Abstract
Purpose
To evaluate the efficacy of the combined intravenous and intra-articular administration of tranexamic acid (TXA) to control the collateral effects and complications of rivaroxaban (RIV) after total knee arthroplasty (TKA) and to compare thromboprophylaxis schemes with and without TXA, RIV and low molecular weight heparin (LMWH).
Materials and methods
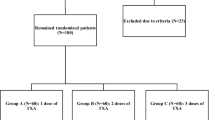
We prospectively studied 158 TKA patients from 2014 to 2018. The patients were randomly assigned into three groups. Group A (46 patients) was administered intravenous and intra-articular TXA and RIV postoperatively; group B (58 patients) was administered TXA as in group A and LMWH postoperatively; and group C (54 patients) was administered saline as in group A and RIV postoperatively. We evaluated blood loss, transfusion requirements and hemorrhagic complications.
Results
Hct and Hb values significantly decreased in group C compared to groups A and B, without any difference between groups A and B. Suction drain blood volume output was significantly higher in group C compared to group A and B, without any difference between group A and B. Hemorrhagic complications were more common in group C. No patient experienced clinical findings of VTE.
Conclusion
Combined intravenous and intra-articular administration of TXA is safe and effective in TKA, with fewer hemorrhagic complications compared to placebo. Thromboprophylaxis with RIV and LMWH is similar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total knee arthroplasty (TKA) improves mobility and quality of life and eliminates knee pain in patients with knee arthritis; however, a major postoperative complication is venous thromboembolism (VTE) [1]. Without thromboprophylaxis, the incidence of deep venous thrombosis (DVT) and pulmonary embolism (PE) ranges up to 22% and 10%, respectively [1,2,3,4,5,6,7,8]. The risk of thrombotic events can be decreased though, using prophylactic agents, such as low molecular weight heparin (LMWH) and fondaparinux that have reduced the cumulative incidence of VTE to 2.3% at 3 months after TKA [1]. Recently, new oral anticoagulants (dabigatran, rivaroxaban, apixaban) have been introduced for thromboprophylaxis in orthopedics [1,2,3]. Their use is favorable because of their easy administration, predictable pharmacokinetics, no requirement for monitoring, better patients’ compliance, and lower food and drug interactions [1,2,3]. However, the use of rivaroxaban (RIV) has been associated with a higher risk of hemorrhagic complications compared to LMWH [1,2,3,4,5,6,7,8].
Combined intravenous and intra-articular administration of tranexamic acid (TXA) in TKA patients has been associated with significant decrease in total blood loss, lower transfusion requirements, decreased postoperative hemoglobin (Hb) value, and shorter hospital stay [9,10,11,12,13,14]. Its excellent clinical efficacy and safety in comparison with intravenous TXA administration alone has been also demonstrated [9,10,11,12,13,14]. Additionally, the use of TXA in TKA patients has not been associated with adverse reactions [9,10,11,12,13, 15, 16]. However, to the best of our knowledge, the combined effect of TXA with thromboprophylaxis agents in TKA has not been specifically studied. Therefore, we performed this study to evaluate the efficacy of the combined intravenous and intra-articular administration of TXA to control the collateral effects and complications of RIV after TKA and to compare thromboprophylaxis schemes with and without TXA, RIV and LMWH.
Materials and methods
We prospectively studied a consecutive cohort of 158 patients with knee arthritis that underwent TKA at the authors’ institutions from January 2014 to January 2018. Inclusion criterion was primary varus gonarthrosis. Exclusion criteria were previous surgery at the knee, thrombocytopenia, anemia (Hb < 10 g/dl), warfarin therapy, coagulopathy, previous VTE and significant comorbidities such as ischemic heart disease, cerebrovascular accident, liver cirrhosis and renal disease. The mean follow-up was 2 years (range 7 months to 4 years); no patient was lost to follow-up. All patients gave written informed consent for their data to be included in this study. This study was approved by the Institutional Review Board/Ethics Committee of the authors’ institution.
