight; Roche, Mannheim, Germany) dissolved in citrate buffer (pH 4.5). Age-matched mice injected with citrate buffer served as non-diabetic controls. Successful induction was confirmed by blood glucose over 250 mg/dl at 1 week after STZ treatment. Glucose levels and body weight were monitored throughout the study. Mice were killed at 3 or 6 months after diabetes induction for analysis of alteration in gene expression and the presence of mild retinopathy. At the end of the study, HbA1c was determined by in2it (I) A1c-Test (Bio-Rad Laboratories, Hercules, CA, USA). Eyes were obtained and stored at −80 °C.
Quantitative retinal morphometry
Retinal vessels were isolated using trypsin digestion technique as previously described [16, 20]. Briefly, retinas were isolated from eyes fixed in 4 % formaldehyde for 48 h. Subsequently, they were digested with 3 % trypsin dissolved in 0.2 M Tris buffer (pH 7.0) at 37 °C for 3 h. The pure retinal vasculature were obtained by washing the retinas with dropping water and dried on objective slides. The retinal vessels were finally stained with periodic acid–Schiff and hematoxylin. The number of pericytes was counted in 10 random 40× microscopic fields of the retina using an image-analyzing system (AnalysisPro; Olympus Opticals, Hamburg, Germany) and expressed in pericytes numbers per mm2 capillary area. The number of acellular capillaries (AC) was counted in 10 random microscopic fields using an integration ocular with a grid of 100 squares. The AC segments were expressed in mm2 of retinal area. Samples were evaluated in a masked fashion.
siRNA-mediated NDPKB knockdown in HUVEC
Human umbilical vein endothelial cells (HUVEC) were obtained from umbilical cords of healthy newborns with informed consent of their mothers. The use of HUVEC was approved by the local medical ethics committee (Medical faculty Mannheim, University of Heidelberg, Germany). Isolation and culture of HUVEC were described previously [21]. Cells until passage 3 were used for the experiments. 50 % confluent cells were transfected with NDPKB siRNA or control siRNA using lipofectamine (Life Technologies, Darmstadt, Germany) according to the manufacturer’s protocol. An NDPKB-specific siRNA (AGG UAG UGU AAU CGC CUU G) or a scrambled siRNA (AAC UGG UUG ACU ACA AGU CUU; Eurofins MWG, Operon, Ebersberg, Germany) was used. Four hours after transfection, the cells were supplemented with 10 % FCS. Twenty-four hours after transfection, HUVEC were serum-starved (0.5 % FCS) overnight followed by stimulation with 30 mM d-glucose or 5.5 mM d-glucose for 24 h.
Immunoblotting
Western blot was performed using retinal or HUVEC proteins extracted with RIPA buffer as previously described [12]. Briefly, proteins were separated by SDS-PAGE and electrically transferred onto nitrocellulose membranes. After blocking with Roti-block (Roth, Karlsruhe, Germany), membranes were incubated with primary antibodies overnight. Immune complexes were incubated with corresponding secondary antibodies and visualized using a chemiluminescent peroxidase substrate (2015200, Roche, Mannheim, Germany; or 34095, Thermo Scientific, Rockford, USA). Protein expression was quantified using ImageJ (NIH, USA). For GlcNAc quantification, all bands in each lane were quantified. Specific primary antibodies were mouse-anti-NDPKB (Kamiya Biomedical, Seattle, WA, USA), rabbit-anti-NDPKA (Santa Cruz, Heidelberg, Germany), goat-anti-Ang2 (Santa Cruz, Heidelberg, Germany), mouse-anti-GlcNAc (Abcam, Cambridge, UK) and mouse-anti-Tubulin (Sigma-Aldrich, Saint Louis, MO, USA). The corresponding secondary antibodies were rabbit-anti-mouse, goat-anti-rabbit or rabbit-anti-goat from Sigma-Aldrich (Saint Louis, MO, USA).
