Abstract
The aim of this study is to establish whether abnormal mineral metabolism is present in patients with type 1 DM with normal renal function and in the absence of microalbuminuria. Serum levels of 1,25-dihydroxyvitamin D, osteoprotegerin (OPG) and receptor activator for nuclear factor kappa β ligand (RANKL) and other determinants of bone metabolism were measured in 35 patients with type 1 DM and in 25 age-, sex- and ethnicity-matched healthy controls. Serum OPG (1.98 vs. 2.98 pmol/l: P = 0.001), 1,25-dihydroxyvitamin D (41.1 vs. 48.2 pmol/l: P = 0.035) and magnesium (0.84 vs. 0.89 mmol/l P = 0.029) levels were significantly lower in patients with type 1 DM compared to normal controls. RANKL levels were similar in both groups. The groups did not differ with respect to calcium, phosphate, PTH, 25-hydroxyvitamin D, tubular reabsorption of phosphate and cross-linked N-telopeptides of type 1-collagen levels. Abnormalities of mineral metabolism including low serum OPG and 1,25-dihydroxyvitamin D levels occur in patients with type 1 DM with normal renal function and in the absence of microalbuminuria. These abnormalities may promote altered bone metabolism and vascular pathology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In patients with diabetes mellitus (DM), vascular calcification (VC) is associated with increased morbidity and mortality due to vascular disease [1]. The etiopathogenesis of VC is multifactorial and involves an interplay of several factors including metabolic abnormalities, inflammation, mechanical and oxidative stress, endocrine and paracrine regulators, abnormal mineral metabolism and other factors promoting and inhibiting mineralisation in the vasculature [2].
Osteoprotegerin (OPG) and receptor activator for nuclear factor kappa β ligand (RANKL) are important regulators of mineral metabolism in both bone and vascular tissue [3]. In the bone milieu, continuous remodelling is carried out primarily through RANKL, one of the major osteoclast maturation factors promoting the bone resorption. OPG functions as a soluble decoy receptor for RANKL and inhibits its effects. Hence, OPG promotes bone formation, whereas, RANKL promotes bone resorption [3]. Parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D are important modulators of OPG/RANKL system along with other factors including TGF-β, glucocorticoids, oestrogen, TNF-α and IL-1 [4]. In contrast, in the vasculature, RANKL promotes calcification, whilst OPG has a protective effect [3].
Abnormal mineral metabolism has been reported to occur early in the course of chronic kidney disease (CKD) in patients with and without DM [5]. The stage at which these abnormalities appear in DM is unclear. Our aim in this study was to establish whether any such abnormalities are present in patients with DM without detectable renal pathology. To achieve this, we measured serum levels of biochemical markers of mineral metabolism, including vitamin D metabolites, OPG and RANKL in patients with type 1 DM and normoalbuminuria, and compared these to those in healthy controls.
Methods
Type 1 DM patients
We studied 35 white, type 1 DM patients, recruited from general practice surgeries in the Hertfordshire, UK. None of the patients had microalbuminuria (defined as albumin creatinine ratio (ACR) <2.5 in males and <3.5 mg/mmol in females on two or more consecutive tests, within a period of one to 3 months) [6]. Patients were screened using the following exclusion criteria—serum creatinine >125 μmol/l, history of renal disease, current pregnancy, the presence of microalbuminuria, clinical evidence of other microvascular complication of DM, malignancy, recent non-steroidal anti-inflammatory drug usage and prior or current use of immunosuppressive therapy.
Healthy control subjects
Twenty-five white, healthy control subjects were drawn from the clinical staff in Lister hospital, Hertfordshire, UK. None of the healthy subjects were on any medication.
Ethical approval and consent
The study protocol was approved by Hertfordshire research ethics committee, UK. Informed, written consent was obtained from all the participants (patients with DM and controls), after full explanation about the study and its purpose, in accordance with declaration of Helsinki 1975.
Collection of data
A detailed medical history was recorded including, age, gender, duration of diabetes, history and duration of hypertension and type of antihypertensive therapy along with other concomitant medications. A complete clinical examination was also carried out. An estimated creatinine clearance was calculated by Cockcroft–Gault formula [7], and estimated glomerular filtration rate was calculated by the MDRD-4 formula for all the study subjects [8].