At admission, the patients were randomly allocated into 3 study groups using a sealed envelope method. Group A patients (n = 46) were administered 10 mg/kg TXA intravenously (iv) before the inflation of the tourniquet and 1000 mg TXA intra-articularly (ia) in a 20 ml solution through the suction drainage after the completion of TKA and wound closure; postoperatively, these patients were administered 10 mg RIV daily for 25 days (a total of 30 days from the operation). Group B patients (n = 58) were administered TXA as in group A; postoperatively, these patients were administered LMWH subcutaneously (sc) daily as in group A. Group C patients (n = 54) were administered natural saline (NS) instead of TXA as in group A; postoperatively, these patients were administered RIV as in group A. Groups were similar with respect to the examined variables, without any potentially confounding variables between groups at baseline (Table 1).
All patients underwent a unilateral, cemented TKA using the same implants (EVOLUTION® Medial-Pivot Knee System, Microport Orthopedics Inc, Arlington, TN, USA). An intramedullary alignment rod was used for the femoral and an extramedullary guiding system for the tibial preparation in all cases. The tourniquet was inflated before the incision and was not released before skin closure and application of compressive dressings. A single intra-articular drain was used that was clamped for the first 3 h postoperatively; thereafter, the drain was left open and removed 48 h after surgery, no matter what the drain output was. Postoperative rehabilitation included mobilization with a walker, muscle strengthening and range of motion exercises as tolerated by the first postoperative day. The patients were discharged from the hospital when they were able to walk independently with a walker (mean 5 days; range 4–7 days postoperatively).
At baseline and within hospitalization (days 1–4), we measured hemoglobin (Hb) and hematocrit (Hct) values, prothrombin time (PT), activated partial thromboplastin time (APTT), platelet count, and total suction drain blood volume. We calculated total Hb loss by the difference between Hb at baseline and Hb at postoperative day 4, because at that time we consider blood volume to be normalized. The cutoff point for allogenic blood transfusion was set at Hb value < 8 g/dl. After discharged, clinical evaluation was done at 1, 3 and 6 months, and yearly thereafter. Clinical follow-up included wound healing and hemorrhagic complications including ecchymosis or hematoma formation around the knee, cerebrovascular events, gastrointestinal or urinary hemorrhage, and clinical signs of DVT and PE [17]. Statistical analysis was performed using IBM SPSS Software, version 20 (IBM Corp., NY, USA). The Kruskal–Wallis test was used to compare categorical variables, and the one-way ANOVA test to compare continuous variables between the three study groups. A p value < 0.05 was considered indicative for statistical significance.
Results
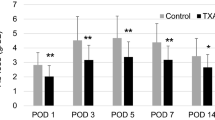
Hct and Hb values statistically significantly decreased in group C compared to groups A and B, without any difference between groups A and B (Fig. 1a, b, Table 2). Transfusions (2 units of packed red blood cells) were necessary in one patient of group B and two patients of group C. Suction drain blood volume output was significantly higher in group C compared to groups A and B, without any difference between groups A and B (Fig. 1c). The mean suction drain blood volume output was 440 ml, 511 ml and 763 ml for groups A, B and C, respectively.
a A graph shows Hct decrease was significantly higher in group C compared to groups A and B. b A graph shows that Hb decrease was significantly higher in group C compared to groups A and B. c A graph shows that suction drain blood volume output was significantly higher in group C compared to groups A and B
By direct comparison of data, hemorrhagic complications were more common in group C. One patient of group A experienced knee swelling and hematoma formation that gradually subsided during the follow-up. Three patients of group C experienced knee swelling and hematoma formation, one patient experienced extended pre-patellar ecchymosis and two patients experienced urinary tract hemorrhage (macroscopic hematuria). No patient of group B experienced any hemorrhagic complications. No patient of any group experienced any clinical signs of DVT or PE that required further imaging investigation during the follow-up period.
