Abstract
Continuous subcutaneous insulin infusion (CSII) is effective and safe in children and adults with type 1 diabetes. Notwithstanding, some patients decide to discontinue using CSII. We evaluated the discontinuation rate, and its related factors, in a large group of children and adolescents with type 1 diabetes using CSII in Italy. Data on all patients with type 1 diabetes younger than 18 years were collected by 28 Pediatric Diabetologic referral Centers located throughout Italy. The primary endpoint was to measure the discontinuation rate using CSII. Among the study population (n = 6,644), 985 (14.8%) were using CSII. Sixty patients discontinued using CSII, representing the 6.1%. The discontinuation rate significantly increased (P = 0.002) with age: 0–6 years, 1/84 (1.2%), 7–11 years, 8/262 (3.1%), 12–18 years, 51/579 (8.8%). The average time to discontinuation was 1.8 ± 1.4 years. The average age of patients who discontinued using CSII was higher than in patients still on CSII (12.1 ± 3.2 vs. 10.3 ± 3.8, P = 0.0001), while their diabetes duration was significantly shorter (8.6 ± 2.7 vs. 10.2 ± 3.7, P = 0.0001). HbA1c decreased only in patients still on CSII (8.7 ± 1.3% vs. 7.8 ± 1.3%, P = 0.02), but not in patients who discontinued using CSII (8.5 ± 1.6% vs. 8.2 ± 1.3%, P = 0.213). HbA1c might be one important indicator helpful to identify patients at higher risk discontinuing using CSII.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thirty years after its introduction, the use of continuous subcutaneous insulin infusion (CSII) appears to be the most physiologic method currently available to deliver insulin. This data keeps increasing, especially among children and adolescents. When properly used, the technique is safe and effective in patients with type 1 diabetes [1–7]. Even if there are several advantages to pump therapy [8], barriers to success still exist in the pediatric population. Indeed, a recent meta-analysis showed a less significant advantage of CSII over multiple daily insulin injections (MDI) on younger subjects [9].
Even if there is a decrease in insulin requirement around 26% in pubertal patients and around 19% in prepubertal children using CSII versus MDI [10], CSII therapy is more expensive than MDI therapy. Therefore, it is important to investigate why certain patients discontinue using CSII. Additionally, no clear criteria have been established to help the physician to identify the ‘right’ patient for CSII and to lower the discontinuation rate.
To address this issue, most of the pediatric scientific Societies (the European Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric Endocrine Society, and the International Society for Pediatric and Adolescent Diabetes, together with the European Association for the Study of Diabetes and the American Diabetes Association) published a consensus statement that summarizes the recommendations for CSII in pediatric and adolescent patients with type 1 diabetes [11]. Nevertheless, some patients still decide to discontinue using the pump. Few studies have investigated CSII discontinuation rates and reasons, especially in pediatric patients [12–16].
The aim of our study was to evaluate the discontinuation rate and its reasons in a large group of pediatric patients with type 1 diabetes using CSII. We also evaluated their clinical and disease-specific characteristics to investigate any relation with the decision to discontinue CSII therapy.
Subjects and methods
This is a multicenter observational retrospective study. The survey was taken during a 9-year period (December 1998–December 2007). Data on all patients with type 1 diabetes aged 18 years or younger were collected by 28 Pediatric Diabetologic Units. These units belong to the Study Group on Diabetes of the Italian Society of Paediatric Endocrinology and Diabetology. They are the referral centers for the diagnosis and treatment of pediatric diabetes, and are located throughout Italy, thus ensuring almost complete coverage of the country [17].
Each Centre reviewed the charts of their patients with type 1 diabetes, and the following data were collected from medical records at baseline and every 6 months for a follow-up time up to 4 years: date of birth, date of onset of diabetes, metabolic control evaluated as value of glycated hemoglobin (HbA1c), measured at each Centre using DCA-2000 Analyser, Siemens/Bayer, Italy, type of insulin therapy, insulin requirement, the age at which the pump therapy began, age at discontinuation using CSII and, when appropriate, reasons for discontinuing, body mass index, evaluated as standard deviation score (BMI-SDS), hypoglycemic and DKA episodes. Severe hypoglycemia was defined as a blood glucose <70 mg/dl (3.9 mmol/l) with a loss of consciousness. DKA was defined as blood pH <7.3 with bicarbonate <15 mEq/l and need for intravenous fluid and insulin infusion. Data collection was completed by December 2009.