Laboratory investigations
A single overnight fasting blood sample was obtained to measure fasting blood glucose (FBG), HbA1c, full blood count (FBC), blood urea, serum creatinine, albumin, C-reactive protein (CRP), bilirubin, alanine transaminase (ALT), alkaline phosphatase, parathyroid hormone (PTH), calcium, phosphate, magnesium levels and lipid profile (total cholesterol, high-density lipoprotein cholesterol, triglycerides and low-density lipoprotein cholesterol). Serum samples were centrifuged at 4,000 rpm for 15 min and stored at −80°C for subsequent analysis. A spot urine sample was collected for estimation of urinary albumin excretion, urine phosphate levels, urinary creatinine, urinary magnesium and Ntx (cross-linked N-telopeptides of type 1 collagen).
Laboratory methodology
FBC measurement was performed on the ABX Pentra® (Horiba Diagnostics, Northampton, UK). Routine biochemistry analyses (sodium, potassium, urea, creatinine, ALT, gamma glutamyl transferase (GGT), lipid profile, CRP, FBG and urine microalbumin) were performed on the Olympus AU 2700® multi-analyser (Olympus Diagnostics, Watford, UK). PTH concentrations were measured on the Beckman Access® 2 immunoassay system (Beckman Coulter (High Wycombe, UK). OPG, RANKL, 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D assays were performed in duplicate on the automated ELISA (enzyme-linked immunosorbent assay) analyser, Triturus® (Grifols, Cambridge, UK).
All analyses were performed as a single batch. These analytes were measured in duplicate to exclude the occurrence of random analytical error. The following within-run analytical coefficients of variation ranges were obtained for the respective concentration ranges. OPG (range 0.81–6.64 pmol/l, CV 0.2–9.4%), RANKL (range 0.5–9.17 pmol/l, CV 0.6–10.5%) and Ntx (range 35.0–342 nm BCE, CV 1.4–8.5%).
Statistical analysis
The statistical analysis was performed by SPSS (Statistical Package for Social Sciences) Version 16 (SPSS Inc., Chicago, USA). The results of the analysis have been presented as mean (95% confidence interval) for parametric data and as median values for non-parametric data. Kruskal–Wallis and Mann–Whitney U-tests were employed for non-parametric data to compare the differences between the groups. For categorical data, chi-squared test was employed to assess the differences between the groups. P values ≤0.05 were considered to be statistically significant.
Results
The comparative baseline characteristics, routine biochemistry and haematology of patients with type 1 DM and healthy controls are given in Table 1. There were no significant differences between the groups with respect to age, gender distribution, BMI, smoking history, blood pressure, biochemical parameters of renal and hepatic function, serum albumin, CRP and haemoglobin levels.
Routine bone biochemistry
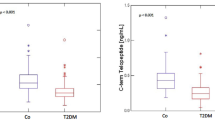
Serum calcium and phosphate levels were not statistically different between the two groups, though mean serum alkaline phosphatase levels were higher in those with DM (94 ± 27 vs. 72 ± 25 IU/l P = 0.002) (Table 2). In patients with DM, there was no correlation between total alkaline phosphatase levels and any other parameter of mineral metabolism, though there was a significant correlation with serum ALT levels (r = 0.492, P = 0.003), suggesting that the liver isoenzyme rather than the bone isoenzyme may have been the elevated moiety. Bone-specific alkaline phosphatase was not measured. PTH levels were not statistically different between patients with DM and controls (4.1 ± 1.9 vs. 3.5 ± 2.2 pmol/l, respectively), neither were urinary Ntx levels (165 ± 88 vs. 173 ± 81 units/l, respectively) or tubular reabsorption of phosphate (87.2 ± 6.6 vs. 88.6 ± 5.5%, respectively).
Vitamin D levels
There were no differences between the groups with respect to serum 25-hydroxyvitamin D levels. However, median 1,25-dihydroxyvitamin D levels were lower in type 1 (41.1 vs. 48.2 pmol/l: P = 0.035) compared to controls (Table 2). There were no significant correlations between 1,25-dihydroxyvitamin D levels and any other markers of mineral metabolism or renal function in either patients with diabetes or controls.
