Abstract
Diabetic retinopathy (DR) is a multifactorial disease characterized by reactive gliosis and disbalance of angiogenesis regulators, contributing to endothelial dysfunction and microvascular complications. This study was organized to elucidate whether poly(ADP-ribose) polymerase-1 (PARP-1) inhibition could attenuate diabetes-induced damage to macroglia and correct angiogenic disbalance in diabetic rat retina. After 8 weeks of streptozotocin (STZ)-induced diabetes, Wistar male rats were treated with PARP-1 inhibitors, nicotinamide (NAm) or 3-aminobenzamide (3-AB) (100 and 30 mg/kg/daily i.p., respectively), for 14 days. After the 10-weeks experiment period, retinas were undergone an immunohistochemical staining for glial fibrillary acidic protein (GFAP), while western blots were performed to evaluate effects of PAPR-1 inhibitors on the levels of PARP-1, poly(ADP-ribosyl)ated proteins (PARs), GFAP, and angiostatin isoforms. Diabetes induced significant up-regulation and activation of retinal PARP-1, reactive gliosis development, and GFAP overexpression compared to non-diabetic control. Moreover, extensive fragmentation of both PARP-1 and GFAP (hallmarks of apoptosis and macroglia reactivation, respectively) in diabetic retina was also observed. Levels of angiostatin isoforms were dramatically decreased in diabetic retina, sustaining aberrant pro-angiogenic condition. Both NAm and 3-AB markedly attenuated damage to macroglia, evidenced by down-regulation of PARP-1, PARs and total GFAP compared to diabetic non-treated group. PARP-1-inhibitory therapy prevented formation of PARP-1 and GFAP cleavage-derived products. In retinas of anti-PARP-treated diabetic animals, partial restoration of angiostatin’s levels was shown. Therefore, PARP-1 inhibitors counteract diabetes-induced injuries and manifest retinoprotective effects, including attenuation of reactive gliosis and improvement of angiogenic status, thus, such agents could be considered as promising candidates for DR management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy (DR) is the most frequent complication of diabetes and the leading cause of visual impairments and acquired blindness in working-age adults especially in industrialized countries [1, 2]. Increasing prevalence of diabetes worldwide highlights the importance of seeking for new therapeutic strategies beyond current standards of diabetes management. Development of DR is linked to a complex interplay between neurons, glia and vascular components of the retina. However, the exact molecular mechanisms, underlying development of diabetes-induced retinal alterations are not completely understood [3]. Accumulating body of evidence suggests that activation and/or overexpression of poly(ADP-ribose) polymerase-1 (PARP-1) in the retina in response to excessive DNA damage induce cell death [4]. Moreover, in apoptosis, PARP-1 is cleaved by activated caspase-3 and such limited proteolysis of PARP-1 through this cleavage renders the enzyme inactive and this further facilitates apoptotic cell death [5]. Numerous studies indicate that glial cells are involved in pathological events in DR, playing protective or degenerative role. The role of retinal macroglial cells (Müller cells and astrocytes) are of particular importance because they maintain the integrity of blood-retinal barrier and operate as communicators between neurons and vessels [6, 7]. It is of interest that Müller cells and astrocytes show dramatically different reactions in response to hyperglycemia in vivo. Müller cells have been demonstrated to be increased in number and manifest gliosis phenotype during the first month of hyperglycemia while astrocytes have tendency to become atrophic [8, 9]. Activated Müller cells may release proinflammatory cytokines, chemokines, complement cascade, and reactive oxygen species (ROS) that activate an apoptotic death program, compromise the integrity of blood-retinal barrier, and may promote retinal degeneration [10]. The most characteristic of reactive gliosis is elevated expression of glial fibrillary acidic protein (GFAP), the principal component of cytoskeletal intermediate filaments synthesized by mature macroglia cells, which is responsible for many of the progressive glial changes, including alterations of morphology, and necessary for adaptive responses directed towards protection of neurons against apoptosis [11].
It is generally accepted that glial reactivity can serve as an indication of altered glial function, including interfered glia-mediated angiogenesis [12]. Excessive capillary growth due to activation of angiogenesis in diabetic retina may result in retinal detachment and intraocular haemorrhage [13]. Intense release of angiogenic inductors, in particular, vascular endothelial growth factor (VEGF), by reactive glial cells leads to enhancement of retinal neovascularization, increased vascular permeability and vascular leakage [14]. In the normal eye, angiogenic effect of VEGF is counterbalanced by inhibitors, and angiostatins are amongst them. Angiostatins are a group of plasminogen fragments consisting of various number of its five kringle (K) domains. Angiostatins are derived due to plasminogen/plasmin reduction and limited digesting by various proteinases [15]. Functionally active angiostatin-like molecules have been found in retina and other eye structures, where they specifically inhibit proliferation of vascular endothelial cells, induce expression of potent angiogenesis inhibitor, pigment epithelium-derived factor (PEDF), and down-regulate VEGF [16, 17]. Loss of angiostatin-generating potential of retinal cells is strongly believed to be an important factor leading to microvascular abnormalities. Indeed, compensation of angiostatin deficit by intravitreal delivery of angiostatin-encoding gene constructions has been found to ameliorate pathological angiogenesis and reduce vascular leakage in retinas of rats with experimental diabetes as well as oxygen-induced retinopathy [18, 19]. Thus, possible non-invasive treatment strategy of DR would be aimed to improve expression of endogenous anti-angiogenic regulators via affecting key molecules, which modulate glial response. PARP-1 inhibition is thought to be one of the promising approaches to restore retinal angiogenic balance, because strong correlation between PARP-1 targeting and attenuation of glial reactivity has been reported [20]. So far, prior studies in this area have solely been focused on pro-angiogenic molecules, mainly VEGF [21], but not on their counteracting regulators. To our knowledge, possible link between retinal PARP-1 activation/overexpression, glial reactivity and angiostatic potential in diabetes has not been yet investigated. Earlier, we have revealed that the amide form of vitamin B3 (nicotinamide) and its derivatives exert pronounced neuroprotective action and ameliorate diabetes-induced neuropathy [22]. The present study was designed to examine the effects of PARP-1 inhibitors, 3-aminobenzamide (3-AB) and nicotinamide (NAm), on glial reactivity and expression of angiostatin proteins in retinas of diabetic rats.