Immunofluorescence
HUVEC transfected with NDPKB siRNA or control siRNA were seeded on gelatin-coated glass coverslips 20 h after transfection. After 6 h, cells were serum-starved (0.5 % FCS) overnight and subsequently cultured in 30 mM d-glucose or 5.5 mM d-glucose for 24 h. Afterwards, cells were washed and fixed with 4 % formaldehyde. The cells were then blocked and permeabilized with 2.5 % BSA and 0.3 % Triton X-100 for 1 h and incubated with primary antibodies overnight at 4 °C. After washing, cells were incubated with secondary antibodies conjugated with FITC for 1 h at room temperature. Finally, the cells were washed and mounted with mowiol (Calbiochem, Germany). The primary antibodies were rabbit-anti-NDPKB ([12], kindly donated by Dr. Postel, New Brunswick, NJ, USA) and goat-anti-Ang2 (Santa Cruz, Heidelberg, Germany). The secondary antibodies were swine-anti-rabbit (Dako, Glostrup, Denmark) and donkey-anti-goat (Acris, Herford, Germany). Photos were taken by confocal laser scanning microscopy (Leica Microsystems, Germany).
Statistical analysis
Data are presented as mean ± SEM. One-way ANOVA with Bonferroni post-test was performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). p values < 0.05 were considered significant.
Results
NDPKB deficiency induced diabetes-like vascular pathology aggravates in experimental DR
To evaluate the contribution of NDPKB in diabetic retinal vascular damage, we assessed the hyperglycemia-induced pericyte loss and formation of AC in NDPKB−/− retinas at 6 months after diabetes induction by STZ treatment.
As shown in Table 1, diabetes induced body weight loss, elevated blood glucose and HbA1c levels in the NDPKB−/− and wild-type (WT) mice to a similar extent. There was no difference between NDPKB−/− and WT controls. As expected, diabetes induced a significant decrease in pericyte coverage in WT retinas. Interestingly, non-diabetic NDPKB−/− retinas showed a similar decrease in pericyte coverage as diabetic WT retinas (p < 0.05). Diabetes further aggravated the vascular damage in NDPKB−/− mice. The number of pericytes in diabetic NDPKB−/− retinas was significantly lower than in diabetic WT retina as well as in non-diabetic NDPKB−/− retinas (p < 0.01, p < 0.05, respectively, Fig. 1). Moreover, diabetes significantly enhanced the formation of AC in WT retinas at 6 months after diabetes induction. The number of AC in non-diabetic NDPKB−/− retinas was similar to WT retinas. However, diabetic NDPKB−/− retinas displayed a significant enhancement in the number of AC compared with diabetic WT retinas as well as non-diabetic NDPKB−/− retinas (p < 0.05, p < 0.001, respectively). Thus, NDPKB deficiency significantly aggravates the damage caused by hyperglycemia, indicating a rather protective role of NDPKB in the development of DR.
NDPKB deficiency induced DR-like vascular pathology, which was aggravated in experimental diabetic retinopathy. Examples of retinal digest preparations are shown. Arrowheads indicate pericytes and arrows show AC. The quantification of pericyte coverage and acellular capillary segments is demonstrated in fold change. n = 5–8. *p < 0.05; **p < 0.01; ***p < 0.001. WT, wild type; KO, NDPKB−/−; NC, non-diabetic; DC, diabetic. Scale bar 50 µm
NDPKB depletion results in increased Ang2 expression in retinas and cultured endothelial cells
The early up-regulation of Ang2 is causally involved in the pericytes loss in diabetic retina [16, 22]. We therefore analyzed protein levels of Ang2 in NDPKB−/− retinas at 3 months after diabetes induction. Ang2 expression was significantly up-regulated in diabetic WT retinas compared with non-diabetic controls (p < 0.01, Fig. 2, left panels). However, Ang2 expression was also significantly elevated in non-diabetic NDPKB−/− retinas compared with non-diabetic WT controls (p < 0.05). Hyperglycemia did not further increase Ang2 expression in NDPKB−/− retinas.