The OPG: RANKL axis
Median serum OPG levels were lower in type 1 DM (1.98 pmol/l) than in controls (2.98 pmol/l: P = 0.001; Fig. 1). Median RANKL levels were similar in both groups (3.44 vs. 4.05 pmol/l: P = NS). The median OPG/RANKL ratio was higher in controls but not significant (0.75 vs. 0.56). In patients with DM, OPG levels were positively correlated with 25-hydroxyvitamin D levels (r = −0.462: P = 0.005) but not with 1,25-dihydroxyvitamin D levels (Table 2). The correlation was not significant in controls. There were no other significant correlations of OPG or RANKL with other markers of mineral metabolism in either patients with diabetes or controls, though there was a weak correlation between OPG/RANKL ratio with both MDRD4-eGFR (r = −0.341: P = 0.045) and ACR (r = −0.372: P = 0.028), and between RANKL and ACR (r = −0.391: P = 0.02) in patients with DM, though not in controls. In the whole group, OPG correlated significantly with duration of diabetes (r = −0.371: P = 0.004), HbA1c (r = −0.293: P = 0.023), magnesium (r = 0.329: P = 0.010), 25-hydroxyvitamin D (r = 0.379: P = 0.003), total cholesterol (r = 0.388: P = 0.002) and LDL (r = 0.405: P = 0.003).
Magnesium levels
Mean serum magnesium levels were lower in patients with DM (0.84 vs. 0.89 mmol/l P = 0.029) than in controls. Urinary magnesium levels were higher in patients with DM (3.04 ± 1.93 vs. 2.04 ± 1.53: P = 0.037) (Table 2). The fractional excretion of magnesium (FEMg) was higher in patients with DM, but not significantly so (2.46 ± 1.33% vs. 2.08 ± 1.23%). There were no significant correlations between serum magnesium or FEMg with any other parameter of mineral metabolism in patients with DM or controls. In patients with DM, serum magnesium levels were inversely correlated with blood sugar levels (r = −0.427: P = 0.011). FEMg was positively correlated with duration of DM (r = −0.398: P = 0.018). There was also a negative correlation between FEMg and both MDRD4-eGFR (r = −0.358: P = 0.035) and Cockcroft and Gault creatinine clearance (r = −0.435: P = 0.009) in patients with DM but not in controls.
Discussion
We have described features of abnormal mineral metabolism in type 1 DM patients with normal renal function, in the absence of microalbuminuria and in the context of normal serum calcium, phosphate and PTH levels. The abnormalities include high serum total alkaline phosphate levels, low serum magnesium levels along with high urinary magnesium levels, low 1,25-dihydroxyvitamin D levels in spite of normal 25-hydroxyvitamin D levels and low serum OPG levels. The OPG/RANKL ratio was lower in patients with DM but not significant.
Low bone mineral density (BMD) is now a recognised feature of type 1 DM [9]. The pathophysiology of low BMD in type 1 DM is poorly understood. Chronic hyperglycaemia may potentially modulate the bone metabolism by its effect on Osteoblasts through osmotic and non-osmotic pathways [10]. Impaired gene expression with suppressed function and maturation of the osteoblasts, as a result of chronic hyperglycaemia, appears to be an important feature of bone loss in DM [10]. In addition, longer duration of diabetes and poor metabolic control may have an adverse impact upon the bone metabolism, resulting in low BMD [11]. Experimental studies have suggested the anabolic effects of insulin and insulin-like growth factor-1 (IGF-1) on the bone. Absence of these hormones, as seen in type 1 DM, may contribute to decreased BMD in these patients [12].
The bone and the vasculature are intricately linked through a network of common mediators of mineral metabolism. These mediators, such as OPG and RANKL, work in a concerted manner to maintain normal mineral homeostasis in the bone and vasculature [13]. OPG is a potent inhibitor of vascular calcification and bone loss by its virtue of serving as a decoy receptor for RANKL; and thus minimising its effect in terms of bone resorption and promotion of calcification in the vasculature. The process of bone formation and resorption and vascular calcification is tightly regulated by the OPG/RANKL system [13]. An imbalance in the OPG to RANKL ratio may potentially impact upon these processes.
Our finding, of significantly low OPG levels compared to controls, is in contrast with those of Rasmussen et al. [14] who reported high levels of OPG in patients with type 1 DM. However, in the Rasmussen study, the type 1 patients studied had more advanced disease with evidence of widespread microvascular complications unlike those we have reported here. Furthermore, a control group was not included [14]. One possible explanation relates to insulin therapy. It has been shown that exogenous insulin may reduce serum levels of OPG in this setting [15].