Materials and Methods
Chemicals
All chemicals used were of analytical reagent grade quality and purchased from Sigma Chemical Co. (USA), except for those listed below or otherwise specified in the text.
Experimental Design
All procedures were carried out in accordance with the national and international guidelines and laws concerning animal welfare and are ethically acceptable. Experiments in vivo were performed according to the ARVO Statement for the use of Animals in Ophthalmic and Vision Research. The experiments were performed on male Wistar rats (270–350 g of b.w.), which were fed a standard diet and had free access to food and water. After 1 week of acclimation, diabetes was induced by a single intraperitoneal (i.p.) injection of freshly prepared solution of streptozotocin (STZ) in citrate buffer (pH 4.5) at 70 mg/kg b.w. The animals were maintained on 12-h light/dark cycle and randomly divided into the following groups (n = 5 in each group): control group (Control); diabetic group [Diabetes (D)]; diabetic groups after 8 weeks of diabetes development were treated by i.p. injection with 3-aminobenamide (D + 3-AB) or with nicotinamide (D + NAm) at doses 30 and 100 mg/kg body weight−1 day−1, respectively, for 2 weeks. The dose levels of PARP-1 inhibitors and treatment period were chosen on the basis of available data indicating absence of any sub-toxic or toxic effects [23, 24]. The rats with blood glucose level over 20.2 ± 2.1 mmol/l were taken into experiments. After 10 weeks experimental rats were sacrificed via cervical dislocation under mild diethyl ether narcosis.
Protein Samples Preparations
Freshly isolated retinas (n = 5 for each treatment) were placed in liquid nitrogen and homogenated in 25 mM tris–HCl (pH 7.4) containing 0.15 M NaCl, 1 % sodium dodecylsulphate, 1 mM ethyleneglycoltetraacetic acid, 2.5 mM ethylenediaminetetraacetic acid, 6.5 μM aprotinin, 1.5 μM pepstatin A, 23 μM leupeptin, 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml soybean trypsin inhibitor, 1 μM sodium orthovanadate (tissue:buffer 1:5, w/v). The homogenates were sonicated three times for 30 s using ultrasonic disintegrator “Sartorius” (Labsonic® M, Göttingen, Germany) and centrifuged at 16,000g for 30 min at 4 °C. The protein concentration in each retinal lysate was determined spectrophotometrically as described elsewhere [25]. The supernatants were diluted 1:1 in Laemmli sample buffer, supplemented with 0.1 M dithiothreitol, and boiled for 5 min. In parallel, samples for angiostatin detection were diluted in non-reducing Laemmli sample buffer. Protein samples were frozen and stored at −20 °C before analysis.
Anti-angiostatin Antibody Production
Antibodies for angiostatin detection were generated in rabbits, and immunization was carried out according to previously described procedure [26]. Briefly, plasminogen from fresh rat plasma was purified on the Lys-sepharose column. Isolated plasminogen was used as antigen for rabbit immunization. Polyclonal rabbit antibodies were purified from blood serum of immunized rabbits by chromatography on angiostatin-conjugated immunoaffine sorbent. Developed antibodies recognized each of the five kringle domains in plasminogen fragments as well as the same epitopes within the tertiary structure of precursor protein.
Western Blot
Protein samples (50 μg/track) were run in 5–18 % denaturing gels and transferred onto nitrocellulose membrane (GE Healthcare, Amersham Bioscience, RPN 203D) with 0.45 μm pore diameter. After blotting, membranes were blocked in 5 % w/v non-fat dried milk for 90 min at 37 °C and probed with anti-PARP (Cell Signaling Technology, #9542, 1:1000), anti-PAR polymer (Trevigen Inc., 4335-MC-100, 1:1000), anti-GFAP (Santa Cruz, sc-9065, 1:1000) or anti-beta actin (Abcam, ab20272, 1:5000) antibodies diluted in phosphate buffer saline containing 0.05 % Triton X-100 (v/v) (PBST) according to manufacturer’s recommendations. Anti-angiostatin antibody was also diluted in PBST and used in final concentration 5 μg/ml. After overnight incubation at 4 °C, membranes were washed five times with PBST and then incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody diluted in PBST for 60 min. Then, unbound antibodies were removed by seven-times washes in PBST for 5 min each. Immunoreactive bands were visualized using the enhanced chemiluminescence method (ECL). Densitometric analysis of the autoradiographs was performed with using of densitometry software TotalLab TL120 (Nonlinear Inc, USA) and normalized to the intensity of the respective bands obtained for β-actin. Each trace was corrected for background by subtracting a tracing of nonreactive area on the blot. Antigens of various molecular weights were identified by extrapolation of plots of relative mobilities of prestained proteins with known molecular weight (PageRuler Prestained Protein Ladder, Fermentas, Germany).
