Abstract
The aim of this study was to analyze the presence of lithogenic metabolic factors in the blood and urine of patients with osteopenia versus osteoporosis. This is a cross-sectional study including 67 patients who were divided into two groups according to the presence of either osteopenia or osteoporosis as measured by bone densitometry: group 1—40 patients with osteopenia (22 men and 18 women) and group 2—27 patients with osteoporosis (13 men and 14 women). Metabolic studies were performed on the blood and urine; statistical analysis was performed comparing means and conducting linear correlation and multivariate analyses with SPSS. Statistical significance was considered to be p ≤ 0.05. The mean age of patients in group 1 was 52.9 ± 12.8 years versus 50.3 ± 11.4 in group 2; the difference was not statistically significant. In group 2, higher levels of osteocalcin, β-crosslaps, urinary calcium, fasting urine calcium/creatinine, 24 h urine calcium/creatinine and 24 h oxaluria were observed compared to group 1. In the multivariate analysis, only the β-crosslaps and urinary calcium were independently associated with osteoporosis. It would be advisable to determine the urinary calcium levels in patients with osteoporosis since altered levels may necessitate modifying the diagnostic and therapeutic approach to osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a primary or secondary progressive bone disease associated with many factors. Its incidence and prevalence have been increasing, and there are different risk factors that can be attributed to this increase. Osteoporosis is diagnosed based on the T-score [<−2.5 standard deviation (SD)] from measuring the bone mineral density by dual energy X-ray absorptiometry. Although it affects more women since estrogen is an important risk factor, osteoporosis has become more common in men in recent years [1, 2]. In secondary osteoporosis, the presence of idiopathic hypercalciuria is striking, which is also the most common metabolic abnormality in patients with calcium nephrolithiasis [3–6]. Hypercalciuria, which is urine calcium excretion higher than 260 mg in 24 h, is now subcategorized as absorptive hypercalciuria and fasting hypercalciuria, since the previous classification suggested by Pak is controversial [7]. The presence of fasting hypercalciuria has been observed in a significant percentage of patients with bone mineral density loss and calcium stones [8], which must be taken into account not only in this particular group of patients, but also in patients without lithiasis. In children, hypercalciuria has been observed to be an important factor determining bone growth and development [9–11]. Taken together, it appears that hypercalciuria may be present in a significant percentage of patients with bone mineral density loss. It is unclear, however, whether hypercalciuria together with other factors may favor the development of kidney calcium stones. The objective of this study was to analyze in a group of patients with bone mineral density loss, the differences in metabolic parameters in the blood and urine based on whether osteopenia (−1 to −2.5 standard deviation) or osteoporosis (<−2.5 standard deviation) was present.
Materials and methods
Study group
We conducted a cross-sectional study from January 1, 2013 to December 31, 2013 that included a total of 67 patients between ages 18–70 years with loss of bone mineral density and without a history of nephrolithiasis, who were treated in the Endocrinology and Rheumatology department. This group of patients was a part of a larger study that included patients with recurrent calcium nephrolithiasis and those without nephrolithiasis or loss of bone mineral density. In the current study, a subanalysis was performed in the group of patients with only the loss of bone mineral density.
-
Inclusion criteria Men and women between ages 18 and 70 years with bone mineral density loss and without calcium stones.
-
Exclusion criteria Patients with ages under 18 years or over 70 years, congenital bone disease, congenital renal disease, hyperparathyroidism, inflammatory bowel disease, renal tubular acidosis, treatment with bisphosphonates, hormone replacement therapy, treatment with thiazides, treatment with potassium citrate, treatment with corticosteroids or treatment with calcium and vitamin D.
The group of 67 patients with bone mineral density loss was divided into either the osteopenic or osteoporotic group:
-
Group 1: 40 patients (22 men and 18 women) with osteopenia (T-score between −1 and −2.5 SD).
-
Group 2: 27 patients (13 men and 14 women) with osteoporosis (T-score <−2.5 SD).
*Menopause was defined as the absence of menstruation after 12 months of amenorrhea.