NDPKB depletion results in increased Ang2 expression in retinas and cultured endothelial cells. Representative immunoblots and quantification of Ang2 expression in the diabetic NDPKB−/− retina (left panel) and NDPKB-depleted HUVEC stimulated with high glucose (right panel). n = 5–6. *p < 0.05; **p < 0.01. WT, wild type; KO, NDPKB−/−; NC, non-diabetic; DC, diabetic; HG, high glucose; NG, normal glucose; siNDPKB, NDPKB siRNA; control, control siRNA
Yao et al. [18] demonstrated that Ang2 is up-regulated by high glucose in EC. To investigate whether the alterations in Ang2 expression in NDPKB−/− retinas occur in EC, we examined the expression of Ang2 in NDPKB-depleted HUVEC. In control cells, high glucose increased the expression of Ang2 (p < 0.05). Compared with control cells under normal glucose, NDPKB-depleted HUVEC also showed an enhanced expression of Ang2 (p < 0.001). This elevated level remained similar under high glucose (Fig. 2, right panels). Furthermore, as shown by immunocytochemistry in supplemental Fig. 1, Ang2 was localized in the Weibel–Palade bodies in HUVEC. An overall silencing of NDPKB was evident in the NDPKB knockdown HUVEC (Fig. 3). In accordance with the previous data [18], an enhanced expression of Ang2 in control cells stimulated with high glucose was evident. NDPKB depletion led to an up-regulation of Ang2 expression in Weibel–Palade bodies, which was not further elevated by high glucose.
NDPKB depletion elevates Ang2 expression in Weibel–Palade bodies. Visualization of NDPKB and Ang2 expression in NDPKB-depleted endothelial cells stimulated with high glucose by immunofluorescence staining. n = 3. HG, high glucose; NG, normal glucose; siNDPKB, NDPKB siRNA; control, control siRNA. Scale bar 50 µm
NDPKB depletion leads to excess protein GlcNAcylation in retinas and cultured endothelial cells
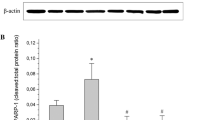
Based on the significance of the hexosamine pathway activation in the development of DR and UDP-GlcNAc as a link between glucose metabolism and nucleotide metabolism, we assessed protein GlcNAcylation in the retina. Enhanced protein GlcNAcylation was detected in diabetic WT retinas (p < 0.01 vs non-diabetic retinas, Fig. 4). Concomitant with the data showing retinal vascular damage in non-diabetic NDPKB−/− mice, an increase in GlcNAcylation was detected in non-diabetic NDPKB−/− retinas compared with WT controls (p < 0.05). Similar to the up-regulated Ang2 content, the increased protein GlcNAcylation in non-diabetic NDPKB−/− retinas was not further elevated in diabetic NDPKB−/− retinas. The data reveal that NDPKB deficiency-induced retinal damage correlates with Ang2 expression and impaired glucose metabolism in the retina.
NDPKB depletion enhances protein GlcNAcylation in retinas and cultured endothelial cells. Representative immunoblots and quantification of protein GlcNAcylation in the diabetic NDPKB−/− retina (left panel) and NDPKB-depleted endothelial cells stimulated with high glucose (right panel). n = 4–5. *p < 0.05; **p < 0.01. WT, wild type; KO, NDPKB−/−; NC, non-diabetic; DC, diabetic; HG, high glucose; NG, normal glucose; siNDPKB, NDPKB siRNA; control, control siRNA
To investigate whether the alterations in protein GlcNAcylation occur in EC, we examined the impact of NDPKB depletion on protein GlcNAcylation in HUVEC. High glucose induced a significant elevation in protein GlcNAcylation in HUVEC (p < 0.05 vs. control). Compared with control cells, an increase in protein GlcNAcylation was detected upon NDPKB knockdown (p < 0.05). In accordance with the findings in retina, high glucose did not induce further increase in protein GlcNAcylation in NDPKB-depleted EC (Fig. 4). These findings indicate that the activation of the hexosamine pathway in EC is under control of NDPKB. Apparently, NDPKB deficiency mimics the impact of hyperglycemic conditions.