Tissue Collection and Immunofluorescence Assay
For immunohistochemical detection of GFAP, rat retinas were fixed in 4 % paraformaldehyde for 2 h and washed in PBS for 1 h. 6-μm-thick retinal tissue sections from formalin-fixed, paraffin-embedded blocks were transferred to poly(Lys)-covered slides to be used for staining. Sections were dewaxed in xylene and progressively hydrated in ethanol aqueous solutions. They were then washed three times with PBS and heated for 1 min in 10 mmol/l citrate buffer (pH 6.0) containing 0,25 % (v/v) Triton X-100 at 100 °C in microwave oven to unmask antigens. The sections were blocked in 3 % bovine serum albumin (BSA)–PBST for 60 min at room temperature and incubated with PBST-diluted anti-GFAP (1/250) antibodies overnight at 4 °C in a humified chamber. Slices were rinsed three to four times with PBST and incubated with fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG secondary antibody diluted 1/500 in PBST for 60 min at room temperature. Control for unspecific binding of the secondary antibody was performed by excluding primary antibodies. After washing, the sections were counterstained with Hoechst-33342 to visualize cell nuclei. Glasses with sections were mounted in DABCO/PVA mounting medium (Sigma-Aldrich, Missouri, USA) and analyzed with LSM 510 META laser scanning confocal microscope (Carl Zeiss, Oberkochen, Germany) using a 63× oil objective. Electronic shutters and image acquisition were under the control of Zeiss ZEN 2009 Light Edition software. Images analysis was equally to whole panel normalized with level tool. All experimental procedures in this study were performed under the same conditions and in at least three parallels. Figures were mounted with Adobe PhotoShop 7.0 (San Jose, CA, USA). Manipulation of the images was restricted to threshold and brightness adjustments to the whole image.
Statistical Analysis
Protein contents evaluated by western blots are expressed in arbitrary units (a.u.) and presented in histograms as mean ± standard deviation (SD), n represents the number of animals. Quantitative results were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni post-hoc tests. P values <0.05 were considered to indicate statistical significance.
Results
Body Weights and Blood Glucose Levels
The experimental diabetes was characterized by development of hyperglycemia and body weight loss. It was shown that after 10 weeks of diabetes the average body weight was 1.24 times lower than that of diabetic group before STZ-injection at the beginning of experiments (P < 0.05). At the end of experiments, 3.8-fold increase in the blood glucose level was achieved (20.2 ± 2.1 vs. 5.3 ± 0.3, P < 0.05). NAm and 3-AB affected neither body weight nor blood glucose level in diabetic rats as compared with untreated diabetic animals. There were no deaths of animals along the whole experimental period.
NAm and 3-AB Affect PARP-1 Expression, Fragmentation, and Activation in Diabetic Rat Retina
To investigate whether relationship between PARP-1 activation and reactive gliosis under DR exists, we firstly explored PARP-1 expression and activity. Western blot analysis demonstrated significant up-regulation of PARP-1 expression (Fig. 1a) and increasing ratio between cleaved PARP-1 fragment (89 kDa) vs. full-length PARP-1 (116 kDa) in diabetic retinas compared to non-diabetic group (0.073 ± 0.021 vs. 0.039 ± 0.007 respectively, P < 0.05) (Fig. 1b). Administration of PARP-1 inhibitors to diabetic rats decreased PARP-1 expression to near basal level and prevented formation of its cleaved products in retina.
Effects of PARP-1 inhibitors on the PARP-1 expression and fragmentation in the retina of rats with STZ diabetes: a representative western blot of PARP-1; b results of the densitometric analysis of immunoblots, in which bar plot shows the ratio between cleaved PARP-1 (89 kDa) and full-length PARP-1 (116 kDa). Equal loading was confirmed by reprobing for β-actin. Data are expressed as mean ± SD (n = 5). *P < 0.05 vs. control; #P < 0.05 vs. diabetes
Using western blot analysis, amounts of poly(ADP-ribosyl)ated proteins (PARs) were measured as an indirect parameter of PARP activity. As shown in Fig. 2a, predominant PARs appeared to be the species of apparent molecular weights from 130 to 72 kDa, however several minor bands (55–17 kDa) were also visualized. Results of densitometric analysis of the blots depicted in Fig. 2b revealed that both 130–72 and 37–26 kDa PAR polymers were increased in diabetic rats compared to control (2.22 ± 0.17 vs. 0.34 ± 0.05 a.u. respectively, P < 0.05). However, PAR accumulation in diabetic rat retinas appeared to be reduced significantly (P < 0.05) by NAm or 3-AB treatment (0.85 ± 0.22 and 0.84 ± 0.30 a.u., respectively) compared to this parameter for non-treated diabetic rats and did not differ from healthy control (P > 0.05). It is not excluded that in the condition of STZ-induced diabetes, auto-poly(ADP-ribosyl)ation of PARP-1 and its fragments could also occur.
Effects of PARP-1 inhibitors on the levels of poly(ADP-ribosyl)ated proteins (PAR) in the retina of rats with STZ diabetes: a representative western blot of PAR; b results of the densitometric analysis of immunoblots showing PAR polymer contents. Equal loading was confirmed by reprobing for β-actin. Bar plot shows the relative protein level, data are expressed as mean ± SD in a.u. (n = 5). *P < 0.05 vs. control; #P < 0.05 vs. diabetes
PARP-1 Inhibitors Alleviate Diabetes-Induced Reactive Gliosis in Rat Retina
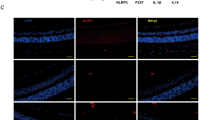
The results of immunofluorescence assay of retinal GFAP are depicted in Fig. 3. Imaging of fixed retinal slices showed dramatically increased overall GFAP immunostaining in retinas of diabetic rats (panel c) compared with non-diabetic animals (panel b). Gross differences between GFAP immunoreactivity in diabetic retinas and healthy control retinas indicates that STZ-induced hyperglycemia caused Müller cell gliosis at 10 weeks of diabetes. Further, we detected the extent of macroglial activation in retinas of diabetic rats treated with 3-AB or NAm. It is obviously seen that both PARP-1 inhibitors reduced GFAP immunoreactivity in retinas of rats with STZ-induced diabetes (Fig. 3, panels d, e).
