Abstract
Aims
Glargine 300 U/mL (Gla-300) has been recently approved for use in children and adolescents with type 1 diabetes (T1D). However, real-world effectiveness data are scarce, and aim of this analysis was to assess clinical outcomes in young patients with T1D switching from 1st generation basal insulin (1BI) to Gla-300.
Methods
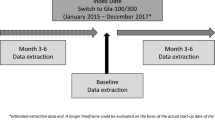
ISPED CARD is a retrospective, multicenter study, based on data anonymously extracted from Electronic Medical Records. The study involved a network of 20 pediatric diabetes centers. Data on all patients aged < 18 years with T1D switching from 1BI to Gla-300 were analyzed to assess clinical characteristics at the switch and changes after 6 and 12 months in glycated hemoglobin (HbA1c), fasting blood glucose (FBG), and standardized body mass index (BMI/SDS). Titration of basal and short-acting insulin doses was also evaluated.
Results
Overall, 200 patients were identified. The mean age at the switch to Gla-300 was 13 years, and mean duration of diabetes was 3.9 years. Average HbA1c levels at switch were 8.8%. After 6 months, HbA1c levels decreased by − 0.88% (95% CI − 1.28; − 0.48; p < 0.0001). The benefit was maintained after 12 months from the switch (mean reduction of HbA1c levels − 0.80%, 95% CI − 1.25; − 0.35, p = 0.0006). Trends of reduction in FBG levels were also evidenced both at 6 months and 12 months. No significant changes in short-acting and basal insulin doses were documented.
Conclusions
The study provides the first real-world evidence of the effectiveness of Gla-300 in children and adolescents with T1D previously treated with 1BI. The benefits in terms of HbA1c levels reduction were substantial, and sustained after 12 months. Additional benefits can be expected by improving the titration of insulin doses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the treatment of children and adolescents with type 1 diabetes (T1D), the achievement and maintenance of good glycemic control play a key role in delaying or reducing the risk of acute complications and microvascular and macrovascular complications [1]. As the gold standard of therapy, the International Society for Pediatric and Adolescent Diabetes (ISPAD) recommends intensive insulin treatment set from the very onset of diabetes [2].
T1D patients require multiple daily insulin injections (≥ 4 under the basal-bolus regimen) or continuous subcutaneous insulin infusion (CSII), frequent or continuous checking of blood glucose levels associated with control of dietary regimen and carbohydrate intake, increased physical activity, and the acquisition of skills needed for the self-management of the disease and the use of technological devices [3]. All these factors may have a significant impact on young patients and their families. In fact, they often experience a significant level of anxiety which impairs their quality of life (QoL) [4, 5] and results in suboptimal diabetes and glucose control [6, 7]. Real-world data show that only 30% of children are able to reach the international glycemic targets [8].
New formulations of basal insulins (BIs) have the potential to improve the care of T1D. Two recent BIs have been developed in the latest years: degludec 100 U/mL (Deg-100) and glargine 300 U/mL (Gla-300). Pharmacokinetic (PK) and pharmacodynamic (PD) studies [9,10,11] and randomized clinical trials on adult [12] and pediatric [13, 14] T1D populations supported the evidence of advantages of both second-generation basal insulins (2BIs) related to their physiological profile, greater dosing flexibility, and better safety than first generation basal insulin (1BI). These features are considered an opportunity to support the achievement of optimal glycemic control and the reduction of acute and chronic complications, while improving the QoL of T1D populations [15].
In Italy, Deg-100 was approved in 2015 for T1D and T2D adults and in February 2017 in T1D patients aged ≥ 1 year, while glargine 300 U/mL (Gla-300) was approved in 2017 for T1D and T2D adults and in December 2020 for T1D patients aged ≥ 6 years.
Real-world evidence (RWE) is a relevant complement to randomized clinical trials [16] and may offer an overview of the impact of use of 2BIs when used in routine clinical practice.
While several studies have investigated the effectiveness and safety of switching from 1 to 2BI in adult T1D and T2D patients, information on children and adolescents with T1D is scant, particularly regarding the switch to Gla-300.