Discussion
We demonstrated that NDPKB participates in retinal vascular damage through regulation of glucose metabolism. Firstly, NDPKB−/− mice showed enhanced protein GlcNAcylation and Ang2 expression. Secondly, the pericyte coverage of the capillaries was significantly decreased in NDPKB−/− retinas and, consequently, the formation of AC was aggravated. Experimental diabetes further enhanced these vascular damages in NDPKB−/− mice.
Our study implies a pivotal role of NDPKB for the development of retinal vascular damage. The NDPKB deficiency in the retina mimics aspects of the early pathology of DR, indicating that NDPKB is crucial for the maintenance of retinal vascular homeostasis. Originally, NDPKB was identified as a housekeeping enzyme required for nucleoside triphosphate homeostasis [23]. It is meanwhile evident that the enzyme has a variety of additional functions based on its high potential to be part of large protein complexes and thereby specifically regulate cellular functions [24]. NDPKB therefore has multiple roles such as acting as protein histidine kinase, DNA nuclease, gene transcription regulator and G protein activator [23, 25–28]. Some studies have proved that NDPKB exerts specific functions in various physiological and pathological conditions in the vasculature [29–34]. NDPKB plays a role in microangiopathy, such as DR, and controls angiogenesis and vascular permeability [12]. Furthermore, Rayner and colleagues demonstrated that NDPKB facilitates the vasoprotective effects of estrogen [29, 30]. The data we report herein from NDPKB−/− mice, both in non-diabetic and in diabetic conditions, fully support the vasoprotective properties of NDPKB.
Our study clearly showed that the retinal vascular damage caused by NDPKB depletion is linked to up-regulation of Ang2 in the retina and EC. It was demonstrated before that intravitreal injection of recombinant Ang2 in the non-diabetic adult mouse [16] and constitutive overexpression of Ang2 in the non-diabetic mOpsinAng2 mouse retina (overexpression of human Ang2 under the mouse opsin promoter in the photoreceptor cells) [35] both induced pericytes loss and mimicked DR. Indeed, the increase in Ang2 is essential for the pericyte dropout in diabetic retina [16] and hyperglycemia further enhanced vascular damage in the mOpsinAng2 mouse [35]. Herein, we observed an up-regulation of Ang2 in NDPKB−/− retinas and, similar to the diabetic mOpsinhAng2 retina, an accelerated retinal vascular pathology in hyperglycemia. In accordance with the data from NDPKB−/− mice, depletion of NDPKB in cultured EC resulted in elevation of Ang2, indicating possible importance of endothelial NDPKB for the stabilization of the retinal vasculature. Nevertheless, the overall levels of Ang2 in the retina are likely more essential for the vascular damage than the sources of Ang2. Several studies indicate wherever elevated Ang2 comes from, e.g., photoreceptor cells (mOpsinhAng2 mouse), EC and Müller cells (DR), it causes pericyte loss. In line with this interpretation, even intravitreal injection into the extraretinal space led to pericyte dropout. The house keeping enzyme NDPKB is expressed predominantly in the ganglion cell layer and inner nuclear layer of the retina [36], where vascular and glial cells are located. The up-regulation of Ang2 in the diabetic retina occurs predominantly in EC and Müller glia cells [16]. We could not localize the up-regulated Ang2 in NDPKB−/− retina to specific cell types due to the lack of appropriate Ang2 antibodies for retinal immunohistochemistry. Nevertheless, an up-regulation of Ang2 in Müller cells and EC in NDPKB−/− retinas is conceivable due to the general knockout of the gene.