GFAP immunostaining in the retina cross-sections: a control for non-specific binding of the secondary FITC-conjugated antibody; b retina of non-diabetic rat (control); c retina of diabetic rat; d retina of diabetic rat treated with 3-AB; e retina of diabetic rat treated with NAm. Increased GFAP-immunoreactivity is well-recognized in retina at 10 weeks of diabetes compared to control, indicating diabetes-induced Müller cell gliosis. In retina of diabetic animals administered to PARP-1 inhibitors, marked decrease in intensity of GFAP immunolabeling is observed. Double staining for GFAP (green), nuclei were counterstained with Hoechst-33342 (blue). Bar represents 20 μm (Color figure online)
Western blot analysis of retinal protein samples confirmed an increased GFAP level detected by immunohistochemistry. There is strong line of evidence indicating that not only intact GFAP polypeptide overexpression can be used as an indicator of reactive gliosis but also its cleavage-derived products [27], which contribute to the overall GFAP immunoreactivity. Therefore, we evaluated levels of each sort of immunoreactive GFAP polypeptides individually. Diabetes-induced elevation in 49 kDa GFAP polypeptide content in comparison with age-match control was shown (3.87 ± 1.37 vs. 2.29 ± 0.01 a.u. respectively, P > 0.05), however this increment did not achieve the level of statistical significance due to a high-order dispersion within the group of diabetic animals (Fig. 4a, b). Administration of NAm or 3-AB had no effects on intact GFAP levels (3.09 ± 0.35 and 3.13 ± 0.09 a.u. respectively). However, vast amounts of cleaved GFAP polypeptides in the range of molecular weights 47–37 kDa were observed in diabetic retina, but they were hardly detectable in healthy retina (3.89 ± 1.08 vs. 0.16 ± 0.10 a.u. respectively, P < 0.05) (Fig. 4c). Both NAm and 3-AB treatments significantly diminished (P < 0.05) levels of 47–37 kDa GFAP in diabetic retinas (0.18 ± 0.06 and 0.46 ± 0.08 a.u. respectively), possibly through prevention of its native subunit against degradation. Finally, we calculated and compared total GFAP expression (Fig. 4d). Diabetes appeared to induce threefold increase in total retinal GFAP compared to control (7.76 ± 0.29 vs. 2.45 ± 0.09 a.u., P < 0.05), whereas treatment of diabetic rats with 3-AB or NAm significantly (P < 0.05) reduced overall GFAP levels (3.59 ± 0.01 and 3.27 ± 0.29 a.u.). Taking together, these results show that PARP-1 inhibitory treatment seemed to improve the aspect of macroglial cells and their reactivity in retinas of rats with STZ-induce diabetes.
Effects of PARP-1 inhibitors on the GFAP expression and fragmentation in the retina of rats with STZ diabetes: a representative western blot of retinal GFAP; b results of the densitometric analysis of the intact 49 kDa GFAP; c results of the densitometric analysis of the cleaved 47–37 kDa GFAP isoforms; d total GFAP content. Bar plot shows the relative protein level, data are expressed as mean ± SD in a.u. (n = 5). *P < 0.05 vs. control; #P < 0.05 vs. diabetes
PARP-1 Inhibitors Improve Angiostatin Levels in Retinas of Rats with STZ-Induced Diabetes
To evaluate influence of PARP-1 inhibitors on the retinal levels of neovascularization suppressors in diabetic rats, angiostatin levels were measured by western blot analysis followed by gel electrophoresis of retina protein samples obtained in non-reducing conditions. Using polyclonal antibody recognized all kringle domains of plasminogen, an angiostatin’s precursor molecule, in most samples at least two different bands were observed (Fig. 5a). These angiostatin-like molecules appeared to be polypeptides migrated at approximately 65 or 50 kDa when run under non-reducing conditions and may correspond to angiostatins K1-5 and K1-4 respectively. Immunoblotting showed a pronounced decrease in angiostatin levels in diabetic retinas. Densitometric analysis of blots represented in Fig. 5b indicates marked decline of both angiostatin isoforms, K1-4 and K1-5, in diabetic retinas compared to non-diabetic rats (1.35 ± 0.06 vs. 5.06 ± 0.15 and 1.14 ± 0.06 vs. 2.82 ± 0.31 a.u. respectively, P < 0.05). Concomitant treatment of diabetic rats with NAm or 3-AB succeeded to improve angiogenic status in retinas by increasing angiostatin release. There was significant (P < 0.05) elevation in K1-4 levels in retinas of diabetic rats treated with 3-AB (2.64 ± 0.15 a.u.) or NAm (3.27 ± 0.08 a.u.). In parallel, K1-5 levels in diabetic retinas appeared to be up-regulated in result of NAm or 3-AB treatment (1.96 ± 0.26 and 2.14 ± 0.16 a.u. respectively). Based on the data obtained, we affirm that PARP-1 inhibitors at the concentrations employed partially restored angiostatin levels in retinas of rats with STZ-induced diabetes. Also, we emphasized a trend of inverse association between amount of GFAP expression and fragmentation, both considered as an indicators of reactive gliosis, and angiostatin production in retinas of rats with STZ-induced diabetes.
Effects of PARP-1 inhibitors on the angiostatin levels in the retina of rats with STZ diabetes: a representative western blot of angiostatin-like molecules, which are composed of plasminogen kringle domains; b results of the densitometric analysis of the band corresponding to angiostatin K1-4; c results of the densitometric analysis of the band corresponding to angiostatin K1-5. Bar plot shows the relative protein level, data are expressed as Mean ± SD in a.u. (n = 5). *P < 0.05 vs. control; #P < 0.05 vs. diabetes. Plasminogen (Plg) (1 μg) was loaded as a positive control for primary antibody binding
Discussion
The idea of this research was driven by the numerical observations indicating that sustained angiogenesis is involved in the development of proliferative DR, while PARP inhibitors are recognized to be promising therapeutic or preventive options to manage this diabetic complication as they are reported to have antiangiogenic potential [21, 28]. The aim of this study was to address the question whether inhibition of excessive PAPR-1 activity has the ability to ameliorate glial reactivity and to improve angiostatin production in retina of diabetic rats. We highlight here for the first time that PAPR-1 inhibition-mediated beneficial antiangiogenic efficacy can be linked with improvement to retinal angio-suppressing status during hyperglycemia possibly through alleviating macroglia reactivity.
