Abstract
Compared to the 2-h oral glucose tolerance test (OGTT), the assessment of HbA1c was proposed as a less time-consuming alternative to detect pathologies in carbohydrate metabolism. This report aims to assess the predictive accuracy of HbA1c to detect alterations in glucose disposition early after gestational diabetes mellitus (GDM) pregnancy. A detailed metabolic characterization was performed in 77 women with previous GDM (pGDM) and 41 controls 3–6 month after delivery: 3-h OGTT, frequently sampled intravenous glucose tolerance test. Follow-up examinations of pGDMs were performed up to 10 years. HbA1c (venous samples, HPLC) was assessed at baseline as well as during the follow-up period (475 patient contacts). Moderate associations were observed between HbA1c and measurements of plasma glucose during the OGTT at the baseline examination: The strongest correlation was found for FPG (r = 0.40, p < 0.001), decreasing after ingestion. No associations were detected between HbA1c and OGTT dynamics of insulin or C-peptide. Moreover, baseline HbA1c showed only modest correlation with insulin sensitivity (r = −0.25, p = 0.010) and disposition index (r = −0.26, p = 0.007). A linear model including fasting as well as post-load glucose levels was not improved by HbA1c. However, pGDM females with overt diabetes manifestation during the follow-up period showed more pronounced increasing HbA1c in contrast to females remaining normal glucose tolerant or developing prediabetes. It is suggested that the performance of HbA1c assessed early after delivery is inferior to the OGTT for the detection of early alterations in glucose metabolism. However, an increase in HbA1c levels could be used as an indicator of risk for diabetes manifestation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
History of gestational diabetes mellitus (GDM) confers a markedly increased risk for the later development of type 2 diabetes [1]. Thus, appropriate risk stratification is recommended to identify early postpartum impairments in carbohydrate metabolism [2]. Conventionally, the postpartum 75-g oral glucose tolerance test (OGTT) is used to identify impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) as intermediate states of hyperglycemia with strong relation to insulin resistance and β-cell failure [3, 4]. Interventional studies have shown that both criteria for prediabetes (PD) can be safely treated by lifestyle intervention or pharmacological treatment [5] to sufficiently prevent the later manifestation of overt diabetes in several populations including females with the history of GDM [6].
In 2010, the ADA [7] as well as in 2011 the WHO [8] consistently added HbA1c levels ≥6.5 % (48 mmol/mol) to their diagnostic recommendations of overt diabetes. Hence, there is increasing interest in using the HbA1c assay as an alternative method for defining prediabetic hyperglycemia. Its use in clinical practice is attractive as it is an internationally well standardized and less time-consuming test without requiring fasting [7, 9]. However, evidence for large interindividual physiologic variability [10] should be concerned as major limitation. Thus, recommendations on a HbA1c-based definition of PD are contradictory between different health care organizations [7–9].
To date, little is known regarding the postpartum reassessment of glucose metabolism via HbA1c. In the majority of women, history of GDM is characterized by modest alterations in insulin sensitivity and β-cell function, whereas plasma glucose levels remain within the normal (nondiabetic) range. Hence, it is questionable, whether these subtle metabolic alterations could be adequately mirrored by the long-term average of glucose levels instead of using a standardized glucose stress test. It was hypothesized that HbA1c might be useful for detecting early postpartal prediabetic conditions; however, two recent reports on this topic showed only moderate to fair concordance between HbA1c and OGTT criteria for PD in women with the history of GDM [11, 12].
Actually, information on the relationship between HbA1c and parameters of glucose disposition as well as the long-term implications in this specific risk collective is still missing. Thus, this report aimed to assess the associations of early postpartum HbA1c levels with impaired carbohydrate metabolism as primary objective. As secondary objectives of this report, we aimed to assess time-dependent changes of HbA1c levels during 10 years of follow-up as well as its accuracy to correctly identify at baseline subjects with later development of diabetes.