The underlying mechanism for Ang2 up-regulation in NDPKB deficiency remains elusive. Whether NDPKB has the ability to regulate gene transcriptional elements through the NDPKB/PuF binding site as reported by Postel et al. has been disputed [26, 37, 38]. In addition, the expression of Ang2 is regulated by other signaling pathways like the activation of the transcription factors Sp1/Sp3 and FOXO1 [18, 39].
The activation of the hexosamine pathway under high glucose elevates GlcNAcylation of proteins, leading to alterations in protein functionality. Yao et al. revealed that the modification of Sp3 by GlcNAcylation is increased under high glucose. This alters the DNA partitioning of the Sp3-mSin3A complex in the Ang2 promoter GC box and leads to enhanced transcription of Ang2 [18]. In our study, we detected that the up-regulation of Ang2 by NDPKB depletion is associated with enhanced protein GlcNAcylation in mouse retina and cultured EC. These data indicate that NDPKB might influence Ang2 expression by regulating glucose metabolism. Based on the potential role of NDPKB in the synthesis of UTP, NDPKB might be involved in the regulation of glucose metabolism by controlling UDP-GlcNAc formation. Nevertheless, it is difficult to understand how the loss of an UTP-producing enzyme can increase the availability of UDP-GlcNAc. It should rather cause the opposite. In addition, NDPKB has been shown to participate specifically in several signaling pathways. Firstly, NDPKB forms a complex with G protein βγ dimers in which it acts as a protein histidine kinase, activating G protein via the supply of GTP to the α subunit [27, 28, 40, 41]. Thus, the cAMP formation through the Gs-protein cascade was significantly reduced upon NDPKB depletion. Almost 50 % of the basal cellular cAMP formation depended on NDPKB [27]. Interestingly, the cellular cAMP concentration is reduced in EC cultured in high glucose [42]. Thus, it might be speculated that the retinal vascular damage in NDPKB−/− mice might be a result of impaired cAMP-dependent pathways. Many genes involved in cAMP-dependent pathways such as PKA and CREB are also GlcNAcylated, and this modification causes functional alterations [43]. Secondly, our previous data indicated an interaction between NDPKB, caveolin1 and heterotrimeric G proteins [27]. Caveolin1 is the main structural component of caveolae and required for caveolae formation. Caveolae play fundamental roles in the compartmentalization and organization of signaling pathways and endocytosis. As dysfunction or abnormalities of G protein activation and caveolae formation have been implicated in DR [44, 45], alterations in caveolin transport to the plasma membrane under NDPKB deficiency may lead to cellular dysfunction. We are presently examining whether caveolae formation is disordered in NDPKB-deficient endothelial cells in vivo and in vitro, and the mechanisms underlying NDPKB-mediated defective caveolin trafficking and cellular endocytosis. Thirdly, NDPKB is able to interact with members of Bcl-2 family of proteins and thereby regulates apoptosis [7], which has been previously linked to the elimination of vascular cells in DR [46].
Taking together, our data identify NDPKB as a protective factor in the endothelium which apparently controls Ang2 expression and the hexosamine pathway. The precise mechanisms underlying NDPKB-dependent diabetic retinal vascular damages remain, however, elusive. Therefore, the identification of the signaling pathways inducing the up-regulation of Ang2 by NDPKB depletion is currently pursued in our laboratory.