Retinal glia (Müller cells, astrocytes and microglia) functions as communicator between neurons and vessels [29]. However, several studies revealed that in diabetic retina, Müller cells and astrocytes showed opposite reactions that points to the differences in their pathophysiological significance during diabetes. Rungger-Brändle et al. [30] have demonstrated that, at the end of the fourth week of experimental diabetes (STZ 60 mg/kg) in rats, the density of Müller cells and microglia was significantly increased in retina as compared with intact animals, while the number of astrocytes were markedly reduced and continued to decline over the next few months. Ly et al. [8] demonstrated that slight Müller cell gliosis was evident after 4 weeks of diabetes and became more pronounced by 6 weeks of diabetes. They also showed that in contrast to Müller cells, astrocytes play an early role in DR progression. Significant loss of astrocytes was observed 4 weeks after the induction of diabetes. Astrocyte degeneration was associated with increased retinal HIF-1α expression, hypoxia in ganglion cell layer, reduced connexin expression resulting in abnormal gap junction formation. In the study of Dorrell et al. [9], loss of astrocytic template in ischemic retina was found to correlate with disorganized vessel formation and pathological revascularization. Reactive Müller cells, in turn, may aggravate progression of proliferative DR by over-expressing VEGF resulting in retinal neovascularization, vascular lesions and leakage, and inflammation [10].
A publication of Kim et al. [20] demonstrated that PARP-1 is involved in neuron-astroglial death and reactive gliosis development. In the current study, we could not clarify a role of each type of retinal glial cells in gliosis development. However, based on the numerical literature evidences, up-regulation of GFAP could be definitely considered as manifestation of Müller glia reactivity in diabetic retina. Moreover, our findings are consistent with former observations that there is an important link between PARP activation/overexpression and glial response in retina. It has been documented before that generation of cleaved GFAP products could serve as a supplementary marker of reactive gliosis in both retina and CNS [11, 31]. Therefore, we evaluated the extent of reactive gliosis by measuring overall GFAP immunoreactivity as well as content of its intact and cleaved polypeptides. Although levels of 49 kDa GFAP appeared unchanged between groups, the significant increase of its fragments was observed in non-treated rats with diabetes, thus, contributing total GFAP abundance. Both NAm and 3-AB were shown to reduce total GFAP and preserve its cleavage in diabetic retina. Recent data suggest that caspases are responsible for limited proteolysis of GFAP, which is aimed to induce the disassembly of the cytoplasmic intermediate filament network that, in turn, is characteristic of apoptosis [32]. Taking into account that PARP-1 and GFAP are both caspase substrates [33], results of our research clearly indicate that reduction of protein degradation in retinas of hyperglicemic rats might be due to attenuation of apoptosis processes by PARP-1 inhibitors and reflect beneficial outcomes of the chosen treatment.
Retinal microvessels are entirely covered by astrocytes and Müller cells to form endothelial–glial complex, and glial cells have the ability to modulate angiogenesis depending on physiological or pathological conditions. In healthy retina, quiescent astrocytes have been shown to counteract VEGF-induced pro-angiogenic stimuli via formation and releasing of exosomes loaded with anti-angiogenic components, such as endostatin and PEDF that inhibit regrowth of new vessels, and their loss or dysfunction are believed to contribute to microvascular abnormalities [34]. Under pathological conditions, reactive Müller cells acquire pro-angiogenic phenotype that is thought to be a compensatory response on ischemic/hypoxic conditions [10]. However, surplus endothelium activation can give rise to abnormal retinal neovascularization that, in turn, leads to fibrous scar formation and culminates in retinal detachment. In such instance, we propose that it may be possible to restore pool of angiogenesis inhibiting molecules by ceasing glial reactivity in diabetic retinas. To evaluate effects of NAm and 3-AB on anti-angiogenic status in retinas of diabetic rats, we measured levels of angiostatin proteins, which are known to be potent anti-angiogenic factors that can inhibit endothelial cell proliferation and migration. Angiostatins are plasminogen-derived fragments of plasminogen best known for its role in fibrinolytic system. Angiostatins comprise plasminogen kringles 1–5, 1–4, 1–3, 2–3, as well as individual kringles. Originally known as tumor angiogenesis inhibitors [35], angiostatins are already considered to be important participants in controlling vascular homeostasis in the eye and play an important role in various diabetic complications as reviewed by [36]. It has been found that increased angiostatin production accompanied by VEGF down-regulation might take place during retinal scatter photocoagulation in proliferative DR [16, 19]. The authors therefore stated that intraocular formation of angiostatins is not permanent but seems to be inducible. In contrary, in other study, angiostatin (50 kDa isoform) has been shown to be normally localized in the nerve fiber layer, ganglion cell layer, inner and outer plexiform layers, and the photoreceptor layer of the cat, cow, dog and rat, while horse and pig retinas showed additional staining in the matrix of the inner nuclear layer [37]. Thus, the results of our study support the latter observations that angiostatins are constitutively produced angiogenesis inhibitors in normal retina, which may contribute to the anti-angiogenic environment in the healthy mammal eye. So far, the population of retinal cells responsible for angiostatin generation is unknown and at present, this issue is under our investigation.