The switch from 1BI to Deg-100 in children and adolescents with T1D under routine clinical practice conditions has already been proven to be effective and safe in several studies [17,18,19,20,21,22]. As for the switch from 1BI to Gla-300 on children or adolescents, beyond the registration trial (EDITION JUNIOR) [13], only one real world study is available, involving newly diagnosed young patients [23].
In adult T1D populations, two real-world studies were performed. The RESTORE-1 study [24] was a retrospective chart review of more than 1000 Italian people with T1D switching from 1BIs to either Gla-300 or Deg-100. Treatment with both 2BIs was associated with similar improvements in glycemic control, without weight gain. Of note, there were no severe hypoglycemic events for Gla-300 and seven events for Deg-100 (p = 0.02). The OneCare Spanish study [25] evaluated continuous glucose monitoring (CGM) data for assessing glycemic variability in people with T1D who were switched from a 1BI (Gla-100 or detemir) to a 2BI (Gla-300 or Deg-100). It showed that the effectiveness and safety of Gla-300 are more similar than different from Deg-100, with a slightly better nocturnal glucose profile, in suboptimally controlled T1D patients switching from a 1BI to Gla-300. More recently, the In Range randomized trial compared Gla-300 and Deg-100 in adults with T1D. Using clinically relevant CGM metrics, the study showed that Gla-300 was non-inferior to IDeg-100, with comparable hypoglycaemia and safety profiles.
In Italy, the ISPED CARD initiative has been launched since 2019 [26], in line with other ongoing projects based on big data analyses such as the Italian AMD Annals [27, 28], the European DPV and SWEET projects [7, 29], or the U.S. T1D Exchange project [30].
The ISPED CARD database is periodically updated by downloading retrospective data of Electronic Medical Records (EMRs) of a large and representative network of pediatrics diabetes clinics. Data are used to assess and continuously improve quality of care and to evaluate specific aspects of care, including appropriateness of treatments and long-term outcomes.
The aim of the present analysis was to explore the effectiveness of switching from 1BI to Gla-300 in children and adolescents in the Italian pediatric usual care setting.
Methods
The ISPED CARD is an observational, retrospective, multicenter study, based on data anonymously extracted from EMRs. The study involved a network of 20 diabetes centers (1/3 of all registered Italian pediatrics diabetes clinics) located in different areas of Italy.
All participating centers adopted the same EMR (Smart Digital Clinic—METEDA SRL, San Benedetto del Tronto, Italy), allowing the extraction of a standard, reproducible set of data. Centers and patients were anonymous, and data were extracted and transferred via a standardized and validated secure procedure.
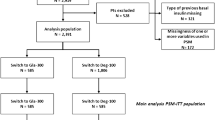
The following inclusion criteria were applied: male or female, aged from 0 to 18 years, diagnosis of T1D, switching to Gla-300 from 1BI (i.e., regular human, glargine-100, detemir, or NPH). Exclusion criteria were switching to Deg-100 or prescription of another basal insulin analogue after initiating Gla-300, and available follow-up shorter than 3 months.
The following characteristics were considered to describe the baseline patient profile: age, gender, diabetes duration, HbA1c, fasting blood glucose (FBG), standardized body mass index (BMI/SDS), lipid profile, blood pressure, insulin therapy (available in EMRs as Anatomical Therapeutic Chemical (ATC) codes).
Endpoints considered included the changes at 6 months (T6) (primary endpoint) and 12 months (T12) in HbA1c, FBG, BMI/SDS, and insulin doses, and proportion of patients at target of HbA1c < 7.5% at each study visit.
The study protocol was approved by all local ethics committees of the participating centers. Due to the study design and the anonymous by design database, based on Italian regulations, the signature of patient informed consent was not requested.
Statistical analysis
Descriptive data are expressed as mean and standard deviation or proportions. Changes in continuous endpoints were assessed using mixed models for repeated measurements. Results are expressed as estimated mean or estimated mean difference from baseline (T0) and 95% confidence interval (95% CI). Paired t tests derived from linear mixed models for repeated measurements were applied for pre-post within-group comparisons.