References
Lascu I, Gonin P (2000) The catalytic mechanism of nucleoside diphosphate kinases. J Bioenerg Biomembr 32(3):237–246
Gilles AM, Presecan E, Vonica A, Lascu I (1991) Nucleoside diphosphate kinase from human erythrocytes. Structural characterization of the two polypeptide chains responsible for heterogeneity of the hexameric enzyme. J Biol Chem 266(14):8784–8789
Janin J, Dumas C, Morera S, Xu Y, Meyer P, Chiadmi M, Cherfils J (2000) Three-dimensional structure of nucleoside diphosphate kinase. J Bioenerg Biomembr 32(3):215–225
Cipollini G, Berti A, Fiore L, Rainaldi G, Basolo F, Merlo G, Bevilacqua G, Caligo MA (1997) Down-regulation of the nm23.h1 gene inhibits cell proliferation. Int J Cancer 73(2):297–302
Fournier HN, Albiges-Rizo C, Block MR (2003) New insights into Nm23 control of cell adhesion and migration. J Bioenerg Biomembr 35(1):81–87
Gervasi F, D’Agnano I, Vossio S, Zupi G, Sacchi A, Lombardi D (1996) nm23 influences proliferation and differentiation of PC12 cells in response to nerve growth factor. Cell Growth Differ 7(12):1689–1695
Kang Y, Lee DC, Han J, Yoon S, Won M, Yeom JH, Seong MJ, Ko JJ, Lee KA, Lee K, Bae J (2007) NM23-H2 involves in negative regulation of Diva and Bcl2L10 in apoptosis signaling. Biochem Biophys Res Commun 359(1):76–82
Krishnan KS, Rikhy R, Rao S, Shivalkar M, Mosko M, Narayanan R, Etter P, Estes PS, Ramaswami M (2001) Nucleoside diphosphate kinase, a source of GTP, is required for dynamin-dependent synaptic vesicle recycling. Neuron 30(1):197–210
Palacios F, Schweitzer JK, Boshans RL, D’Souza-Schorey C (2002) ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat Cell Biol 4(12):929–936
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54(6):1615–1625
Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O (2011) Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem 80:825–858
Feng Y, Gross S, Wolf NM, Butenschon VM, Qiu Y, Devraj K, Liebner S, Kroll J, Skolnik EY, Hammes HP, Wieland T (2014) Nucleoside diphosphate kinase B regulates angiogenesis through modulation of vascular endothelial growth factor receptor type 2 and endothelial adherens junction proteins. Arterioscler Thromb Vasc Biol 34(10):2292–2300
Hammes HP, Feng Y, Pfister F, Brownlee M (2011) Diabetic retinopathy: targeting vasoregression. Diabetes 60(1):9–16
Feng Y, Pfister F, Schreiter K, Wang Y, Stock O, Vom Hagen F, Wolburg H, Hoffmann S, Deutsch U, Hammes HP (2008) Angiopoietin-2 deficiency decelerates age-dependent vascular changes in the mouse retina. Cell Physiol Biochem 21(1–3):129–136
Feng Y, Vom Hagen F, Wang Y, Beck S, Schreiter K, Pfister F, Hoffmann S, Wagner P, Seeliger M, Molema G, Deutsch U, Hammes HP (2009) The absence of angiopoietin-2 leads to abnormal vascular maturation and persistent proliferative retinopathy. Thromb Haemost 102(1):120–130
Hammes HP, Lin J, Wagner P, Feng Y, Vom Hagen F, Krzizok T, Renner O, Breier G, Brownlee M, Deutsch U (2004) Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes 53(4):1104–1110
Pfister F, Feng Y, Vom Hagen F, Hoffmann S, Molema G, Hillebrands JL, Shani M, Deutsch U, Hammes HP (2008) Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes 57(9):2495–2502
Yao D, Taguchi T, Matsumura T, Pestell R, Edelstein D, Giardino I, Suske G, Rabbani N, Thornalley PJ, Sarthy VP, Hammes HP, Brownlee M (2007) High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A. J Biol Chem 282(42):31038–31045
Di L, Srivastava S, Zhdanova O, Sun Y, Li Z, Skolnik EY (2010) Nucleoside diphosphate kinase B knock-out mice have impaired activation of the K+ channel KCa3.1, resulting in defective T cell activation. J Biol Chem 285(50):38765–38771
Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Brownlee M, Deutsch U (2002) Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 51(10):3107–3112
Kroll J, Epting D, Kern K, Dietz CT, Feng Y, Hammes HP, Wieland T, Augustin HG (2009) Inhibition of Rho-dependent kinases ROCK I/II activates VEGF-driven retinal neovascularization and sprouting angiogenesis. Am J Physiol Heart Circ Physiol 296(3):H893–H899
Feng Y, Vom Hagen F, Pfister F, Djokic S, Hoffmann S, Back W, Wagner P, Lin J, Deutsch U, Hammes HP (2007) Impaired pericyte recruitment and abnormal retinal angiogenesis as a result of angiopoietin-2 overexpression. Thromb Haemost 97(1):99–108
Lacombe ML, Milon L, Munier A, Mehus JG, Lambeth DO (2000) The human Nm23/nucleoside diphosphate kinases. J Bioenerg Biomembr 32(3):247–258
Hsu T, Steeg PS, Zollo M, Wieland T (2015) Progress on Nme (NDP kinase/Nm23/Awd) gene family-related functions derived from animal model systems: studies on development, cardiovascular disease, and cancer metastasis exemplified. Naunyn Schmiedebergs Arch Pharmacol 388(2):109–117
Attwood PV, Wieland T (2015) Nucleoside diphosphate kinase as protein histidine kinase. Naunyn Schmiedebergs Arch Pharmacol 388(2):153–160
Berberich SJ, Postel EH (1995) PuF/NM23-H2/NDPK-B transactivates a human c-myc promoter-CAT gene via a functional nuclease hypersensitive element. Oncogene 10(12):2343–2347
Hippe HJ, Wolf NM, Abu-Taha HI, Lutz S, Le Lay S, Just S, Rottbauer W, Katus HA, Wieland T (2011) Nucleoside diphosphate kinase B is required for the formation of heterotrimeric G protein containing caveolae. Naunyn Schmiedebergs Arch Pharmacol 384(4–5):461–472
Hippe HJ, Wolf NM, Abu-Taha I, Mehringer R, Just S, Lutz S, Niroomand F, Postel EH, Katus HA, Rottbauer W, Wieland T (2009) The interaction of nucleoside diphosphate kinase B with Gbetagamma dimers controls heterotrimeric G protein function. Proc Natl Acad Sci USA 106(38):16269–16274
Rayner K, Chen YX, Hibbert B, White D, Miller H, Postel EH, O’Brien ER (2007) NM23-H2, an estrogen receptor beta-associated protein, shows diminished expression with progression of atherosclerosis. Am J Physiol Regul Integr Comp Physiol 292(2):R743–R750
Rayner K, Chen YX, Hibbert B, White D, Miller H, Postel EH, O’Brien ER (2008) Discovery of NM23-H2 as an estrogen receptor beta-associated protein: role in estrogen-induced gene transcription and cell migration. J Steroid Biochem Mol Biol 108(1–2):72–81
Fournier HN, Dupe-Manet S, Bouvard D, Lacombe ML, Marie C, Block MR, Albiges-Rizo C (2002) Integrin cytoplasmic domain-associated protein 1alpha (ICAP-1alpha) interacts directly with the metastasis suppressor nm23-H2, and both proteins are targeted to newly formed cell adhesion sites upon integrin engagement. J Biol Chem 277(23):20895–20902
Zippo A, De Robertis A, Bardelli M, Galvagni F, Oliviero S (2004) Identification of Flk-1 target genes in vasculogenesis: Pim-1 is required for endothelial and mural cell differentiation in vitro. Blood 103(12):4536–4544
Buxton IL, Yokdang N (2011) Extracellular NM23 signaling in breast cancer: incommodus verum. Cancers (Basel) 3(3):2844–2857
Yokdang N, Tellez JD, Tian H, Norvell J, Barsky SH, Valencik M, Buxton IL (2011) A role for nucleotides in support of breast cancer angiogenesis: heterologous receptor signalling. Br J Cancer 104(10):1628–1640
Pfister F, Wang Y, Schreiter K, Vom Hagen F, Altvater K, Hoffmann S, Deutsch U, Hammes HP, Feng Y (2010) Retinal overexpression of angiopoietin-2 mimics diabetic retinopathy and enhances vascular damages in hyperglycemia. Acta Diabetol 47(1):59–64