Another important finding of the present study is that angiostatin-like plasminogen fragments, comprising all five kringle domains of plasminogen, are formed in retina. There is currently no information available on the production of endogenous K1-5 in retina. It is of interest that, of all plasminogen-derived fragments, angiostatins containing K5 possess the strongest inhibitory activity toward endotheliocyte proliferation and thus might play peculiar role in regulation of retinal neovascularization [38]. Growing evidence indicates that K5 itself exerts potent anti-angiogenic activity in retina and has its own receptor, though poorly characterized [39, 40]. Sima et al. [41] have reported that a single intravitreous injection of K1-4 (7.5 μg per eye) significantly decreased VEGF levels and reduced retinal vascular leakage in rats with oxygen-induced and STZ-induced DR. In the other study, very promising results on targeted delivery and transgenic expression of mouse angiostatin K1-4 in reducing retinal vascular leakage in an experimental diabetic rat model have been reported [19]. Though, we were not focused on VEGF expression in the present study, our findings are concordant with the prior report [21] indicating that PARP inhibition prevents both diabetes- and hypoxia-induced retinal VEGF formation, thus representing valuable strategy to counteract angiogenic disbalance in DR. Moreover, our results provide the first evidence that inhibition of PARP-1 activity has more complex outcomes for retinal angiogenic balance, influencing not only expression of growth factors and cytokines, but also is able to affect production of angiostatic substances.
Although PARP-1 is vitally important for maintaining the integrity of the genome in eukaryotic cells, its negative, pro-apoptotic and pro-necrotic effects on cells are also implicated in the development of numerous pathological conditions [42]. In our research, we tested PARP-inhibiting effects of the two different agents, NAm and 3-AB. Results obtained are in line with our previously published data indicating that PARP inhibition counteracts multiple manifestations of peripheral neuropathy and renal hypertrophy in diabetic mice [43]. Effects of the natural PARP antagonist, NAm, appeared to be more profound in comparison with those of 3-AB. Unlike 3-AB, NAm is able to exert beneficial effects in diabetic state through pleiotropic mechanisms, which are beyond direct PARP-1 inhibition. NAm is known to serve essential functions through complex effects of the correspondent vitamin or its metabolites [44], including reduction of neuronal ROS production, Ca2+ influx, apoptosis and cell injury [45]. With regard to our research, NAm could normalize retinal NAD+, improve mitochondrial functions, attenuate retinal cell death and thereby restore normal function of retina [46, 47]. Moreover, anti-diabetic efficacy of NAm may also be associated with formation and better availability of 1-methylnicotinamide, the endogenous product of NAm methylation, due to its ability to attenuate deleterious effects of diabetes in the CNS as described elsewhere [48]. 3-AB is the first generation PARP-1 synthetic inhibitor with absence of unwanted side effects. However, 3-AB has been found to suppress N-methyl-N-nitrosourea-induced retinal damage and completely rescue photoreceptor cell apoptosis via preservation of NF-kappaB activity in rats [49]. It should be noted that other processes than those examined in our study might be affected by 3-AB and NAm, and above all, their antioxidant activities cannot be neglected [50].
There are some limitations to this study, which outline the directions of future research. Cautions must be used in extrapolating results of this animal model on humans. Although the present work has yielded some promising findings indicating successful outcome from PARP inhibition in treatment regime when major molecular alterations linked with DR pathogenesis is already developed, efficacy of PARP inhibitor-based medication applied from the very onset of diabetes needs to be evaluated. Since our research has shown that PARP inhibitors caused encouraging elevation of angiostatic proteins, which are responsible for down-regulation of neovascularization in diabetic retina, ongoing and future studies will be fulfilled in order to clarify an issue whether angiostatins are produced by retinal cells per se through proteolytic fragmentation of plasminogen or these molecules are specifically sequestrated from the blood and internalized into retinal cells. To check these hypotheses, a reductionist cell culture approach could be well suited. Further work is also required to determine if the diabetes-induced alterations in macroglia affect their capacity to produce and/or secreted angiostatins. Angiostatin-like molecules formed both in vitro and in vivo required being isolated and their individual or combine effects have to be thoroughly characterized in terms of their anti-proliferative and anti-migration activities toward endothelial cells. Finally, determination of aminoacid sequences of these plasminogen fragments will provide a better understanding of their subtle molecular structure and mechanisms of proteolytic processing of parent protein in retina.
In conclusion, the present study was organized in vivo to assess effects of PARP-1 inhibitors, 3-AB and NAm, on angiostatin levels and glial reactivity in retina in the rat model of STZ-induced diabetes. Diabetes was found to compromise ability of retinal cells in producing angiostatic proteins. We report first that PARP-1 inhibitors can exert their antiangiogenic capacities by indirect mechanism, i.e. improving levels of intrinsic angiostatic molecules, which were identified as K1-4 and K1-5 fragments of plasminogen. The key findings of this study add to a growing literature that link between PARP-associated processes, retinal gliosis, and angiogenesis plays pathophysiological role in DR development. PARP-inhibiting treatment may become a supplementary therapeutic strategy for the amelioration of diabetes-related eye abnormalities, including retinopathies.