As categorical secondary outcomes, the proportions of patients with HbA1c ≤ 7.5% [53 mmol/mol] at each visit were evaluated using mixed effects models. The results are expressed as probability (which is equivalent to the prevalence) and Odds Ratio (OR) with relative 95% CI.
Results
Overall, from a total of 3,391 patients registered in the ISPED CARD database including data relative to the years 2015–2021, 1,370 (40.4%) switched to 2BI. Among the latter, 200 (14.6%) switched from 1BI to Gla-300 (Fig. 1).
Patient characteristics at the first prescription of Gla-300 are reported in Table 1.
The mean age at switch was 13 years, and two in three patients had over 12 years. The mean duration of T1D was 3.9 years. Average HbA1c levels at switch were 8.8%, and one in three patients had BMI/SDS > 1.5. As for previous basal insulin, 98% of patients switched to Gla-300 from insulin glargine 100 U/mL, and 2% from insulin detemir. Short acting insulin more frequently associated with Gla-300 were lispro (56.0%) and aspart (31.1%). Average basal and short acting insulin doses were 0.4 U/kg and 0.5 U/kg, respectively.
Figure 2 reports the results relative to effectiveness analysis of HbA1c. After 6 months from switching from 1BI to Gla-300, HbA1c levels decreased by − 0.88% (95% CI − 1.28; − 0.48; p < 0.0001). The benefit was maintained after 12 months from the switch (mean reduction of HbA1c levels − 0.80%, 95% CI − 1.25; − 0.35, p = 0.0006). The likelihood of reaching a HbA1c level < 7.5% was 79% (OR = 1.79; 95% CI 1.20–2.67) higher at 6 months as compared to baseline, and 60% (OR = 1.60; 95% CI 0.92–2.78) higher at 12 months (Table 2). However, less than 50% of the patients reached the desired target at T6 (46%) and T12 (43%) (Table 2).
A reduction was also documented in FBG levels both at 6 months and 12 months after the switch; however, statistical significance was not reached. No significant changes were documented for BMI/SDS and both short-acting and basal insulin doses (Table 3).
Conclusions
Efficacy and safety of Gla-300 in children and adolescents with T1D have been investigated in the EDITION JUNIOR randomized trial [13]. However, real world data confirming experimental results are lacking. This analysis represents a first picture of the effectiveness of switching from 1BI to Gla-300 in a T1D pediatric population. Data showed statistically significant improvements in HbA1c levels after 6 months, sustained at 12 months, without weight gain. Larger proportions of patients had HbA1c levels ≤ 7.5% after 6 and 12 months, as compared to baseline. These benefits were obtained despite marginal changes in the prescribed doses of basal and short-acting insulin during the follow-up. Better adherence to insulin treatment and improved pharmacokinetic and pharmacodynamic properties of second-generation basal insulins can represent possible explanations for our findings. Also, it is possible that the introduction of a new basal insulin led to more frequent dose adjustments, with a positive impact on metabolic control. The study also showed a delay in switching to a 2BI (average HbA1c levels of 8.8%).
To our knowledge, this is the first real-world study documenting the effectiveness of switching from 1BI to Gla-300 in children and adolescents with T1D. The only observational study on this topic compared the effectiveness and safety of insulin glargine 300 U/ml versus 100 U/ml in children and adolescents with newly diagnosed T1D. In this study, during the first month of follow-up after diagnosis, the Gla-300 group had a lower percentage of time below range for hypoglycaemia < 54 mg/dl, suggesting that this formulation might reduce the risk of severe hypoglycaemia. Moreover, no cases of severe hypoglycaemia were reported with Gla-300, while one case occurred with Gla-100 [23].
Our study has clinical implications, since it confirms the importance of timely switching from 1 to 2BI in children and adolescents with T1D, in order to improve metabolic control. Of note, despite the better safety profile of 2BIs documented in randomized clinical trials, Gla-300 dose was insufficiently titrated, as also suggested by the mild reduction in fasting blood glucose levels. The recent authorization to the prescription of Gla-300 in children can explain the lack of familiarity with its appropriate use in clinical practice. A greater confidence in the management of new insulins with adequate dose titration would likely produce even more relevant results, allowing the attainment of the desired therapeutic targets in a larger proportion of patients. Of note, suboptimal insulin doses titration was also documented in the EDITION JUNIOR trial, which was associated with only a moderate reduction in FBG levels [13].