Methods
Study participants
A detailed description of the study design is given elsewhere [13]. For this report, we included 77 women 3–6 months after pregnancy with GDM previous gestational diabetes (pGDM) (the 4th International Workshop Conference on GDM with a 75-g OGTT [14] ) as well as 41 women after gestation without GDM (controls) participating in the “Vienna Post-Gestational Diabetes Project.” Subjects included in this report were recruited between January 2000 and December 2003. Females with baseline examination before January 2000 were excluded due to missing of HbA1c. pGDMs were annually invited for reexaminations (OGTT, HbA1c, and biometric parameters) until March 2013 (median time of follow-up of 68 month, IQR 51–136 month). Thus, a total of 475 patient contacts (approximately 6.2 contacts per subject) with measurements of HbA1c were available. We were able to exclude specific conditions (severe anemia, renal failure, infectious diseases) which might affect HbA1c levels after complete examination of the medical history (exclusion criteria).
At the baseline visit, all subjects received a detailed metabolic characterization including examinations of routine laboratory and body composition. A 3-h 75-g OGTT was additionally performed: Glucose, insulin, and C-peptide were measured at fasting (FPG) as well as at 10, 20, 30, 60, 90, 120, 150, and 180 min. Moreover, a frequently sampled intravenous glucose tolerance test (FSIGT) was performed to assess parameters of glucose disposition: Glucose (300 mg/kg body weight) was infused for 30 s starting at time 0 and normal insulin (0.03 U/kg, Humulin R; Eli Lilly, Indianapolis) from 20 to 25 min, respectively. Plasma concentration of glucose and insulin was assessed at fasting as well as at 3, 4, 5, 6, 8, 10, 14, 19, 22, 27, 30, 35, 40, 50, 70, 100, 140, and 180 min. Insulin sensitivity index (SI) was estimated by minimal model analysis from the FSIGT [15], and the disposition index (DI) was calculated as SI × ΔAIRG, where the latter represents the incremental short-term insulin response after glucose bolus: 3–10 min [16]. Insulin sensitivity and secretion were additionally estimated from the OGTT by using the oral glucose insulin sensitivity index (OGIS) [17] and the insulinogenic index (IGI), respectively. IGI was calculated as Δinsulin30/Δglucose30 [18]. The area under the concentration curves during the OGTT was estimated by using the trapezoidal rule.
A manifestation of overt diabetes was diagnosed, if FPG or 2-h post-load glucose levels exceeded 125 or 199 mg/dl. IFG and IGT were defined by glucose ranges between 100–125 mg/dl and 140–199 mg/dl, respectively.
Some data of the present study were reported previously [19–22]; however, the association of HbA1c levels with parameters of disturbed carbohydrate metabolism as well as follow-up examinations until March 2013 was not reported until now.
The study was approved by the local ethics committee (Ethics Committee of the Medical University of Vienna) and performed in accordance with the 1964 Declaration of Helsinki and its later amendments. All subjects gave written informed consent prior to the inclusion in the study.
Laboratory reagents
Plasma glucose was obtained from Beckman, Fullerton, CA, USA; Insulin was obtained from radioimmunoassay, Serono Diagnostics; C-Peptide was obtained from radioimmunoassay, CIS Bioe International. HbA1c (high-performance liquid chromatography) was obtained from Variant II, Bio-Rad, IFCC standardized and DCCT aligned, CV = 1.8 % (HbA1c = 5.6 %).
Statistical analysis
Continuous variables were summarized by means and standard deviations (SD). Differences between two subgroups at the baseline visit [e.g., categorized for PD or normal glucose tolerance) were compared with Student’s t test and differences between more than two groups with analysis of variance (ANOVA), respectively. Data transformations [ln(x + 1)] were performed if skewed distribution was detected within descriptive analysis: SI and DI.
Pearson’s product-moment correlation was used for analyzing linear associations between baseline HbA1c and glucose as well as insulin and C-peptide levels during the 3-h OGTT. Multiple linear regression models were used to assess the associations between HbA1c levels and parameters of glucose disposition including FPG and post-load glucose as further predictors. The additional information derived from HbA1c levels was expressed in changes of adjusted R-squared (AR2), indicating how well a statistical model explains the outcome variable adjusted for the number of independent variables included into the model.
The predictive performance of HbA1c levels with PD (defined by the OGTT as gold standard) at the baseline examination was assessed by receiver operating characteristic (ROC) analysis, and 95 % confidence intervals (95 % CI) were estimated by one million stratified bootstrap replicates by using the pROC package for R [23]. The agreement between HbA1c- and OGTT-based criteria for PD was additionally assessed by Cohen’s κ coefficient.