Methodology
In all patients, medical history and physical examination were performed, and weight, height and body mass index were determined. At baseline, plain abdominal radiography and/or intravenous urography and renal ultrasound were done to rule out kidney stones.
Biochemical analysis of blood and urine along with measurement of the bone density of the hip and lumbar spine was also performed but at a later time point.
-
Parameters measured in blood: creatinine, uric acid, calcium, phosphorus, intact parathyroid hormone (iPTH), alkaline phosphatase, osteocalcin** and β-crosslaps***.
-
**Determined by chemiluminescence assay in an automatic analyzer using LIAISON-Osteocalcin (DIASORIN) (Roche Diagnostic).
-
***Determined by electrochemiluminescence immunoassay (ECLIA) using Elecsys in a MODULAR ANALYTICS E170 automatic analyzer.
-
Parameters measured in fasting urine: calcium, creatinine, oxalate, citrate, uric and calcium/creatinine ratio. After 8 h of nocturnal fasting, low intake of protein (0.8–1 g/weight kg/day) and salt (3–5 g/day) was recommended.
-
Parameters measured in 24 h urine: diuresis, creatinine, uric acid, calcium, citrate, oxalate and calcium/creatinine ratio.
-
Bone density: measured by dual energy X-ray absorptiometry (Hologic QDR 4500).
Statistical analysis
Statistical analysis of the results was conducted using Student’s t test for qualitative–quantitative variables, Chi-square test for qualitative variables, odds ratio for analysis of binary logistic regression results, and using a confidence interval of 95 % for multivariate analysis. Pearson correlation test or failing Spearman rho test was used for linear correlation analysis between quantitative variables. Normality of the variables was checked using Kolmogorov–Smirnov test and analysis of variance with Levene test. We considered results to be statistically significant if p ≤ 0.05. Analyses were conducted with SPSS 17.0 for Windows.
Ethical considerations
All patients read and signed the informed consent to participate in the study. This study was approved by the Ethics Committee of the University Hospital San Cecilio and funded by the Fundación Progreso y Salud (Junta de Andalucía, Spain).
Results
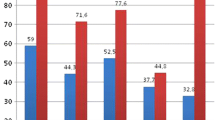
The mean age of the patients included in the study was 52.9 ± 12.8 years in group 1 versus 50.3 ± 11.4 in group 2 without any statistically significant difference. No statistically significant difference was observed regarding sex between groups. There were 32 women out of the total 67 patients. Among the 32 women, 16 had menopause with osteopenia and 11 had menopause with osteoporosis, and the difference was not statistically significant (p = 0.43). When the different parameters studied from the blood and urine of patients in the two groups were evaluated, we observed that patients with osteoporosis had higher levels of osteocalcin and β-crosslaps compared to the osteopenia group, and this was statistically significant (see Table 1). Urine analysis of patients in group 2 showed increased excretion of calcium and oxalate in the 24 h urine samples compared to group 1, which was statistically significant, and the 24 h calcium/creatinine and fasting calcium/creatinine ratios were higher compared to group 1 (see Table 2). Calcium excretion in urine had a positive linear correlation with levels of sodium (R = 0.710; p = 0.0001) and urea (R = 0.778; p = 0.0001) in the urine.
Multivariate analysis of the following variables was performed: osteocalcin, β-crosslaps, fasting urine calcium/creatinine ratio, 24 h urine calcium/creatinine ratio, 24 h urinary calcium and 24 h oxaluria. The two variables independently associated with the occurrence of osteoporosis were fasting urine calcium/creatinine ratio (p = 0.03) and β-crosslaps levels (p = 0.03).
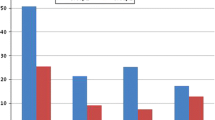
In the Pearson linear correlation analysis, the calcium excretion in 24 h urine was positively related with the levels of β-crosslaps (R = 0.258; p = 0.03) (Fig. 1); the 24 h urine calcium/creatinine and fasting urine calcium/creatinine Fig. 2 ratios showed positive linear correlation with the levels of β-crosslaps (R = 0.279; p = 0.02 and R = 0.282; p = 0.02, respectively).