In adult T1D and T2D, Gla-300 has consistently demonstrated lower hypoglycemia incidence both vs Gla-100 and Deg-100 during titration phase, a peculiar characteristic that should support the physician in doing a safer and progressive titration of insulin doses [24, 31].
Finally, the role of CGM in the treatment of pediatric patients should be discussed. The use of CGM has increased in the last years, at least in developed countries [32]. CGM was shown to be associated with lower HbA1c in both adolescents and young adults [33]. The combined use of innovative insulins and technologies can play an important role in the achievement of therapeutic targets while minimizing the risk of hypoglycemia.
The study has strengths and limitations. The major strength is the large and representative study sample, which was the result of the willingness of Italian pediatric diabetologists to voluntary collaborate to aggregate and critically review data from their clinical practice to improve quality of care. Among the limitations, it should be emphasized that participating centers were mainly located in northern-central Italy; therefore, results could be not generalizable to other Italian areas. Furthermore, additional information on timing of basal insulin administration and treatment adherence was not available and we could not assess whether this aspect could influence study results. In this respect, the achievement of a better metabolic control without substantial titration of insulin dose suggests that increased adherence could represent an important factor. An additional limitation is represented by the lack of safety information. In fact, data derived from self-monitoring of blood glucose/continuous glucose monitoring are still not systematically downloaded on EMRs. However, the collection of safety data in the context of the ISPED CARD initiative is among the objectives of the network. Furthermore, at the time of the analysis Gla-300 had been made available from a short time. Therefore, this analysis intended to provide a preliminary picture of the potential of this new therapeutic option. Comparative effectiveness analysis between Gla-300 and Deg-100 in T1D pediatric population has been planned in the next edition of ISPED CARD campaign.
In conclusion, this study provides the first real-world evidence of the effectiveness of Gla-300 in children and adolescents with T1D previously treated with 1BI. The benefits in terms of HbA1c levels reduction are substantial, and sustained after 12 months. Additional benefits can be expected by improving the titration of insulin doses.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Qualified researchers may request access to patient-level data and related documents (including, e.g., the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications). Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants.
Abbreviations
- 95% CI:
-
95% Confidence interval
- ATC:
-
Anatomical therapeutic chemical codes
- 1BI:
-
First generation basal insulin
- 2BI:
-
Second-generation basal insulins
- BI:
-
Basal insulin
- BMI:
-
Body mass index
- BMI/SDS:
-
Standardized BMI
- CSII:
-
Continuous subcutaneous insulin infusion (Insulin Pump)
- CGM:
-
Continuous glucose monitoring
- Deg-100:
-
Degludec 100 U/mL
- EMR:
-
Electronic medical records
- FBG:
-
Fasting blood glucose
- Gla-300:
-
Glargine 300 U/mL
- GP:
-
General practitioner
- HbA1c:
-
Glycated hemoglobin
- ISPED CARD:
-
Italian society of paediatric endocrinology diabetology continuous clinical monitoring of diabetes
- LDL:
-
Low-density lipoproteins
- MDI:
-
Multiple daily injections of insulin
- NPH:
-
Neutral protamine hagedorn insulin
- OR:
-
Odds Ratio
- PDOC:
-
Pediatric diabetes outpatient clinics
- RWE:
-
Real-world evidence
- SIEDP:
-
Italian society of pediatric endocrinology and diabetology
- T1D:
-
Type 1 diabetes
- T2D:
-
Type 2 diabetes
- T0:
-
Baseline
- T6:
-
Follow-up at 6 months
- T12:
-
Follow-up at 12 months
- QoL:
-
Quality of life
References
de Bock M, Codner E, Craig ME et al (2022) ISPAD Clinical Practice Consensus Guidelines 2022: glycemic targets and glucose monitoring for children, adolescents, and young people with diabetes. Pediatr Diabetes 23:1270–1276. https://doi.org/10.1111/pedi.13455