Kaplan–Meier estimates and the log-rank test were used to examine the association of HbA1c-based categories with the incidence of type 2 diabetes during the follow-up period. An event was defined as manifestation of type 2 diabetes, and data were censored if patients were lost to follow-up. Moreover, linear mixed-effects models with random intercepts and random slopes by subjects were used to assess time-dependent changes of HbA1c during follow-up in different subgroups [i.e., females with pGDM showing NGT during follow-up examination (OGTT criteria) as well as pGDMs developing IFG or IGT or overt diabetes]. An exponential spatial covariance structure was used to model the correlations between repeated measurements. To assess group-specific differences in the slope of HbA1c trajectories, a disease group by time interaction term was included into the model. Individual data of HbA1c trajectories were visualized by spaghetti plots as well.
Statistical analysis was performed with R (V3.0.1) and contributed packages (particularly the R-packages “pROC,” “nlme,” and “survival”) [24]. A two-sided p value ≤0.05 was considered statistically significant, without adjustment for multiplicity.
Results
Cross-sectional analyses at the baseline examination
At the baseline examination, descriptive characteristics of women categorized by HbA1c versus OGTT suggested that both criteria discriminated subjects with impaired cardio-metabolic risk factors. However, subjects fulfilling IFG or IGT were older, more obese, and insulin resistant (Table 1). In addition, postpartum HbA1c levels were significantly higher in females with pGDM as compared to the control group (5.50 ± 0.42 vs. 5.17 ± 0.27, p < 0.001).
As shown in Table 2, correlation analyses of metabolic parameters assessed at the baseline visit revealed only moderate associations of HbA1c with measurements of plasma glucose during the OGTT: The strongest association was observed between HbA1c and FPG (r = 0.40, p < 0.001) decreasing within this examination. Of note, no associations were found between HbA1c and OGTT dynamics of insulin and C-peptide, whereas they were found to be significantly correlated with fasting and post-load glucose levels. With regard to glucose disposition, HbA1c showed weak correlation with log-transformed SI (r = −0.25, p = 0.010) or DI (r = −0.26, p = 0.007). Three different multivariate regression models were assessed to explain log-transformed DI: model A including FPG as independent variable (AR2 = 5.4 %, p = 0.009); model B including FPG and 2 h post-load glucose levels (AR2 = 14.4 %, p < 0.001); model C including FPG, 1 and 2-h post-load glucose levels (AR2 = 21.3 %, p < 0.001). The inclusion of HbA1c as further predictor had no significant impact to improve the predictive performance of these models (model A: AR2 = 7.3 %; model B: AR2 = 14.6 %, model C: AR2 = 21.6 %). Comparable results were observed when log-transformed SI was used as dependent variable: model A (AR2 = 9.4 vs. 9.9 % after including HbA1c); model B (AR2 = 15.5 vs. 15.0 %); model C (AR2 = 18.3 vs. 17.9 %). No interactions were identified between females with pGDM and controls suggesting comparable effects in women after delivery.
Figure 1 shows ROC analysis of HbA1c to explain IFG or IGT as gold standard in pGDMs and revealed that HbA1c performed at best fair to identify early alterations during the OGTT at the baseline examination (AUC: 0.69, 95 % CI 0.56–0.81). The recommended cutoff by the ADA (HbA1c ≥5.7 %, 39 mmol/mol) showed a specificity of 69.6 % (95 % CI 57.1–82.2) and a sensitivity of 57.1 % (95 % CI 38.1–76.2). Another cutoff (HbA1c ≥6.0 %, 42 mmol/mol) recommended by others [9] showed improved specificity (83.9 %, 95 % CI 73.2–92.9), however, lacked in sensitivity (23.8 %, 95 % CI 9.5–42.9) as well. The κ-index was 0.24 (p = 0.031) for IFG/IGT and HbA1c ≥5.7 % (39 mmol/mol) and 0.09 (p = 0.433) for HbA1c ≥6.0 % (42 mmol/mol), respectively.