Primary outcome measure was the rate of discontinuation from CSII, evaluating both timing and reasons. Secondary outcome measures were the HbA1c, insulin requirement, BMI-SDS, adverse events (severe hypoglycemia and/or DKA episodes) before, during and after CSII therapy, when appropriate.
Continuous variables are displayed as frequencies or percentages. T test, Chi-square test, and Fisher’s exact test were used to compare groups. Paired t test and ANOVA were used to analyze changes of continuous variables over time. A level of 0.05 determined statistical significance.
Results
At each Centre, a pediatric team took care of CSII initiation for each patient and his/her follow-up. No differences have been observed among the 28 sites regarding pump prescriptions, nor the sites differed in the make-up of their multi-disciplinary teams or differed in their rate of pump prescriptions.
Among the study population (n = 6,989) (Fig. 1), 985 (14.8%) patients with type 1 diabetes were identified as CSII users. After a careful evaluation of all data collected, 60 patients were identified who chose to discontinue using CSII at some time after pump initiation, representing a percentage of 6.1%. The group that discontinued CSII had a slightly non-significant higher proportion of female patients (52 vs. 48%, P = 0.873). The discontinuation rate significantly increased (P = 0.002) with age, based on age at pump initiation: 0–6 years, 1/84 (1.2%), 7–11 years, 8/262 (3.1%), 12–18 years, 51/579 (8.8%).
The average amount of time for discontinuation was 1.8 ± 1.4 years: 8 out of 60 patients (13.3%) discontinued using CSII in the first 6 months of therapy, 15 patients (25%) at 6–12 months after CSII starting, 17 patients (28.3%) between 1 and 2 years, 9 patients (15%) between 2 and 3 years, and 11 patients (18.3%) over 3 years of CSII. The patients in the 6–12 years age group discontinued using the pump in the first 12 months mostly (68%).
As pump discontinuation occurred within 4 years at most, the reference group (n = 532) consisted of all patients who maintained CSII therapy for at least 4 years. We specifically did not adjust for age or gender as these were factors we wished to examine as possible predictors. No difference has been observed between patients that entered the study and who did not about age, disease duration, baseline HbA1c, insulin requirement and BMI-SDS.
Primary reasons for pump discontinuation were grouped into 6 categories. Major problems (e.g., impairment of metabolic control and exaggerated expectations) accounted for discontinuation in 33%, diabetes burnout occurred in 18.7%, pump breaking in 3.3% and minor problems (e.g., infusion site issues) in 12%. Body image concerns associated with wearing the pump occurred in 23%, and concerns about weight gain occurred in 10%.
Comparing the two groups (discontinue using CSII = dropout group vs. non-discontinue using CSII = non-dropout group), at the moment of CSII initiation, average age of patients in the dropout group was higher than in non-dropout group (12.1 ± 3.2 vs. 10.3 ± 3.8, P = 0.0001), while diabetes duration was significantly shorter (8.6 ± 2.7 vs. 10.2 ± 3.7 years, P = 0.0001) (Table 1). After excluding the data from the 84 preschoolers whom parents management of the diabetes could have influenced the results, the effect of age and disease duration persists in the dropout group.
Before CSII initiation, HbA1c (8.5 ± 1.6 vs. 8.2 ± 1.3%, P = 0.453), insulin requirement (0.90 ± 0.30 vs. 0.87 ± 0.25 U/kg/day, P = 0.639) and BMI-SDS (0.24 ± 0.82 vs. 0.24 ± 0.86, P = 0.912) were similar in the two groups (Table 1).
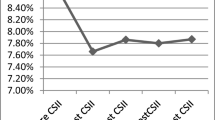
During the 4-year follow-up, patients in the dropout group did not show any significant change in HbA1c, while patients in the non-dropout group showed a significant improvement in HbA1c at 6 months (7.2 ± 1.3%, P = 0.0001), 12 months (7.2 ± 2.3%, P = 0.0001), 18 months (7.7 ± 1.3%, P = 0.0001), and 24 months (7.4 ± 2%, P = 0.0001) (Fig. 2). HbA1c kept to be significantly lower in the non-dropout patients, even when evaluated for those discontinuing CSII with an age-matched subgroup (who did not discontinue CSII) at each time point (0–6 years, comparison not possible because of the only patient in the dropout group, 7–11 years, P = 0.001 by ANOVA, 12–18 years, P = 0.0001 by ANOVA).