Discussion
Calcium is an important and vital component for mineralization of the skeleton, and intrinsic or extrinsic alterations in bone metabolism can affect the blood and urine levels; therefore, it is critical to understand the role of hypercalciuria in bone health [12]. Furthermore, the determination of calcium in urine in patients with bone mineral density loss may have implications on medical treatment and monitoring: resulting in changes in management depending on the presence or absence of hypercalciuria [13]. As previously mentioned, while it is clear that the diagnosis of osteopenia or osteoporosis is made by bone densitometry, it may be useful to identify and measure different markers in the blood and urine of these patients. In a study by Ryan et al. [14], they noted that a group of patients with osteoporosis, hypercalciuria was also present. Studies by Cerdá et al. [15] and Peris et al. [16] detected alterations in iPTH or other markers of bone resorption. In our study, we observed that patients with osteoporosis showed higher levels of markers of bone turnover compared to patients with osteopenia; however, iPTH levels were not different between the two groups. Hence, the loss of bone density does not appear to have a direct relationship with this particular hormone in our group of patients. Other authors have analyzed the presence of hypercalciuria in women with osteoporosis, noting that about 40 % have hypercalciuria [17] and recommend testing for hypercalciuria as a part of a routine evaluation of patients with osteoporosis [18]. In the study presented here, the mean calcium excretion in 24 h urine was much higher in patients with osteoporosis versus patients with osteopenia. In addition, urinary calcium and β-crosslaps (bone resorption marker) were each an independent factor associated with osteoporosis after multivariate analysis. In addition to these findings, higher fasting and 24 h urine calcium/creatinine ratios were observed in patients with osteoporosis compared to patients with osteopenia. Some studies have postulated that there may be an increase in intestinal calcium absorption in women with osteoporosis and hypercalciuria [19], but this has not been confirmed. It seems clear that calcium metabolism is altered in patients with osteoporosis since increased levels of calcium is observed in the urine and has a positive linear relationship with levels of β-crosslaps, suggesting that with more bone resorption, calciuria is greater. This increase in urinary calcium has not produced an increase in the incidence of kidney stones. However, since hypercalciuria is a lithogenic risk factor [6], it may be important to determine the presence hypercalciuria in patients with osteoporosis. The main limitation of this study was the low number of patients. Another limitation was not taking into account the potential effects of food intake in the study group. However, our findings corroborates with the existing data in the literature in regard to the independent association of urinary calcium with osteoporosis. Calciuria could be altered in patients with incipient osteoporosis, independent of iPTH and vitamin D, probably in relation with bone intrinsic metabolism and in correlation with bone mineral density resorption markers, such as β-crosslaps. Usually, the treatment of osteoporosis is based on increasing oral calcium intake and vitamin D. This could lead to increase in calciuria in patients with previously unknown hypercalciuria. Hence, we recommend the evaluation and analysis of calciuria and fasting and 24 h urine calcium/creatinine ratios in patients with osteoporosis, as it may have possible therapeutic implications. Finally, low diet calcium intake results in increased bone mineral density loss and hypercalciuria, so we recommend a normal daily calcium intake (1000–1200 mg at day with natural sources).