Cengiz E, Danne T, Ahmad T et al (2022) ISPAD clinical practice consensus guidelines 2022: insulin treatment in children and adolescents with diabetes. Pediatr Diabetes 23:1277–1296. https://doi.org/10.1111/pedi.13442
Limbert C, Tinti D, Malik F et al (2022) ISPAD clinical practice consensus guidelines 2022: the delivery of ambulatory diabetes care to children and adolescents with diabetes. Pediatr Diabetes 23:1243–1269. https://doi.org/10.1111/pedi.13417
Anderson BJ, Laffel LM, Domenger C et al (2017) Factors associated with diabetes-specific health-related quality of life in youth with type 1 diabetes: the global TEENs study. Diabetes Care 40:1002–1009
Driscoll KA, Raymond J, Naranjo D, Patton SR (2016) Fear of hypoglycemia in children and adolescents and their parents with type 1 diabetes. Curr Diab Rep 16:77
Foster NC, Beck RW, Miller KM et al (2019) State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther 21:66–72. https://doi.org/10.1089/dia.2018.0384
Witsch M, Kosteria I, Kordonouri O et al (2016) Possibilities and challenges of a large international benchmarking in pediatric diabetology-The SWEET experience. Pediatr Diabetes 17(Suppl 23):7–15. https://doi.org/10.1111/pedi.12432
Van Loocke M, Battelino T, Tittel SR et al (2021) Lower HbA1c targets are associated with better metabolic control. Eur J Pediatr 180:1513–1520. https://doi.org/10.1007/s00431-020-03891-2
Bailey TS, Pettus J, Roussel R et al (2018) Morning administration of 0.4U/kg/day insulin glargine 300U/mL provides less fluctuating 24-hour pharmacodynamics and more even pharmacokinetic profiles compared with insulin degludec 100 U/mL in type 1 diabetes. Diabetes Metab 44:15–21
Heise T, Hovelmann U, Nosek L, Hermanski L, Bottcher SG, Haahr H (2015) Comparison of the pharmacokinetic and pharmacodynamic profiles of insulin degludec and insulin glargine. Expert Opin Drug Metab Toxicol 11:1193–1201
Tumini S, Carinci S (2017) Unmet needs in children with diabetes: the role of basal insulin. Minerva Pediatr 69:513–530. https://doi.org/10.23736/S0026-4946.17.05112-X
Roussel R, Ritzel R, Boëlle-Le Corfec E, Balkau B, Rosenstock J (2018) Clinical perspectives from the BEGIN and EDITION programmes Trial-level meta-analyses outcomes with either degludec or glargine 300U/mL vs glargine 100U/mL in T2DM. Diabetes Metab 44:402–409
Danne T, Tamborlane WV, Malievsky OA et al (2020) Efficacy and safety of insulin glargine 300 Units/mL (Gla-300) versus insulin glargine 100 Units/mL (Gla-100) in children and adolescents (6–17 years) with type 1 diabetes: results of the EDITION JUNIOR randomized controlled trial. Diabetes Care 43:1512–1519
Urakami T, Mine Y, Aoki M, Okuno M, Suzuki J (2017) A randomized crossover study of the efficacy and safety of switching from insulin glargine to insulin degludec in children with type 1 diabetes. Endocr J 64:133–140
Maffeis C, Rabbone I (2022) Insulin glargine 300 U/mL therapy in children and adolescents with type 1 diabetes. Paediatr Drugs 24:499–512
Berger ML, Sox H, Willke RJ et al (2017) Good practices for real-world data studies of treatment and/or comparative effectiveness: recommendations from the joint ISPOR-ISPE special task force on real-world evidence in health care decision making. Value Health 20:1003–1008. https://doi.org/10.1016/j.jval.2017.08.3019
Thalange N, Deeb L, Iotova V et al (2015) Insulin degludec in combination with bolus insulin aspart is safe and effective in children and adolescents with type 1 diabetes. Pediatr Diabetes 16:164–176. https://doi.org/10.1111/pedi.12263
Predieri B, Suprani T, Maltoni G et al (2018) Switching from glargine to degludec: the effect on metabolic control and safety during 1-year of real clinical practice in children and adolescents with type 1 diabetes. Front Endocrinol (Lausanne) 9:462. https://doi.org/10.3389/fendo.2018.00462
Kochar IS, Sethi A (2018) Real-world efficacy and safety of insulin degludec with mealtime rapid-acting insulin in type 1 diabetes in Indian pediatric population. Int J Pediatr Endocrinol 2018:6. https://doi.org/10.1186/s13633-018-0059-0