References
Fong DS, Aiello LP, Ferris FL, Klein R (2004) Diabetic retinopathy. Diabetes Care 27(10):2540–2553. doi:10.2337/diacare.27.10.2540
Antonetti DA, Klein R, Gardner TW (2012) Diabetic retinopathy. N Engl J Med 366(13):1227–1239. doi:10.1056/NEJMra1005073
Kern TS (2014) Interrelationships between the retinal neuroglia and vasculature in diabetes. Diabetes Metab J 38(3):163–170. doi:10.4093/dmj.2014.38.3.163
Paquet-Durand F, Silva J, Talukdar T, Johnson LE, Azadi S, van Veen T, Ueffing M, Hauck SM, Ekström PA (2007) Excessive activation of poly(ADP-ribose) polymerase contributes to inherited photoreceptor degeneration in the retinal degeneration 1 mouse. J Neurosci 27(38):10311–10319. doi:10.1523/JNEUROSCI.1514-07.2007
Chaitanya GV, Alexander JS, Babu PP (2010) PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun Signal 8:31. doi:10.1186/1478-811X-8-31
Baydas G, Tuzcu M, Yasar A, Baydas B (2004) Early changes in glial reactivity and lipid peroxidation in diabetic rat retina: effects of melatonin. Acta Diabetol 41(3):123–128
Coorey NJ, Shen W, Chung SH, Zhu L, Gillies MC (2012) The role of glia in retinal vascular disease. Clin Exp Optom 95(3):266–281. doi:10.1111/j.1444-0938.2012.00741.x
Ly A, Yee P, Vessey KA, Phipps JA, Jobling AI, Fletcher EL (2011) Early inner retinal astrocyte dysfunction during diabetes and development of hypoxia, retinal stress, and neuronal functional loss. Invest Ophthalmol Vis Sci 52(13):9316–9326. doi:10.1167/iovs.11-7879
Dorrell MI, Aguilar E, Jacobson R, Trauger SA, Friedlander J, Siuzdak G, Friedlander M (2010) Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy. Glia 58(1):43–54. doi:10.1002/glia.20900
Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A (2006) Müller cells in the healthy and diseased retina. Prog Retin Eye Res 25(4):397–424
Sugaya-Fukasawa M, Watanabe T, Tamura M, Egashira S, Hisatomi H (2011) Glial fibrillary acidic protein is one of the key factors underlying neuron-like elongation in PC12 cells. Exp Ther Med 2(1):85–87. doi:10.3892/etm.2010.162
Suárez I, Bodega G, Rubio M, García-Segura LM, Fernández B (1994) Astroglial induction of in vivo angiogenesis. J Neural Transplant Plast 5(1):1–10. doi:10.1155/NP.1994.1
Abcouwer SF (2012) Angiogenic factors and cytokines in diabetic retinopathy. J Clin Cell Immunol 1(11):1–12. doi:10.4172/2155-9899
Simó R, Carrasco E, García-Ramírez M, Hernández C (2006) Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr Diabetes Rev 2(1):71–98
Cao Y, Xue L (2004) Angiostatin. Semin Thromb Hemost 30(1):83–93. doi:10.1055/s-2004-822973
Spranger J, Hammes HP, Preissner KT, Schatz H, Pfeiffer AF (2000) Release of the angiogenesis inhibitor angiostatin in patients with proliferative diabetic retinopathy: association with retinal photocoagulation. Diabetologia 43(11):1404–1407. doi:10.1007/s001250051546
Coppini LP, Visniauskas B, Costa EF, Filho MN, Rodrigues EB, Chagas JR, Farah ME, Barros NM, Carmona AK (2015) Corneal angiogenesis modulation by cysteine cathepsins: in vitro and in vivo studies. Exp Eye Res 134:39–46. doi:10.1016/j.exer.2015.03.012
Drixler TA, Borel Rinkes IH, Ritchie ED, Treffers FW, van Vroonhoven TJ, Gebbink MF, Voest EE (2001) Angiostatin inhibits pathological but not physiological retinal angiogenesis. Invest Ophthalmol Vis Sci 42(13):3325–3330
Shyong MP, Lee FL, Kuo PC, Wu AC, Cheng HC, Chen SL, Tung TH, Tsao YP. Reduction of experimental diabetic vascular leakage by delivery of angiostatin with a recombinant adeno-associated virus vector. Mol Vis 13:133–141
Kim J, Kim Y, Kim J, Kang T (2014) PARP1 activation/expression modulates regional-specific neuronal and glial responses to seizure in a hemodynamic-independent manner. Cell Death Dis 5:e1362. doi:10.1038/cddis.2014.331
Obrosova IG, Minchenko AG, Frank RN, Seigel GM, Zsengeller Z, Pacher P, Stevens MJ, Szabó C (2004) Poly(ADP-ribose) polymerase inhibitors counteract diabetes- and hypoxia-induced retinal vascular endothelial growth factor overexpression. Int J Mol Med 14(1):55–64
Kuchmerovska T, Shymanskyy I, Donchenko G, Kuchmerovskyy M, Pakirbaieva L, Klimenko A (2004) Poly(ADP-ribosyl)ation enhancement in brain cell nuclei is associated with diabetic neuropathy. J Diabetes Complicat 18(4):198–204. doi:10.1016/S1056-8727(03)00039-4
Chiu J, Xu B, Chen S, Feng B, Chakrabarti S (2008) Oxidative stress-induced, poly(ADP-ribose) polymerase-dependent upregulation of ET-1 expression in chronic diabetic complications. Can J Physiol Pharmacol 86(6):365–372. doi:10.1139/Y08-033
Maiese K, Chong ZZ, Hou J, Shang YC (2009) The vitamin nicotinamide: translating nutrition into clinical care. Molecules 14(9):3446–3485. doi:10.3390/molecules14093446
Stoscheck CM (1990) Quantitation of protein. Methods Enzymol 182:50–68
Tykhomyrov AA, Yusova EI, Diordieva SI, Corsa VV, Grinenko TV (2013) Production and characteristics of antibodies against K1-3 fragment of human plasminogen. Biotechnol Acta 6(1):86–96
Tura A, Schuettauf F, Monnier PP, Bartz-Schmidt KU, Henke-Fahle S (2009) Efficacy of Rho-kinase inhibition in promoting cell survival and reducing reactive gliosis in the rodent retina. Invest Ophthalmol Vis Sci 50(1):452–461. doi:10.1167/iovs.08-1973
Rajesh M, Mukhopadhyay P, Bátkai S, Godlewski G, Haskó G, Liaudet L, Pacher P (2006) Pharmacological inhibition of poly(ADP-ribose) polymerase inhibits angiogenesis. Biochem Biophys Res Commun 350(2):352–357. doi:10.1016/j.bbrc.2006.09.049