HbA1c values in patients who discontinued from pump (dropout group; n = 60) and who are still on pump (non-drop group; n = 532); at time point 36 and 48 months, we were not able to find any statistical significance between groups because of the small numbers left in the dropout group; the numbers in the graph upon the upper line represent the numbers of patients who discontinued from pump at each time point. *P = 0.0001 between groups
Insulin requirement and BMI-SDS showed only minimally significant differences at some time point during the follow-up period in both groups (Table 2).
Considering only patients in the dropout group, they showed no improvement in HbA1c, insulin requirement and BMI-SDS after discontinuing CSII. In particular, in the dropout group, the HbA1c was 8.6 ± 1.5% at the moment of discontinuation, and 8.5 ± 1.4% at both 6 and 12 months after discontinuation (Table 2).
No difference has been observed in DKA episodes or severe hypoglycemia intra- and inter-groups, either at baseline or during the follow-up.
Discussion
This study reports the discontinuation rate of CSII therapy in a large retrospective review of Italian type 1 diabetes pediatric patients.
We found that 60 out of 985 patients started on pump therapy discontinued the pump, and the discontinuation rate increased with age. Factors associated with pump discontinuation were older age at the time of starting pump therapy and shorter duration of diabetes before starting therapy. After excluding preschoolers to undo the possible effect of parental management on the pump therapy, this effect has been maintained. The reasons cited for discontinuation were poor metabolic control, diabetes burnout, technical issues, and concern about body weight.
While there were no differences in baseline HbA1c, BMI-SDS, or insulin requirements between groups, patients who discontinued CSII did not change their HbA1c at any time point during the follow-up and after stopping CSII. Patients who remained on pump therapy showed an improvement in glycemic control after pump therapy was initiated. Patients who continued pump therapy also decreased insulin requirements which the patients who discontinued pump therapy did not. There were no differences and frequency of DKA or hypoglycemia between patients who continued or discontinued pump therapy.
The rate of discontinuation from the pump found in this study is quite low, but in accordance with other authors [18]. Few papers have been published that address this particular aspect of insulin pump therapy [12, 13, 15, 16, 18–21], with a discontinuation rate that fully vary from 4.3 to 64%. It is difficult to explain the reasons for such a difference: the different rate of pump prescriptions in different countries, the different methodology used throughout the studies, the lack of national registries of insulin pump users might be some of the reasons. Moreover, most of the studies refer to small groups of patients followed for short time, with the exception of the one by Hofer et al. [18], which reports a 4.3% discontinuation rate in more than 11.700 patients with type 1 diabetes. However, Hofer et al. [18] did not report data only about children and adolescents, like we did in the present study. As a matter of fact, age seems to play an important role in the decision to discontinue using CSII, as we observed in our study with regard to adolescent group (12–18 years) that shows the highest rate of discontinuation, in accordance with Hofer et al. [18] and de Vries et al. [22]. This might be a little surprising since the characteristics of “freedom” and “versatility” of insulin pump therapy seem to match the typical need of this age of life. Perhaps, sometimes, exaggerated expectations might be the ultimate cause of discontinuation of pump therapy in adolescents, showing once again that adolescence is a challenging period with the need of constant parental supervision throughout it [23, 24].
Moreover, combining the evidence that patients who discontinued using the pump showed a shorter disease duration and were older than patients who did not discontinue, we speculate that diabetes onset at an older age may result in a greater burden, with patients showing more difficulties to accept diabetes and its management, especially CSII, that might make them feel more “different”.
As physicians, our main duty would be to help our adolescent patients to better cope with diabetes management. Probably, there is not a magic formula for this, but the best we may do is to aim for an even more tailored education. For sure, each time we did not see any improvement in HbA1c after 6 months of a new therapy, we are facing a failure, and we need to do our best to give that patient reasons to escape long-term complications and improve his/her compliance.