Longitudinal analyses of follow-up data
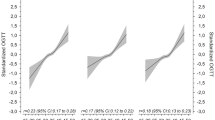
During the follow-up period, 16 (21 %) women with pGDM developed type 2 diabetes, 30 (39 %) subjects showed IFG or IGT (baseline or new manifestation), and further 31 (40 %) women had normal glucose metabolism (NGT). As visualized in Fig. 2, HbA1c trajectories differed between the subgroups: The estimated increase in HbA1c levels (%) per 3 years was significantly more pronounced in subjects who developed overt diabetes as compared to subjects with NGT (β group:time = 0.45, 95 % CI 0.34–0.56, p < 0.001) or IFG/IGT (β group:time = 0.38, 95 % CI 0.27–0.48, p < 0.001). Of note, HbA1c levels at baseline were comparable between the three groups (NGT: 5.4 ± 0.47, IFG/IGT: 5.5 ± 0.42, diabetes: 5.6 ± 0.38, p ANOVA = 0.421). The results remained constant in a sensitivity analysis where NGT (n = 10) or IFG/IGT (n = 6) females with a follow-up period of less than 5 years were excluded. Therefore, baseline HbA1c performed poor for classifying subjects under particularly high risk: HbA1c ≥5.7 % (39 mmol/mol) misclassified 50 % (p Log-Rank = 0.235) and HbA1c ≥6.0 % (42 mmol/mol) misclassified 81 % (p Log-Rank = 0.921) of females with later development of diabetes. Kaplan–Meier plots are provided as supplementary material.
Trajectories of HbA1c levels in females after pregnancy with GDM: a females with pGDM showing NGT during follow-up examination (OGTT criteria); b females with pGDM developing prediabetes (OGTT criteria: IFG or IGT); c females with pGDM developing diabetes (OGTT criteria). Dashed line marks the respective HbA1c cutoff for prediabetes (5.7 %, 39 mmol/mol) and diabetes (6.5 %, 48 mmol/mol)
Discussion
This report aimed to assess the predictive performance of HbA1c in post-GDM risk stratification, keeping in mind the practical advantage of a single parameter containing long-term information of carbohydrate metabolism without requiring fasting or the time-consuming burden of an OGTT. While HbA1c was shown to be related to insulin resistance and disposition index, estimated correlations were small and the explanatory value of HbA1c appeared to be inferior as compared to measurements of plasma glucose in multivariable analysis. Secondarily, we observed that HbA1c was not sufficient to identify subjects with IFG or IGT correctly. However, increasing HbA1c levels during the follow-up examinations showed improved predictability for the later manifestation of overt diabetes as compared to baseline levels.
Our results are in line with observations from the Insulin Resistance and Atherosclerosis Study suggesting that parameters of glucose disposition were more strongly related to fasting or 2-h post-load glucose as compared to HbA1c levels in a mixed population. Of note, analysis of interactions revealed strong effect modifications due to ethnicity and particularly sex (HbA1c was stronger related to insulin sensitivity in women), underlining the major importance of investigating these issues in females after pregnancy with GDM [25]. In addition, Kanat et al. [26] assessed the relationship between β-cell failure and HbA1c in Mexican Americans. Although insulin sensitivity and β-cell secretion decreased steeply in subjects exceeding HbA1c levels of 5.5 % (37 mmol/mol), approximately half of the individuals with HbA1c <5.7 % (39 mmol/mol) had IFG or IGT and markedly impaired β-cell function. Thus, the authors concluded that the OGTT was superior to identify subjects with particularly high risk for future diabetes. In contrast, no major differences in HOMA-IR and insulinogenic index were observed between individuals meeting HbA1c versus OGTT criteria for PD in an Italian cohort. However, the authors mentioned that individuals with overt diabetes identified by the OGTT were at higher risk for disturbed insulin action and insulin secretion as those identified by HbA1c [27].
The lacking performance of baseline HbA1c as a correlate of impaired glucose disposition is also mirrored by its notable weak association with insulin or C-peptide levels during the OGTT as proposed by our results in females after pregnancy with GDM. It is well established that insulin or C-peptide dynamics as well as their relation to changes in plasma glucose are key factors for deriving accurate information of insulin secretion and insulin sensitivity [28]. As both defects represent the underlying pathophysiologic mechanisms already for the development of disturbed carbohydrate metabolism during the pregnant state [29], their reassessment after delivery is pivotal to provide an accurate risk approximation. However, based on our data, this seems to be out of the scope of HbA1c, representing the mean glucose of the last 2–3 month, but giving only a weak surrogate of borderline disorders which are actually only quantifiable by performing a glucose tolerance or clamp test, respectively.