Tsacopoulos M, Poitry-Yamate CL, MacLeish PR, Poitry S (1998) Trafficking of molecules and metabolic signals in the retina. Prog Retin Eye Res 17(3):429–442
Rungger-Brändle E, Dosso AA, Leuenberger PM (2000) Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci 41(7):1971–1980
Chen MH, Hagemann TL, Quinlan RA, Messing A, Perng MD (2013) Caspase cleavage of GFAP produces an assembly-compromised proteolytic fragment that promotes filament aggregation. ASN Neuro 5(5):e00125. doi:10.1042/AN20130032
Mouser PE, Head E, Ha KH, Rohn TT (2006) Caspase-mediated cleavage of glial fibrillary acidic protein within degenerating astrocytes of the Alzheimer’s disease brain. Am J Pathol 168(3):936–946
Zhang X, Barile G, Chang S, Hays A, Pachydaki S, Schiff W, Sparrow J (2005) Apoptosis and cell proliferation in proliferative retinal disorders: PCNA, Ki-67, caspase-3, and PARP expression. Curr Eye Res 30(5):395–403. doi:10.1080/02713680590956306
Barot M, Gokulgandhi MR, Patel S, Mitra AK (2013) Microvascular complications and diabetic retinopathy: recent advances and future implications. Future Med Chem 5(3):301–314. doi:10.4155/fmc.12.206
O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Cao Y, Moses M, Lane WS, Sage EH, Folkman J (1994) Angiostatin: a circulating endothelial cell inhibitor that suppresses angiogenesis and tumor growth. Cold Spring Harb Symp Quant Biol 59:471–482
Tykhomyrov AA, Shram SI, Grinenko TV (2015) Role of angiostatins in diabetic complications. Biomed Khim 61(1):41–56. doi:10.1134/S1990750814020140
Pearce JW, Janardhan KS, Caldwell S, Singh B (2007) Angiostatin and integrin αvβ3 in the feline, bovine, canine, equine, porcine and murine retina and cornea. Vet Ophthalmol 10(5):313–319
Cao Y, Chen A, An SS, Ji RW, Davidson D, Llinás M (1997) Kringle 5 of plasminogen is a novel inhibitor of endothelial cell growth. J Biol Chem 272(36):22924–22928
Zhang SX, Sima J, Shao C, Fant J, Chen Y, Rohrer B, Gao G, Ma JX (2004) Plasminogen kringle 5 reduces vascular leakage in the retina in rat models of oxygen-induced retinopathy and diabetes. Diabetologia 47(1):124–131. doi:10.1007/s00125-003-1276-4
Ma J, Li C, Shao C, Gao G, Yang X (2012) Decreased K5 receptor expression in the retina, a potential pathogenic mechanism for diabetic retinopathy. Mol Vis 18:330–336
Sima J, Zhang SX, Shao C, Fant J, Ma J (2004) The effect of angiostatin on vascular leakage and VEGF expression in rat retina. FEBS Lett 564(1–2):19–23. doi:10.1016/S0014-5793(04)00297-2
Li N, Chen J (2014) ADP-ribosylation: activation, recognition, and removal. Mol Cells 37(1):9–16. doi:10.14348/molcells.2014.2245
Drel VR, Pacher P, Stavniichuk R, Xu W, Zhang J, Kuchmerovska TM, Slusher B, Obrosova IG (2011) Poly(ADP-ribose)polymerase inhibition counteracts renal hypertrophy and multiple manifestations of peripheral neuropathy in diabetic Akita mice. Int J Mol Med 28(4):629–635. doi:10.3892/ijmm.2011.709
Virág L, Szabó C (2002) The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev 54(3):375–429
Shen CC, Huang HM, Ou HC, Chen HL, Chen WC, Jeng KC (2004) Protective effect of nicotinamide on neuronal cells under oxygen and glucose deprivation and hypoxia/reoxygenation. J Biomed Sci 11(4):472–481. doi:10.1159/000077897
Bai S, Sheline CT (2013) NAD(+) maintenance attenuates light induced photoreceptor degeneration. Exp Eye Res 108:76–83. doi:10.1016/j.exer.2012.12.007
Klaidman L, Morales M, Kem S, Yang J, Chang ML, Adams JD (2003) Nicotinamide offers multiple protective mechanisms in stroke as a precursor for NAD+, as a PARP inhibitor and by partial restoration of mitochondrial function. Pharmacology 69(3):150–157
Kuchmerovska T, Shymanskyy I, Chlopicki S, Klimenko A (2010) 1-methylnicotinamide (MNA) in prevention of diabetes-associated brain disorders. Neurochem Int 56(2):221–228. doi:10.1016/j.neuint.2009.10.004
Miki K, Uehara N, Shikata N, Matsumura M, Tsubura A (2007) Poly(ADP-ribose) polymerase inhibitor 3-aminobenzamide rescues N-methyl-N-nitrosourea-induced photoreceptor cell apoptosis in Sprague-Dawley rats through preservation of nuclear factor-kappaB activity. Exp Eye Res 84(2):285–292. doi:10.1016/j.exer.2006.09.023
Ji D, Li GY, Osborne NN (2008) Nicotinamide attenuates retinal ischemia and light insults to neurones. Neurochem Int 52(4–5):786–798. doi:10.1016/j.neuint.2007.09.012
Acknowledgments
The authors are thankful to Kuznetsov K.I. (PhD, research fellow) and Professor Veselovsky N.S. (Bogomoletz Institute of Physiology of NAS of Ukraine) for their cooperation, expert technical assistance, and fruitful discussion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest regarding the publishing of this paper.
Ethical Approval
All applicable international, national, and Institutional Animal Care and Use Committee at Palladin Institute of Biochemistry of NASU (Kyiv, Ukraine; No. 0112U002625) guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Guzyk, M.M., Tykhomyrov, A.A., Nedzvetsky, V.S. et al. Poly(ADP-Ribose) Polymerase-1 (PARP-1) Inhibitors Reduce Reactive Gliosis and Improve Angiostatin Levels in Retina of Diabetic Rats. Neurochem Res 41, 2526–2537 (2016). https://doi.org/10.1007/s11064-016-1964-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1964-3