The relationship between shorter disease duration and pump failure might be explained by the fact that younger children usually show a better compliance to diabetes management than adolescents in whom diabetes burden is especially demanding [25]. On the other hand, CSII therapy requires a strong motivation, attitude, and discipline that by themselves might explain the easier failure that adolescent group experience. Last but not least, younger patients might count on parental help.
Evaluating the causes of CSII discontinuation, unlike what has been reported in the literature [12, 14], pump breaking and technical problems account only for a minor number of patients, showing the great improvement in pump technology observed in the last few years. More than half of the patients discontinued the pump because of psychological concerns. Psychological issues are very important in chronic illnesses [26] (e.g., type 1 diabetes), suggesting the need of multidisciplinary team approach, with a psychologist and dietician side by side with adult or pediatric diabetologist and nurse. Strikingly, one-third of these patients experienced unease with their body image or had fear of weight gain. At this respect, we would like to hint at diabulimia, only recently receiving attention, but not a medically recognized condition yet [27]. The lack of proper insulin treatment in those with diabetes may lead to many harmful physical effects. The misconceptions and exaggerated expectations of teens, especially with excess eating and omitted insulin problems, may lead the patients to the acknowledgment that pumps would not solve this issue at all and choose to discontinue using the pump, as we had observed some years ago in patients using multiple daily injection schemes [28]. The diabetes team task should reassure the adolescent that the correct use of insulin pump prevents him or her from becoming overweight, as stated in recent literature [6] and confirmed in our non-dropout group.
The most important cause that accounts for the majority of patients discontinuing from CSII is an impairment of metabolic control and/or exaggerated expectations from pump efficiency. HbA1c might be the cornerstone upon which lays the selection of the “right” patient that could use CSII therapy and will not discontinue using it after some time. As a matter of fact, it is interesting to observe the trend of HbA1c in the two groups (dropout and non-dropout). At the moment of CSII initiation, the patients in the dropout group presented HbA1c slightly but not significantly higher than patients who did not discontinue using CSII, stating that the starting HbA1c might not be that important, as we previously suggested [29], as well as others recently observed [22]. Instead, an in-depth look at what happens in the following months might be of great interest. The non-dropout group showed a significant improvement in HbA1c just 6 months after CSII initiations and kept lower HbA1c during the whole follow-up. On the contrary, in the dropout group, the HbA1c held steady for the whole time, even after CSII discontinuation. A lack of improvement in the metabolic control after 6–12 months after the starting of the new therapy might suggest the need to reinforce both education and motivation in order to avoid discontinuation using the pump in the following months.
Similar observations could be done regarding insulin requirement that in the dropout group did not change neither after CSII initiation nor during follow-up or after discontinuing from using the pump. On the contrary, switching from MDI to CSII therapy usually involves a decrease in insulin requirement as noted by Colino et al. [10] and in our non-dropout group.
Finally, we would like to highlight the limitations of the present study in order to guide future research on this topic. Indeed, while the large number of patients included likely makes the data valid, the retrospective review aspect is not ideal: several questions cannot be addressed by this study. In particular, it would be interesting to know who made the choice to start pump therapy in patients who continued pump versus those who did not, if there were differences in parental oversight of pump therapy between the 2 groups, or differences in adherence to various aspects of diabetes therapy, i.e., glucose testing, diet and carbohydrate counting, insulin administration, before and during pump therapy between those who continued or discontinued therapy. These areas will clearly need to be the focus of future study if we are to truly understand who is likely to discontinue pump therapy.
In conclusion, considering the reasons for pump therapy failure is an urgent matter, especially when the world Health Care economy is considered at a crisis level. Discussions with patients and families about the best therapy choices, and improving diabetes education resources, is an important tool for keeping costs down in the management of one of the most common chronic illnesses in the world.