References
Olszynski WP, Shawn Davison K, Adachi JD, Brown JP, Cummings SR, Hanley DA, Harris SP, Hodsman AB, Kendler D, McClung MR, Miller PD, Yuen CK (2004) Osteoporosis in men: epidemiology, diagnosis, prevention, and treatment. Clin Ther 26:15–28
Gielen E, Vanderschueren D, Callewaert F, Boonen S (2011) Osteoporosis in men. Best Pract Res Clin Endocrinol Metab. 25:321–335
Painter SE, Kleerekoper M, Camacho PM (2006) Secondary osteoporosis: a review of the recent evidence. Endocr Pract. 12:436–445
Hudec SM, Camacho PM (2013) Secondary causes of osteoporosis. Endocr Pract 19:120–128
Polymeris A, Michalakis K, Sarantopoulou V (2013) Secondary osteoporosis—an endocrinological approach focusing on underlying mechanisms. Endocr Regul. 47:137–148
Arrabal Martín M, Fernández Rodríguez A, Arrabal Polo MA, Ruíz García MJ, Zuluaga Gómez A (2006) Study of the physical-chemical factors in patients with renal lithiasis. Arch Esp Urol 59:583–594
Audran M, Legrand E (2000) Hypercalciuria. Joint Bone Spine. 67:509–515
Arrabal-Polo MA, del Carmen Cano-García M, Canales BK, Arrabal-Martín M (2014) Calcium nephrolithiasis and bone demineralization: pathophysiology, diagnosis, and medical management. Curr Opin Urol 24:633–638
Zerwekh JE (2010) Bone disease and hypercalciuria in children. Pediatr Nephrol 25:395–401
Skalova S, Palicka V, Kutilek S (2005) Bone mineral density and urinary N-acetyl-beta-D-glucosaminidase activity in paediatric patients with idiopathic hypercalciuria. Nephrology (Carlton) 10:99–102
Penido MG, Lima EM, Marino VS, Tupinambá AL, França A, Souto MF (2003) Bone alterations in children with idiopathic hypercalciuria at the time of diagnosis. Pediatr Nephrol 18:133–139
Ryan LE, Ing SW (2012) Idiopathic hypercalciuria and bone health. Curr Osteoporos Rep. 10:286–295
Bilić-Curcić I, Milas-Ahić J, Smolić M, Smolić R, Mihaljević I, Tucak-Zorić S (2009) Urolithiasis and osteoporosis: clinical relevance and therapeutic implications. Coll Antropol. 33(Suppl 2):189–192
Ryan CS, Petkov VI, Adler RA (2011) Osteoporosis in men: the value of laboratory testing. Osteoporos Int 22:1845–1853
Cerdà D, Peris P, Monegal A, Albaladejo C, Martínez de Osaba MJ, Surís X, Guañabens N (2011) Increase of PTH in post-menopausal osteoporosis. Rev Clin Esp 211:338–343
Peris P, Ruiz-Esquide V, Monegal A, Alvarez L, Martínez de Osaba MJ, Martínez-Ferrer A, Reyes R, Guañabens N (2008) Idiopathic osteoporosis in premenopausal women. Clinical characteristics and bone remodelling abnormalities. Clin Exp Rheumatol 26:986–991
Odabasi E, Turan M, Tekbas F, Kutlu M (2009) Evaluation of secondary causes that may lead to bone loss in women with osteoporosis: a retrospective study. Arch Gynecol Obstet 279:863–867
Carvalho M, Kulak CA, Borba VZ (2012) Prevalence of hypercalciuria in postmenopausal women with osteoporosis. Arq Bras Endocrinol Metabol. 56:1–5
Odvina CV, Poindexter JR, Peterson RD, Zerwekh JE, Pak CY (2008) Intestinal hyperabsorption of calcium and low bone turnover in hypercalciuric postmenopausal osteoporosis. Urol Res 36:233–239
Acknowledgments
This article is a part of doctoral thesis by Maria Sierra Girón-Prieto in the Doctorado of Medicina Clinica and Salud Publica. Granada University. This investigation has been funded by Fundacion Progreso y Salud. Consejeria de Salud. Junta de Andalucia. Pi 0766/2013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
It is a low ethically responsible study in which the patients gave their informed consent after reading the Patient Information Sheet. The study was approved by the Hospital Ethics Committee.
Conflict of interest
The authors have no conflicts of interest to report.
Rights and permissions
About this article
Cite this article
Girón-Prieto, M.S., del Carmen Cano-García, M., Poyatos-Andújar, A. et al. The value of hypercalciuria in patients with osteopenia versus osteoporosis. Urolithiasis 45, 279–283 (2017). https://doi.org/10.1007/s00240-016-0909-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-016-0909-2