Likewise, the strong association of IFG and IGT with parameters of glucose disposition was previously confirmed by a number of investigations, also suggesting different underlying deteriorations for both prediabetic conditions (IFG: impaired early phase of insulin secretion and hepatic insulin sensitivity; IGT: impaired early and late phase of secretion as well as impaired insulin sensitivity in the muscle) [4, 5]. Also, a recent metaanalysis compared progression rates to type 2 diabetes of IFG, IGT, and raised HbA1c (≥6.0, 42 mmol/mol) and suggested lower incidence rates for subjects meeting the HbA1c criterion, while subjects with IFG and IGT showed the highest rates of conversion [30]. In our study, the accordance between HbA1c and IFG or IGT was only moderate: Females with HbA1c ≥6.0 % (42 mmol/mol) are likely presenting IFG or IGT, but there are many false negatives, while postpartum HbA1c ≥5.7 % (39 mmol/mol) lacks in specificity as well. This was also shown by a French study suggesting that postpartum screening with HbA1c (alone or in combination with FPG) gives no accurate model for risk stratification [12]. Also, another study in 54 pGDM women reported at best fair agreement between HbA1c and OGTT criteria for PD with specificity of 68 % (comparable to our observation) and sensitivity of 65 % (slightly higher as observed in our study but within the confidence margin) [11]. The remarkable discordance between the methods was also concerned in larger epidemiological investigations containing different ethnic groups [25, 31, 32]. As a possible explanation, authors discussed large physiologic variability of HbA1c [10, 33] due to differences in age [34] and race [35] in addition to genetic [36], and hematological factors [37, 38]. These and other parameters (e.g., iron deficiency [39]) might also influence HbA1c levels in the postpartum period and thus contribute to the weak association with glycemic parameters and dynamics of insulin and C-peptide as well.
In the present study, we also found that subjects with later development of overt type 2 diabetes showed a notably increase in HbA1c, suggesting that the longitudinal reassessment might be of importance in the medical decision-making process. Increasing HbA1c relative to baseline levels possibly derives an interpretation of glucose metabolism which is independent from specific interindividual or postpartal confounders. Therefore, we recommend HbA1c reassessment for early identifying potential conversions to type 2 diabetes after baseline risk stratification via the OGTT. However, as our observations are limited due to the small sample size of subjects developing type 2 diabetes, we suggest that larger follow-up studies on HbA1c changes should take further care on this topic.
To point out strength and limitations, while some of our findings are restricted due to the rare number of events (i.e., cases with incident type 2 diabetes) and hence these results should be interpreted descriptively, the major strength of this observational study is the availability of FSIGT in addition to OGTT-derived parameters of carbohydrate metabolism as well as the long-term reassessment of HbA1c.
We conclude that baseline HbA1c assessed early after delivery is only a weak surrogate of insulin sensitivity and disposition index and gives almost no additive information to glycemic parameters assessed during the OGTT. Moreover, recommended HbA1c cutoffs for PD misclassifies a large number of subjects meeting the OGTT criteria early after pregnancy with GDM. Thus, our study corroborates the WHO recommendation that HbA1c is imprecise to exclude borderline deteriorations in carbohydrate metabolism [8]. However, as pGDM females developing overt diabetes showed significantly increasing HbA1c levels during the follow-up period, we suggest that the longitudinal assessment of HbA1c changes provides additive information for an early diagnosis of diabetes manifestation. This, however, needs to be confirmed in a larger study.