References
Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R (2003) Insulin pump therapy: a meta-analysis. Diabetes Care 26:1079–1087
Plotnick LP, Clark LM, Brancati FL, Erlinger T (2003) Safety and effectiveness of insulin pump therapy in children and adolescents with type 1 diabetes. Diabetes Care 26:1142–1146
Nahata L (2006) Insulin therapy in pediatric patients with type I diabetes: continuous subcutaneous insulin infusion versus multiple daily injections. Clin Pediatr 45:765–774
Ludvigsson J, Samuelsson U (2007) Continuous insulin infusion (CSII) or modern type of multiple daily injections (MDI) in diabetic children and adolescents: a critical review on a controversial issue. Pediatr Endocrinol Rev 5:666–678
Jeitler K, Horvath K, Berghold A, Gratzer TW, Neeser K, Pieber TR, Siebenhofer A (2008) Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia 51:941–951
Pańkowska E, Błazik M, Dziechciarz P, Szypowska A, Szajewska H (2009) Continuous subcutaneous insulin infusion vs multiple daily injections in children with type 1 diabetes: a systematic review and meta-analysis of randomized control trials. Pediatr Diabetes 10:52–58
Bruttomesso D, Costa S, Baritusso A (2009) Continuous subcutaneous insulin infusion (CSII) 30 years later: still the best option for insulin therapy. Diabetes Metab Res Rev 25:99–111
Pickup JC, Sutton AJ (2008) Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med 25:765–774
Monami M, Lamanna C, Marchionni N, Mannucci E (2010) Continuous subcutaneous insulin infusion versus multiple daily insulin injections in type 1 diabetes: a meta-analysis. Acta Diabetol 47(suppl 1):S77–S81
Colino E, Alvarez MA, Carcavilla A, Alonso M, Ros P, Barrio R (2010) Insulin dose adjustment when changing from multiple daily injections to continuous subcutaneous insulin infusion in the pediatric age group. Acta Diabetol 47(suppl 1):S1–S6
Phillip M, Battelino T, Rodriguez H, Danne T, Kaufman F; European Society for Paediatric Endocrinology; Lawson Wilkins Pediatric Endocrine Society; International Society for Pediatric and Adolescent Diabetes; American Diabetes Association (2007) European Association for the study of diabetes: use of insulin pump therapy in the pediatric age-group: consensus statement from the European Society for Paediatric Endocrinology, the Lawson Wilkins Pediatric Endocrine Society, and the International Society for Pediatric and Adolescent Diabetes, endorsed by the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 30:1653–1662
Wood JR, Moreland EC, Volkening LK, Svoren BM, Butler DA, Laffel LBM (2006) Durability of insulin pump use in pediatric patients with type 1 diabetes. Diabetes Care 29:2355–2360
Ronsin O, Jannot-Lamotte MF, Vague P, Lassman-Vague V (2005) Factors related to CSII compliance. Diabetes Metab 31:90–95
Schifferdecker E, Schmidt K, Boehm BO, Schatz H (1994) Long-term compliance of intensified insulin therapy. Diabetes Res Clin Pract 23:17–23
Floyd JC Jr, Cornell RG, Jacober SJ, Griffith LE, Funnell MM, Wolf LL, Wolf FM (1993) A prospective study identifying risk factors for discontinuance of insulin pump therapy. Diabetes Care 16:1470–1478
Guinn TS, Bailey GJ, Mecklenburg RS (1988) Factors related to discontinuation of continuous subcutaneous insulin-infusion therapy. Diabetes Care 11:46–51
Rabbone I, Iafusco D, Toni S, Lombardo F, Lorini R, Italian Study Group on Diabetes of Italian Society Pediatric of Endocrinology and Diabetology (ISPED) (2010) Italian (retrospective) survey on continuous subcutaneous insulin infusion (CSII) therapy in children and adolescents with type 1 diabetes mellitus. Infusystem International 9:9–13
Hofer SE, Heidtmann B, Raile K, Fröhlich-Reiterer E, Lilienthal E, Berghaeuser MA, Holl RW, DPV-Science-Initiative and the German working group for insulin pump treatment in pediatric patients (2010) Discontinuation of insulin pump treatment in children, adolescents, and young adults. A multicenter analysis based on the DPV database in Germany and Austria. Pediatr Diabetes 11:116–121