Discussion
TKA may be associated with substantial blood loss and need for transfusion; however, blood loss and transfusion requirements have been associated with immunologic reactions, disease transmission, and increased risk of surgical complications, time for hospitalization and overall cost of treatment [9,10,11,12,13,14]. In this setting, the use of TXA is promising, as a safe and effective method to reduce hemorrhage [9,10,11,12,13, 15, 16, 18,19,20,21], especially in combined intravenous and intra-articular administration [9,10,11,12,13]. In the present study, we evaluated the effect of TXA associated with RIV or LMWH in TKA patients. Our results showed that combined intravenous and intra-articular administration of TXA is associated with lower blood loss and fewer transfusion requirements and hemorrhagic complications compared to placebo (saline) in TKA patients, without any difference in thromboprophylaxis with RIV and LMWH. These results support the role of TXA in TKA patients regardless of the thromboprophylactic agent administered postoperatively in these patients.
ΤΧΑ is an inhibitor of the conversion of plasminogen to plasmin through the reversible blockade of fibrinolysin-binding sites on the plasminogen. Previous studies have reported the efficacy of either intravenous or intra-articular ΤΧΑ in TKA patients including significantly reduced blood loss, transfusion requirements and hospital stay without any related complications [11, 22,23,24,25,26,27]. However, to the best of our knowledge, only one published study has reported on the efficacy of combined intravenous and intra-articular TXA administration in TKA patients [26]. All the above studies concluded that TXA is effective and safe and significantly reduces postoperative blood loss and transfusion requirements, as well as wound hematoma formation [22, 23, 28,29,30,31]. Less transfusion requirements, fewer laboratory examinations and shorter hospital stay are advantages associated with TXA administration that may lead to reduction in the overall cost of treatment [21, 32,33,34]. After TKA, a 3-h suction drain clamping can result in temporary hemostasis and may control hematoma formation without increasing thromboembolic events or the risk of hematoma formation and wound healing complications [35,36,37]. There is evidence that TXA administration alone could reduce blood loss and transfusion requirements [25], but it seems that TXA plus suction drain clamping, as in this series, can achieve better results in terms of hemostasis [23].
Using appropriate thromboprophylaxis in TKA patients has decreased the incidence of DVT to 1–3% and of PE to 0.2–1.1% [38]. Conventional anticoagulants, such as LMWH, warfarin, aspirin, as well as newer agents, such as fondaparinux, RIV, apixaban and dabigatran, are available for thomboprophylaxis [16, 39]. The most widely used agents and current preferences in most guidelines are LMWH; however, LMWH have been associated with limitations such as the injectable administration, risk of heparin-induced thrombocytopenia (approximately 0.2%), and inactivation of factors related to clotting [40]. Additionally, the incidence of significant hemorrhagic complications with the use of any type of thromboprophylaxis is reported from 1.8 to 5.1%, which may be underestimated in the well-controlled reports, and this incidence may be increased with the more effective agents [41, 42]. Newer oral anticoagulants have shown equal efficacy and safety compared to LMWH, improved patients’ quality of life and treatment compliance, and healthcare cost reduction [43,44,45,46]. RIV showed superior efficacy as compared to enoxaparin for the prevention of DVT after TKA, however at the cost of an increased risk of hemorrhagic and wound healing complications, even though these were not reported at a statistically significant difference [6, 8, 38, 47, 48]. RIV has also been associated with a higher rate of hemorrhagic complications when used without TXA after TKA [30, 49]. Similarly, dabigatran led to a significant increase in postoperative wound leakage (20%) that resulted in increased hospital stay [50]. To reduce the risk of oral anticoagulants-related complications switch-therapy modalities have been recommended; the patients can take advantage of the safety of LMWH in the first postoperative days, when it is most likely to present wound bleeding events and then switch to the more convenient oral anticoagulants during the outpatient follow-up period [17].
In conclusion, combined intravenous and intra-articular administration of TXA is safe and effective in TKA, with fewer hemorrhagic complications compared to placebo. Thromboprophylaxis with RIV and LMWH is similar.