Urakami T, Kuwabara R, Aoki M, Okuno M, Suzuki J (2016) Efficacy and safety of switching from insulin glargine to insulin degludec in young people with type 1 diabetes. Endocr J 63:159–167. https://doi.org/10.1507/endocrj.EJ15-0245
Schmitt J, Scott ML (2019) Insulin degludec in adolescents with Type 1 diabetes: Is newer better? - A retrospective self-control case series in adolescents with a history of diabetic ketoacidosis. Horm Res Paediatr 92:179–185. https://doi.org/10.1159/000504707
Elahi S, Patel AD, Guandalini C et al (2019) Impact of switching youth with diabetes to insulin degludec in clinical practice. Endocr Pract 25:226–229. https://doi.org/10.4158/EP-2018-0417
Rabbone I, Pozzi E, Savastio S et al (2022) A comparison of the effectiveness and safety of insulin glargine 300 U/ml versus 100 U/ml in children and adolescents with newly diagnosed type 1 diabetes: a retrospective, observational, short-term study. Diabetes Obes Metab 24:2474–2477. https://doi.org/10.1111/dom.14839
Laviola L, Porcellati F, Bruttomesso D et al (2021) Comparative effectiveness of switching from first-generation basal insulin to glargine 300 U/ml or degludec 100 U/ml in Type 1 diabetes: the RESTORE-1 study. Diabetes Ther 12:509–525. https://doi.org/10.1007/s13300-020-00982-z
Conget I, Mangas MÁ, Morales C et al (2021) Effectiveness and safety of insulin glargine 300 U/ml in comparison with insulin degludec 100 U/ml evaluated with continuous glucose monitoring in adults with type 1 diabetes and suboptimal glycemic control in routine clinical practice: the OneCARE study. Diabetes Ther 12:2993–3009. https://doi.org/10.1007/s13300-021-01153-4
Nicolucci A, Graziano G, Lombardo F et al (2024) ISPED CARD Study Group Continuous improvement of quality of care in pediatric diabetes: the ISPED CARD clinical registry. Acta Diabetol 61:599–607. https://doi.org/10.1007/s00592-023-02233-6
Rossi MC, Nicolucci A, Arcangeli A et al (2008) Baseline quality-of-care data from a quality-improvement program implemented by a network of diabetes outpatient clinics. Diabetes Care 31:2166–2168. https://doi.org/10.2337/dc08-0469
Rossi MC, Candido R, Ceriello A et al (2015) Trends over 8 years in quality of diabetes care: results of the AMD Annals continuous quality improvement initiative. Acta Diabetol 52:557–571. https://doi.org/10.1007/s00592-014-0688-6
Stahl-Pehe A, Kamrath C, Prinz N et al (2022) Prevalence of type 1 and type 2 diabetes in children and adolescents in Germany from 2002 to 2020: A study based on electronic health record data from the DPV registry. J Diabetes 14:840–850. https://doi.org/10.1111/1753-0407.13339
Beck RW, Tamborlane WV, Bergenstal RM et al (2012) The T1D exchange clinic registry. J Clin Endocrinol Metab 97:4383–4389. https://doi.org/10.1210/jc.2012-1561
Joshi SR, Singh G, Marwah A, Mittra S, Suvarna VR, Athalye SN (2023) Comparative clinical efficacy and safety of insulin glargine 300 U/ml (Toujeo) versus insulin glargine 100 U/ml in type 2 diabetes and type 1 diabetes: a systematic literature review and meta-analysis. Diabetes Obes Metab 25:1589–1606. https://doi.org/10.1111/dom.15007
Bratke H, Margeirsdottir HD, Assmus J, Njølstad PR, Skrivarhaug T (2021) Does current diabetes technology improve metabolic control? A cross-sectional study on the use of insulin pumps and continuous glucose monitoring devices in a nationwide pediatric population. Diabetes Ther 12:2571–2583. https://doi.org/10.1007/s13300-021-01127-6
Laffel LM, Kanapka LG, Beck RW et al (2020) Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA 323:2388–2396. https://doi.org/10.1001/jama.2020.6940
Acknowledgements
The authors thank the participating centers and all the collaborators from SANOFI, CORESEARCH and METEDA involved in the study. Additional Assistance: CORESEARCH (Pescara, Italy) was the Clinical Research Organization involved in the data management and statistical analysis (Giuseppe Lucisano, Giusi Graziano), medical writing (Maria Chiara Rossi, Antonio Nicolucci) and regulatory activities (Rosalia Di Lallo, Clara Santavenere) of the study. Meteda (San Benedetto, Italy) developed the software for the data extraction.