Jones SE, Jomary C, Grist J, Stewart HJ, Neal MJ (2000) Identification by array screening of altered nm23-M2/PuF mRNA expression in mouse retinal degeneration. Mol Cell Biol Res Commun MCBRC 4(1):20–25
Postel EH, Berberich SJ, Rooney JW, Kaetzel DM (2000) Human NM23/nucleoside diphosphate kinase regulates gene expression through DNA binding to nuclease-hypersensitive transcriptional elements. J Bioenerg Biomembr 32(3):277–284
Steeg PS, Zollo M, Wieland T (2011) A critical evaluation of biochemical activities reported for the nucleoside diphosphate kinase/Nm23/Awd family proteins: opportunities and missteps in understanding their biological functions. Naunyn Schmiedebergs Arch Pharmacol 384(4–5):331–339
Potente M, Urbich C, Sasaki K, Hofmann WK, Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM, Dimmeler S (2005) Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest 115(9):2382–2392
Wieland T (2007) Interaction of nucleoside diphosphate kinase B with heterotrimeric G protein betagamma dimers: consequences on G protein activation and stability. Naunyn Schmiedebergs Arch Pharmacol 374(5–6):373–383
Wieland T, Nurnberg B, Ulibarri I, Kaldenberg-Stasch S, Schultz G, Jakobs KH (1993) Guanine nucleotide-specific phosphate transfer by guanine nucleotide-binding regulatory protein beta-subunits. Characterization of the phosphorylated amino acid. J Biol Chem 268(24):18111–18118
Wang HZ, Wu KY, Lin CP, Fong JC, Hong SJ (1997) Alteration of glucose uptake in cultured human corneal endothelial cells grown in high glucose media via cAMP-dependent pathway. Kaohsiung J Med Sci 13(9):566–571
Hart GW, Akimoto Y (2009) The O-GlcNAc modification. In: Varki A, Cummings RD, Esko JD et al (eds) Essentials of glycobiology, 2nd edn. Cold Spring Harbor Laboratory Press, New York
Kowluru A, Kowluru RA, Yamazaki A (1992) Functional alterations of G-proteins in diabetic rat retina: a possible explanation for the early visual abnormalities in diabetes mellitus. Diabetologia 35(7):624–631
Klaassen I, Hughes JM, Vogels IM, Schalkwijk CG, Van Noorden CJ, Schlingemann RO (2009) Altered expression of genes related to blood-retina barrier disruption in streptozotocin-induced diabetes. Exp Eye Res 89(1):4–15
Kern TS, Du Y, Miller CM, Hatala DA, Levin LA (2010) Overexpression of Bcl-2 in vascular endothelium inhibits the microvascular lesions of diabetic retinopathy. Am J Pathol 176(5):2550–2558
Acknowledgments
The authors thank Doris Baltus, Heike Rauscher and Kristina Stephan-Schnatz for excellent technical support. This study was supported by grants from the European Foundation for the Study of Diabetes (TW, YF) and the Deutsche Forschungsgemeinschaft (SFB TR23, TP B6 (TW) and A9 (SWS), and GRK 1874 SP2 (TW, YF)).
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights disclosure
Regarding HUVEC isolation, all procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Regarding animal studies, all applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent disclosure
Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Managed by Massimo Porta.
Yi Qiu and Di Zhao have contributed equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.

592_2015_752_MOESM1_ESM.jpg
Supplemental Fig. 1: localization of Ang2 in Weibel-Palade bodies in endothelial cells. Weibel-Palade-bodies were visualized by staining with von Willebrand factor (vWF) (JPEG 103 kb)
Rights and permissions
About this article
Cite this article
Qiu, Y., Zhao, D., Butenschön, VM. et al. Nucleoside diphosphate kinase B deficiency causes a diabetes-like vascular pathology via up-regulation of endothelial angiopoietin-2 in the retina. Acta Diabetol 53, 81–89 (2016). https://doi.org/10.1007/s00592-015-0752-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-015-0752-x