References
Messerschmidt C, Friedman RJ (2015) Clinical experience with novel oral anticoagulants for thromboprophylaxis after elective hip and knee arthroplasty. Arterioscler Thromb Vasc Biol 35(4):771–778. https://doi.org/10.1161/ATVBAHA.114.303400
Papadopoulos DV, Kostas-Agnantis I, Gkiatas I, Tsantes AG, Ziara P, Korompilias AV (2017) The role of new oral anticoagulants in orthopaedics: an update of recent evidence. Eur J Orthop Surg Traumatol 27(5):573–582. https://doi.org/10.1007/s00590-017-1940-x
Venker BT, Ganti BR, Lin H, Lee ED, Nunley RM, Gage BF (2017) Safety and efficacy of new anticoagulants for the prevention of venous thromboembolism after hip and knee arthroplasty: a meta-analysis. J Arthroplasty 32(2):645–652. https://doi.org/10.1016/j.arth.2016.09.033
Frost C, Song Y, Barrett YC, Wang J, Pursley J, Boyd RA, LaCreta F (2014) A randomized direct comparison of the pharmacokinetics and pharmacodynamics of apixaban and rivaroxaban. Clin Pharmacol 6:179–187. https://doi.org/10.2147/CPAA.S61131
Feng W, Wu K, Liu Z, Kong G, Deng Z, Chen S, Wu Y, Chen M, Liu S, Wang H (2015) Oral direct factor Xa inhibitor versus enoxaparin for thromboprophylaxis after hip or knee arthroplasty: systemic review, traditional meta-analysis, dose-response meta-analysis and network meta-analysis. Thromb Res 136(6):1133–1144. https://doi.org/10.1016/j.thromres.2015.10.009
Huisman MV, Quinlan DJ, Dahl OE, Schulman S (2010) Enoxaparin versus dabigatran or rivaroxaban for thromboprophylaxis after hip or knee arthroplasty: results of separate pooled analyses of phase III multicenter randomized trials. Circ Cardiovasc Qual Outcomes 3(6):652–660. https://doi.org/10.1161/CIRCOUTCOMES.110.957712
Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, Geerts W, Group RS (2008) Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 358(26):2765–2775. https://doi.org/10.1056/NEJMoa0800374
Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, Cushner FD, Lotke PA, Berkowitz SD, Bandel TJ, Benson A, Misselwitz F, Fisher WD, Investigators R (2009) Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 373(9676):1673–1680. https://doi.org/10.1016/S0140-6736(09)60734-0
Marra F, Rosso F, Bruzzone M, Bonasia DE, Dettoni F, Rossi R (2016) Use of tranexamic acid in total knee arthroplasty. Joints 4(4):202–213. https://doi.org/10.11138/jts/2016.4.4.202
Jain NP, Nisthane PP, Shah NA (2016) Combined administration of systemic and topical tranexamic acid for total knee arthroplasty: can it be a better regimen and yet safe? A randomized controlled trial. J Arthroplasty 31(2):542–547. https://doi.org/10.1016/j.arth.2015.09.029
Li JF, Li H, Zhao H, Wang J, Liu S, Song Y, Wu HF (2017) Combined use of intravenous and topical versus intravenous tranexamic acid in primary total knee and hip arthroplasty: a meta-analysis of randomised controlled trials. J Orthop Surg Res 12(1):22. https://doi.org/10.1186/s13018-017-0520-4
Yuan ZF, Yin H, Ma WP, Xing DL (2016) The combined effect of administration of intravenous and topical tranexamic acid on blood loss and transfusion rate in total knee arthroplasty: combined tranexamic acid for TKA. Bone Joint Res 5(8):353–361. https://doi.org/10.1302/2046-3758.58.BJR-2016-0001.R2
Lee SY, Chong S, Balasubramanian D, Na YG, Kim TK (2017) What is the ideal route of administration of tranexamic acid in TKA? A randomized controlled trial. Clin Orthop Relat Res 475(8):1987–1996. https://doi.org/10.1007/s11999-017-5311-z