Bell DS, Ackerson C, Cutter G, Clements RS Jr (1988) Factors associated with discontinuation of continuous subcutaneous insulin infusion. Am J Med Sci 295:23–28
Knight G, Boulton AJM, Ward JD (1986) Experience of continuous subcutaneous insulin infusion in the outpatient management of diabetic teenagers. Diabetes Care 3:82–84
Brink SJ, Stewart C (1986) Insulin pump treatment in insulin-dependent diabetes mellitus. Children, adolescents, and young adults. JAMA 255:617–622
de Vries L, Grushka Y, Lebenthal Y, Shalitin S, Phillip M (2010) Factors associated with increased risk of insulin pump discontinuation in pediatric patients with type 1 diabetes. Pediatr Diabetes Aug 15 [Epub ahead of print]
Scaramuzza A, Iafusco D, Lombardo F, Rabbone I, Toni S, Italian Society of Paediatric Endocrinology and Diabetology (2008) Adolescent use of insulin and patient-controlled analgesia pump technology: a 10-year food and drug administration retrospective study of adverse events. Pediatrics 122:473–474
Pinelli L, Rabbone I, Salardi S, Toni S, Scaramuzza A, Bonfanti R, Cherubini V, Franzese A, Frongia AP, Lafusco D, Sulli N, Tumini S, Curto O, Miassimelli M, Diabetes Study Group of the Italian Society of Paediatric Endocrinology and Diabetology (2008) Insulin pump therapy in children and adolescents with type 1 diabetes: the Italian viewpoint. Acta Biomed 79:57–64
Havelka M, Lucanin JD, Lucanin D (2009) Biopsychosocial model—the integrated approach to health and disease. Coll Antropol 33:303–310
Malik JA, Koot HM (2009) Explaining the adjustment of adolescents with type 1 diabetes: role of diabetes-specific and psychosocial factors. Diabetes Care 32:774–779
Hasken J, Kresl L, Nydegger T, Temme M (2010) Diabulimia and the role of school health personnel. J Sch Health 80:465–469
Iafusco D, Vanelli M, Gugliotta M, Iovane B, Chiari G, Prisco F (2004) Prevalence of eating disorders in young patients with type 1 diabetes from two different Italian cities. Diabetes Care 27:2278
Iafusco D, Confetto S, Prisco F, Lombardo F, Salzano G, De Luca F (2006) The egg or the chicken? Should good compliance to multi-injection insulin therapy be a criterion for insulin pump therapy, or does insulin pump therapy improve compliance? J Pediatr 148:421
Conflict of interest
None of the authors (neither the one listed in the title page nor the one listed in the “Appendix”) has any conflict of interest to declare. The study was not sponsored.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study is conducted for the Diabetes Study Group of the Italian Society of Paediatric Endocrinology and Diabetology (ISPED). All authors are listed in the “Appendix”.
Appendix
Appendix
R. Lera, Alessandria
V. Cherubini, Ancona
C. Zecchino, Department of Pediatrics, Bari
E. Frezza, Policlinico Giovanni XXIII, Bari
S. Zucchini, S. Salardi, Bologna
P. Frongia, Brotzu Hospital, Cagliari
D. Lo Presti, Catania
F. Citriniti, Catanzaro
S. Tumini, F. Chiarelli, Chieti
L. Spallino, Como
N. Lazzaro, Crotone
S. Toni, Firenze
G. d’Annunzio, R. Lorini, Genova
M. Bruzzese, Locri
F. Lombardo, G. Salzano, F. De Luca, Messina
R. Bonfanti, F. Meschi, San Rafael Institute, Milano
A. Scaramuzza, G. V. Zuccotti, University of Milano, Luigi Sacco Hospital, Milano
D. Iafusco, F. Prisco, Second University, Napoli
P. Buono, A. Franzese, Federico II University, Napoli
F. Cadario, Novara
V. Calcaterra, Pavia
L. Iughetti, Reggio Emilia
R. Schiaffini, M. Cappa, Bambin Gesù Hospital, Roma
M. Delvecchio, San Giovanni Rotondo
I. Rabbone, F. Cerutti, Torino
F. Fontana, Tortona
L. Guearraggio, Tradate
A. Salvatoni, Varese
Rights and permissions
About this article
Cite this article
Lombardo, F., Scaramuzza, A.E. & Iafusco, D. Failure of glycated hemoglobin drop after continuous subcutaneous insulin infusion initiation may indicate patients who discontinue: a 4-year follow-up study in children and adolescents with type 1 diabetes. Acta Diabetol 49 (Suppl 1), 99–105 (2012). https://doi.org/10.1007/s00592-011-0344-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-011-0344-3