References
Bellamy L, Casas JP, Hingorani AD, Williams D (2009) Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta analysis. Lancet 373:1773–1779
International Association of Diabetes and Pregnancy Study Groups Consensus Panel (2010) International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33:676–682
Abdul-Ghani MA, Tripathy D, DeFronzo RA (2006) Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 29:1130–1139
Abdul-Ghani MA, DeFronzo RA (2009) Plasma glucose concentration and prediction of future risk of type 2 diabetes. Diabetes Care 32:S194–S198
Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M (2012) Prediabetes a high-risk state for diabetes development. Lancet 379:2279–2290
Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, Pi-Sunyer X, Fowler S, Kahn SE, Diabetes Prevention Program Research Group (2008) Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrin Metab 93:4774–4779
American Diabetes Association (2010) Standards of medical care in diabetes—2010. Diabetes Care 33:S11–S61
World Health Organisation (2011) Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. World health Organisation, Geneva
The International Expert Committee (2009) International expert committee report on the role of the A1C Assay in the diagnosis of diabetes. Diabetes Care 32:1327–1334
Cohen RM, Haggerty S, Herman WH (2010) HbA1c for the diagnosis of diabetes and prediabetes: is it time for a mid-course correction? J Clin Endocrinol Metab 95:5203–5206
Kim C, Herman WH, Cheung NW, Gunderson EP, Richardson C (2011) Comparison of haemoglobin A1c with fasting plasma glucose and 2-h postchallenge glucose for risk stratification among women with recent gestational diabetes mellitus. Diabetes Care 34:1949–1951
Picon MJ, Murri M, Munoz A, Fernandez-Garcia JC, Gomez-Huelgas R, Tinahones FJ (2012) Hemoglobin A1c versus oral glucose tolerance test in postpartum screening. Diabetes Care 35:1648–1653
Winzer C, Wagner O, Festa A, Schneider B, Roden M, Bancher-Todesca D, Pacini G, Funahashi T, Kautzky-Willer A (2004) Plasma adiponectin, insulin sensitivity, and subclinical inflammation in women with prior gestational diabetes mellitus. Diabetes Care 27:1721–1727
Metzger BE, Coustan DR (1998) The organizing committee: summary and recommendations of the fourth international workshop-conference on gestational diabetes mellitus. Diabetes Care 21(2):161–167
Pacini G, Tonolo G, Sambataro M, Maioli M, Ciccarese M, Brocco E, Avogaro A, Nosadini R (1998) Insulin sensitivity and glucose effectiveness: minimal model analysis of regular and insulin-modified FSIGT. Am J Physiol Endocrinol Metab 274:E592–E599
Kahn SE, Prigeon RL, McCulloch DSK (2002) Quantification of the relationship between insulin sensitivity and β-cell function in human subjects: evidence for a hyperbolic function. Diabetes 42:1663–1672
Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ (2001) A model based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 24:539–548
Tura A, Kautzky-Willer A, Pacini G (2006) Insulinogenic indices from insulin and C-peptide:comparison of beta-cell function from OGTT and IVGTT. Diabetes Res Clin Pract 72:298–301
Göbl CS, Bozkurt L, Prikoszovich T, Winzer C, Pacini G, Kautzky-Willer A (2011) Early possible risk factors for overt diabetes after gestational diabetes mellitus. Obstet Gynecol 118:71–78
Bozkurt L, Göbl CS, Tura A, Chmelik M, Prikoszovich T, Kosi L, Wagner O, Roden M, Pacini G, Gastaldelli A, Kautzky-Willer A (2012) Fatty liver index predicts further metabolic deteriorations in women with previous gestational diabetes. PLoS ONE 7:e32710
Tura A, Grassi A, Winhofer Y, Guolo A, Pacini G, Mari A, Kautzky-Willer A (2012) Progression to type 2 diabetes in women with former gestational diabetes: time trajectories of metabolic parameters. PLoS ONE 7:e50419
Göbl CS, Bozkurt L, Prikoszovich T, Tura A, Pacini G, Kautzky-Willer A (2013) Estimating the risk after gestational diabetes mellitus: can we improve the information of the postpartum OGTT? Am J Physiol Endocrinol Metab 304:E524–E530
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform 7:77. doi:10.1186/1471-2105-12-77
R Core Team (2013). R: a language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org/ 6 June 2013
Lorenzo C, Wagenknecht LE, Hanley AJ, Rewers MJ, Karter AJ, Haffner SM (2010) A1C between 5.7 and 6.4 % as a marker for identifying pre-diabetes, insulin sensitivity and secretion, and cardiovascular risk factors: the Insulin Resistance and Atherosclerosis Study (IRAS). Diabetes Care 33:2104–2109