Iseki T, Tsukada S, Wakui M, Yoshiya S (2018) Intravenous tranexamic acid only versus combined intravenous and intra-articular tranexamic acid for perioperative blood loss in patients undergoing total knee arthroplasty. Eur J Orthop Surg Traumatol. https://doi.org/10.1007/s00590-018-2210-2
Shen PF, Hou WL, Chen JB, Wang B, Qu YX (2015) Effectiveness and safety of tranexamic acid for total knee arthroplasty: a prospective randomized controlled trial. Med Sci Monit 21:576–581. https://doi.org/10.12659/MSM.892768
Charoencholvanich K, Siriwattanasakul P (2011) Tranexamic acid reduces blood loss and blood transfusion after TKA: a prospective randomized controlled trial. Clin Orthop Relat Res 469(10):2874–2880. https://doi.org/10.1007/s11999-011-1874-2
Ozler T, Ulucay C, Onal A, Altintas F (2015) Comparison of switch-therapy modalities (enoxaparin to rivaroxaban/dabigatran) and enoxaparin monotherapy after hip and knee replacement. Acta Orthop Traumatol Turc 49(3):255–259. https://doi.org/10.3944/AOTT.2015.14.0219
Digas G, Koutsogiannis I, Meletiadis G, Antonopoulou E, Karamoulas V, Bikos C (2015) Intra-articular injection of tranexamic acid reduce blood loss in cemented total knee arthroplasty. Eur J Orthop Surg Traumatol 25(7):1181–1188. https://doi.org/10.1007/s00590-015-1664-8
Yozawa S, Ogawa H, Matsumoto K, Akiyama H (2018) Periarticular injection of tranexamic acid reduces blood loss and the necessity for allogeneic transfusion after total knee arthroplasty using autologous transfusion: a retrospective observational study. J Arthroplasty 33(1):86–89. https://doi.org/10.1016/j.arth.2017.08.018
Ishida K, Tsumura N, Kitagawa A, Hamamura S, Fukuda K, Dogaki Y, Kubo S, Matsumoto T, Matsushita T, Chin T, Iguchi T, Kurosaka M, Kuroda R (2011) Intra-articular injection of tranexamic acid reduces not only blood loss but also knee joint swelling after total knee arthroplasty. Int Orthop 35(11):1639–1645. https://doi.org/10.1007/s00264-010-1205-3
George DA, Sarraf KM, Nwaboku H (2015) Single perioperative dose of tranexamic acid in primary hip and knee arthroplasty. Eur J Orthop Surg Traumatol 25(1):129–133. https://doi.org/10.1007/s00590-014-1457-5
Wu Y, Yang T, Zeng Y, Li C, Shen B, Pei F (2017) Clamping drainage is unnecessary after minimally invasive total knee arthroplasty in patients with tranexamic acid: a randomized, controlled trial. Medicine (Baltimore) 96(7):e5804. https://doi.org/10.1097/MD.0000000000005804
Zhang Y, Zhang JW, Wang BH (2017) Efficacy of tranexamic acid plus drain-clamping to reduce blood loss in total knee arthroplasty: a meta-analysis. Medicine (Baltimore) 96(26):e7363. https://doi.org/10.1097/MD.0000000000007363
Lin PC, Hsu CH, Chen WS, Wang JW (2011) Does tranexamic acid save blood in minimally invasive total knee arthroplasty? Clin Orthop Relat Res 469(7):1995–2002. https://doi.org/10.1007/s11999-011-1789-y
Lin PC, Hsu CH, Huang CC, Chen WS, Wang JW (2012) The blood-saving effect of tranexamic acid in minimally invasive total knee replacement: is an additional pre-operative injection effective? J Bone Joint Surg Br 94(7):932–936. https://doi.org/10.1302/0301-620X.94B7.28386
Xie J, Ma J, Huang Q, Yue C, Pei F (2017) Comparison of enoxaparin and rivaroxaban in balance of anti-fibrinolysis and anticoagulation following primary total knee replacement: a pilot study. Med Sci Monit 23:704–711
Sarzaeem MM, Razi M, Kazemian G, Moghaddam ME, Rasi AM, Karimi M (2014) Comparing efficacy of three methods of tranexamic acid administration in reducing hemoglobin drop following total knee arthroplasty. J Arthroplasty 29(8):1521–1524. https://doi.org/10.1016/j.arth.2014.02.031