OPG levels have been reported to correlate significantly with increasing age [16] and given the fact that the patient group in our study was much younger compared to the previous study [14], which may be one of the reasons for our finding low levels of OPG. In addition, a huge analytical variation has been reported in OPG assays due to lack of standardization, [17] and this may conceivably contribute to the discrepant reports in OPG levels from various studies. We found a correlation between OPG and 25-hydroxyvitamin D levels but not with 1,25-dihydroxyvitamin D levels. The relevance of this is unknown.
Abnormal mineral metabolism with low levels of 1,25-dihydroxyvitamin D and high PTH levels has been reported to occur in the early stages of CKD [5]. Our findings though, of low 1,25-dihydroxyvitamin D levels in the context of normal renal function and in the absence of microalbuminuria suggest either that the primary cause of these low levels in this setting, is non-renal (perhaps related to hypomagnesaemia—see below), or that, as previously suggested, more subtle changes in renal function perhaps at the tubulointerstitial level, precede the onset of microalbuminuria, yet influence the production of 1,25-dihydroxyvitamin D at this site [18].
The low serum magnesium levels in patients with type 1 DM is in agreement with a previous study, which reported low serum magnesium in normoalbuminuric type 1 DM [19]. The low levels of magnesium may be due to augmented urinary excretion, as seen in our cohort, coupled with impaired intestinal absorption of magnesium when compared to healthy individuals. Magnesium deficiency may potentially impair the mineral homeostasis, leading to impaired PTH secretion, and low serum concentrations of 1,25-dihydroxyvitamin D [20].
We have demonstrated a series of abnormalities in the regulators of mineral metabolism in patients with type 1 DM with normal renal function and in the absence of microalbuminuria. It remains to be determined whether these abnormalities have a common pathogenetic basis or whether different mechanisms are responsible for each. Their clinical impact also remains to be elucidated, though the potential for adverse clinical effects is significant. OPG had a significant correlation with important players in the vessel wall such as magnesium, 25-hydroxyvitamin D, total cholesterol and LDL cholesterol, suggesting a nexus between these vascular parameters. Clinically, OPG levels may serve as a surrogate marker of bone and vascular health in patients with DM. 1,25-dihydroxyvitamin D has major effects on mineral metabolism and wide-ranging pleiotropic effects. Consequences of deficiency include impaired intestinal calcium absorption, impaired bone function, impaired immunity and many other potential problems related to the bone and skeletal system [21].
OPG and RANKL are key regulators of bone metabolism and vascular disease and the OPG/RANKL axis plays an important role in the osteogenic modulation of vasculature in DM [22]. In bone, RANKL functions as a major osteoclast maturation factor, promoting osteoclast activity and bone resorption, whereas OPG opposes the action of RANKL, acting as a soluble decoy receptor [23]. RANKL promotes calcification in the vessel wall, whilst OPG protects against it [13]. OPG has indirect anti-apoptotic and protective effects on the EC by serving as a decoy receptor for TNF-related apoptosis-inducing ligand (TRAIL) and minimising its effect on EC [24]. Hence, low OPG levels may tend to enhance bone resorption, endothelial dysfunction and vascular calcification all with potential deleterious clinical consequences.
Our study has a number of limitations, including small sample size, measurement of limited number of markers of bone formation and resorption, non-measurement of bone mineral density. In addition, this is a cross-sectional pilot study exploring the status of mineral metabolism in normoalbuminuric patients with type 1 DM; hence, it is difficult to establish causal relationships between altered mineral metabolism and disturbances of bone and vascular milieu.
In summary, we have demonstrated abnormalities in the regulators of vascular calcification and bone metabolism of potential clinical relevance in patients with type 1 DM with normal renal function and in the absence of microalbuminuria. The precise effects of these disturbances on endothelial function, bone metabolism and vascular calcification need further investigation. Our observations warrant further studies in larger population groups to confirm and extend the findings.