The ISPED CARD Study Group: Participating centers—year 2022 (in alphabetic order by town):
Bracciolini GP, Alessandria; Cherubini V, Ancona ; Bobbio A, Aosta; Zucchini S, Bologna; Suprani T, Cesena; De Donno V, Cuneo; Lombardo F, Messina; Bonfanti R, Milano; Franzese A, Napoli; Rabbone I, Novara; Graziani V, Ravenna; Zampolli M, San Fermo Della Battaglia (CO); Rutigliano I, San Giovanni Rotondo (FG); de Sanctis L, Torino; Guerraggio LP, Tradate (VA); Franceschi R, Trento; Tornese G, Trieste; Franco F, Udine; Maffeis C, Verona; Arnaldi C, Viterbo.
Funding
Statistical analysis and medical writing assistance was funded by Sanofi S.r.l., Milan, Italy.
Author information
Authors and Affiliations
Consortia
Contributions
All authors made substantial contributions to the conception and design of the work. Riccardo Bonfanti, Fortunato Lombardo, Ivana Rabbone, and Stefano Zucchini contributed to the data collection. Giusi Graziano conducted the statistical analyses. Maria Chiara Rossi and Antonio Nicolucci drafted the article. All authors revised the article critically for important intellectual content. All authors approved the final version to be published. All authors agreed all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Corresponding author
Ethics declarations
Conflict of interest
Maria Chiara Rossi and Antonio Nicolucci have received funding for research from Sanofi, NovoNordisk, Alfasigma, Pikdare, AstraZeneca, Shionogi, SOBI, and Theras. Riccardo Bonfanti has served on advisory board panels for Sanofi, Novonordisk, Lilly, Medtronic, Abbott, Movi and has received speaker’s fees by Novonordisk, Sanofi, Lilly, Movi, Medtronic, Theras and financial support for research by Movi and Lilly. Giusi Graziano has nothing to disclose. Monica Larosa is an employee of Sanofi and may hold shares and/or stock options in the company. Fortunato Lombardo has received consultancy fees from Movi. Stefano Zucchini has served on advisory board panels for Sanofi and Movi. Giacomo Vespasiani is medical consultant of Meteda. Ivana Rabbone has received honoraria for participating in the speaker bureau and consulting fees as a member of Eli Lilly, Menarini, Medtronic, Theras, Novo Nordisk and Sanofi advisory boards.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. The study protocol was approved by all local ethics committees of the participating centers.
Informed consent
Due to the study design and the anonymous by design database, based on Italian regulations, the signature of patient informed consent was not requested.
Additional information
Managed By Massimo Federici.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rossi, M.C., Bonfanti, R., Graziano, G. et al. Effectiveness of switching from first-generation basal insulin to Glargine 300 U/mL in children and adolescents with type 1 diabetes: results from the ISPED CARD database. Acta Diabetol 61, 1169–1176 (2024). https://doi.org/10.1007/s00592-024-02304-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-024-02304-2