Kanat M, Winnier D, Norton L, Arar N, Jenkinson C, DeFronzo RA, Abdul-Ghani MA (2011) The relationship between β-cell function and glycated hemoglobin: results from the veterans administration genetic epidemiology study. Diabetes Care 34:1006–1010
Bianchi C, Miccoli R, Bonadonna RC, Giorgino F, Frontoni S, Faloia E, Marchesini G, Dolci MA, Cavalot F, Cavallo GM, Leonetti F, Del Prato S, GENFIEV Investigators (2012) Pathogenetic mechanisms and cardiovascular risk: differences between HbA(1c) and oral glucose tolerance test for the diagnosis of glucose tolerance. Diabetes Care 35:2607–2612
Ahrén B, Pacini G (2004) Importance of quantifying insulin secretion in relation to insulin sensitivity to accurate assess beta cell function in clinical studies. Eur J Endocrinol 150:97–104
Kautzky-Willer A, Handisurya A (2009) Metabolic diseases and associated complications: sex and gender matter! Eur J Clin Invest 39:631–648
Morris DH, Khunti K, Achana F, Srinivasan B, Gray LJ, Davies MJ, Webb D (2013) Progression rates from HbA1c 6.0–6.4% and other prediabetes definitions to type 2 diabetes: a meta analysis. Diabetologia 56:1489–1493
Mann DM, Carson AP, Shimbo D, Fonseca V, Fox CS, Mutner P (2010) Impact of A1C screening criterion on the diagnosis of pre-diabetes among U.S. adults. Diabetes Care 33:2190–2195
James C, Bullard KM, Rolka DB, Geiss LS, Williams DE, Cowie CC, Albright A, Gregg EW (2011) Implications of alternative definitions of prediabetes for prevalence in U.S. adults. Diabetes Care 34:387–391
Lapolla A, Mosca A, Fedele D (2011) The general use of glycated haemoglobin for the diagnosis of diabetes and other categories of glucose intolerance: still a long way to go. Nutr Metab Cardiovasc Dis 21:467–475
Seals DR, Hagberg JM, Allen WK, Hurley BF, Dalsky GP, Ehsani AA, Holloszy JO (1984) Glucose tolerance in young and older athletes and sedentary men. J Appl Physiol 56:1521–1525
Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, Lachin JM, Montez MG, Brenneman T, Barrett-Connor E (2007) Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the diabetes prevention program. Diabetes Care 30:2453–2457
Soranzo N, Sanna S, Wheeler E, Gieger C, Radke D, Dupuis J, Bouatia-Naji N, Langenberg C, Prokopenko I, Stolerman E, Sandhu MS, Heeney MM, Devaney JM, Reilly MP, Ricketts SL (2010) Common variants at ten genomic loci influence haemoglobin A1C levels via glycemic and non-glycemic pathways. Diabetes 59:3229–3239
Cohen RM, Franco RS, Khera PK, Smith EP, Lindsell CJ, Ciraolo PJ, Palascak MB, Joiner CH (2008) Red cell lifespan heterogeneity in haematologically normal people is sufficient to alter HbA1c. Blood 112:4284–4291
Hardikar PS, Joshi SM, Bhat DS, Raut DA, Katre PA, Lubree HG, Jere A, Pandit AN, Fall CHD, Yajnik CS (2012) Spuriously high prevalence of prediabetes diagnosed by HbA1c in young Indian partly explained by haematological factors and iron deficiency anemia. Diabetes Care 35:797–802
Kim C, Bullard KM, Herman WH, Berckles GL (2010) Association between iron deficiency and A1C levels among adults without diabetes in the National Health and Nutrition Survey, 1999–2006. Diabetes Care 33:780–785
Acknowledgments
We acknowledge to Mrs. Astrid Hofer, Study Nurse, Medical University of Vienna, Department of Internal Medicine III, Mr. Thomas Prikoszovich, MD, Medical University of Vienna, Department of Internal Medicine III, and Mrs. Anita Thomas, Bsc, Department of Internal Medicine III, for helping with data assessment. The study was supported by the Austrian Science Fund (P14515-MED) to AKW.
Conflict of interest
Christian S. Göbl, Latife Bozkurt, Rajashri Yarragudi, Andrea Tura, Giovanni Pacini and Alexandra Kautzky-Willer declare they have no conflict of interest.
Statement of Human and Animal Rights
All procedures followed were in accordance with the ethical standards of the Ethics Committee of the Medical University of Vienna and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Statement of Informed consent
Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Managed by Antonio Secchi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Göbl, C.S., Bozkurt, L., Yarragudi, R. et al. Is early postpartum HbA1c an appropriate risk predictor after pregnancy with gestational diabetes mellitus?. Acta Diabetol 51, 715–722 (2014). https://doi.org/10.1007/s00592-014-0574-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-014-0574-2