References
Chen NX, Moe SM (2003) Arterial calcification in diabetes. Curr Diab Rep 3(1):28–32
Vattikuti R, Towler DA (2004) Osteogenic regulation of vascular calcification: an early perspective. Am J Physiol Endocrinol Metab 286(5):E686–E696
Collin-Osdoby P (2004) Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res 95(11):1046–1057
Khosla S (2001) Minireview: the OPG/RANKL/RANK system. Endocrinology 142(12):5050–5055
Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL (2007) Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 71(1):31–38
Vanholder R (2006) Chronic kidney disease in adults—UK guidelines for identification, management and referral. Nephrol Dial Transplant 21(7):1776–1777
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16(1):31–41
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 130(6):461–470
Hamilton EJ, Rakic V, Davis WA, Chubb SA, Kamber N, Prince RL, Davis TM (2009) Prevalence and predictors of osteopenia and osteoporosis in adults with Type 1 diabetes. Diabet Med 26(1):45–52
Botolin S, McCabe LR (2006) Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem 99(2):411–424
Brandao FR, Vicente EJ, Daltro CH, Sacramento M, Moreira A, Adan L (2007) Bone metabolism is linked to disease duration and metabolic control in type 1 diabetes mellitus. Diabetes Res Clin Pract 78(3):334–339
Verhaeghe J, Suiker AM, Visser WJ, Van HE, Van BR, Bouillon R (1992) The effects of systemic insulin, insulin-like growth factor-I and growth hormone on bone growth and turnover in spontaneously diabetic BB rats. J Endocrinol 134(3):485–492
Schoppet M, Preissner KT, Hofbauer LC (2002) RANK ligand and osteoprotegerin: paracrine regulators of bone metabolism and vascular function. Arterioscler Thromb Vasc Biol 22(4):549–553
Rasmussen LM, Tarnow L, Hansen TK, Parving HH, Flyvbjerg A (2006) Plasma osteoprotegerin levels are associated with glycaemic status, systolic blood pressure, kidney function and cardiovascular morbidity in type 1 diabetic patients. Eur J Endocrinol 154(1):75–81
Xiang GD, Sun HL, Zhao LS (2007) Changes of osteoprotegerin before and after insulin therapy in type 1 diabetic patients. Diabetes Res Clin Pract 76(2):199–206
Szulc P, Hofbauer LC, Heufelder AE, Roth S, Delmas PD (2001) Osteoprotegerin serum levels in men: correlation with age, estrogen, and testosterone status. J Clin Endocrinol Metab 86(7):3162–3165
Dovio A, Sartori ML, Angeli A (2003) Correspondence re: A. Lipton et al., Serum osteoprotegerin levels in healthy controls and cancer patients. Clin. Cancer Res., 8: 2306-2310, 2002. Clin Cancer Res 9(6):2384–2385
Singh DK, Winocour P, Farrington K (2008) Mechanisms of disease: the hypoxic tubular hypothesis of diabetic nephropathy. Nat Clin Pract Nephrol 4(4):216–226
Sjogren A, Floren CH, Nilsson A (1986) Magnesium deficiency in IDDM related to level of glycosylated hemoglobin. Diabetes 35(4):459–463
Fatemi S, Ryzen E, Flores J, Endres DB, Rude RK (1991) Effect of experimental human magnesium depletion on parathyroid hormone secretion and 1,25-dihydroxyvitamin D metabolism. J Clin Endocrinol Metab 73(5):1067–1072
Al-Badr W, Martin KJ (2008) Vitamin D and kidney disease. Clin J Am Soc Nephrol 3(5):1555–1560
Schoppet M, Al-Fakhri N, Franke FE, Katz N, Barth PJ, Maisch B, Preissner KT, Hofbauer LC (2004) Localization of osteoprotegerin, tumor necrosis factor-related apoptosis-inducing ligand, and receptor activator of nuclear factor-kappaB ligand in Monckeberg’s sclerosis and atherosclerosis. J Clin Endocrinol Metab 89(8):4104–4112
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89(2):309–319
Malyankar UM, Scatena M, Suchland KL, Yun TJ, Clark EA, Giachelli CM (2000) Osteoprotegerin is an alpha vbeta 3-induced, NF-kappa B-dependent survival factor for endothelial cells. J Biol Chem 275(28):20959–20962
Acknowledgments
This study was funded by Renal Research Unit, Lister Hospital, Stevenage, UK, and partly supported by an unrestricted grant from Eli Lily.
Conflict of interest statement
All authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, D.K., Winocour, P., Summerhayes, B. et al. Low serum osteoprotegerin levels in normoalbuminuric type 1 diabetes mellitus. Acta Diabetol 47 (Suppl 1), 105–110 (2010). https://doi.org/10.1007/s00592-009-0146-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-009-0146-z