Yen SH, Lin PC, Chen B, Huang CC, Wang JW (2017) Topical tranexamic acid reduces blood loss in minimally invasive total knee arthroplasty receiving rivaroxaban. Biomed Res Int 2017:9105645. https://doi.org/10.1155/2017/9105645
Hu KZ, Sun HY, Sui C (2017) Effects of five treatment regimens on blood loss and blood transfusion in total knee arthroplasty: a preliminary study in China. Int J Clin Pharmacol Ther 55(5):433–441. https://doi.org/10.5414/CP202813
Wang JW, Chen B, Lin PC, Yen SH, Huang CC, Kuo FC (2017) The efficacy of combined use of rivaroxaban and tranexamic acid on blood conservation in minimally invasive total knee arthroplasty a double-blind randomized, controlled trial. J Arthroplasty 32(3):801–806. https://doi.org/10.1016/j.arth.2016.08.020
Yen SH, Lin PC, Kuo FC, Wang JW (2014) Thromboprophylaxis after minimally invasive total knee arthroplasty: a comparison of rivaroxaban and enoxaparin. Biomed J 37(4):199–204. https://doi.org/10.4103/2319-4170.125627
Lin ZX, Woolf SK (2016) Safety, efficacy, and cost-effectiveness of tranexamic acid in orthopedic surgery. Orthopedics 39(2):119–130. https://doi.org/10.3928/01477447-20160301-05
Goyal N, Chen DB, Harris IA, Rowden N, Kirsh G, MacDessi SJ (2016) Clinical and financial benefits of intra-articular tranexamic acid in total knee arthroplasty. J Orthop Surg (Hong Kong) 24(1):3–6. https://doi.org/10.1177/230949901602400103
Vigna-Taglianti F, Basso L, Rolfo P, Brambilla R, Vaccari F, Lanci G, Russo R (2014) Tranexamic acid for reducing blood transfusions in arthroplasty interventions: a cost-effective practice. Eur J Orthop Surg Traumatol 24(4):545–551. https://doi.org/10.1007/s00590-013-1225-y
Jeon YS, Park JS, Kim MK (2017) Optimal release timing of temporary drain clamping after total knee arthroplasty. J Orthop Surg Res 12(1):47. https://doi.org/10.1186/s13018-017-0550-y
Pornrattanamaneewong C, Narkbunnam R, Siriwattanasakul P, Chareancholvanich K (2012) Three-hour interval drain clamping reduces postoperative bleeding in total knee arthroplasty: a prospective randomized controlled trial. Arch Orthop Trauma Surg 132(7):1059–1063. https://doi.org/10.1007/s00402-012-1501-z
Raleigh E, Hing CB, Hanusiewicz AS, Fletcher SA, Price R (2007) Drain clamping in knee arthroplasty, a randomized controlled trial. ANZ J Surg 77(5):333–335. https://doi.org/10.1111/j.1445-2197.2007.04053.x
Russell RD, Hotchkiss WR, Knight JR, Huo MH (2013) The efficacy and safety of rivaroxaban for venous thromboembolism prophylaxis after total hip and total knee arthroplasty. Thrombosis 2013:762310. https://doi.org/10.1155/2013/762310
Flevas DA, Megaloikonomos PD, Dimopoulos L, Mitsiokapa E, Koulouvaris P, Mavrogenis AF (2018) Thromboembolism prophylaxis in orthopaedics: an update. EFORT Open Rev 3(4):136–148. https://doi.org/10.1302/2058-5241.3.170018
Kinov P, Tanchev PP, Ellis M, Volpin G (2014) Antithrombotic prophylaxis in major orthopaedic surgery: an historical overview and update of current recommendations. Int Orthop 38(1):169–175. https://doi.org/10.1007/s00264-013-2134-8
Callaghan JJ, Dorr LD, Engh GA, Hanssen AD, Healy WL, Lachiewicz PF, Lonner JH, Lotke PA, Ranawat CS, Ritter MA, Salvati EA, Sculco TP, Thornhill TS, American Colleg of Chest Physicians (2005) Prophylaxis for thromboembolic disease: recommendations from the American College of Chest Physicians—are they appropriate for orthopaedic surgery? J Arthroplasty 20(3):273–274
Levine MN, Raskob G, Beyth RJ, Kearon C, Schulman S (2004) Hemorrhagic complications of anticoagulant treatment: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest 126(3 Suppl):287S–310S. https://doi.org/10.1378/chest.126.3_suppl.287s
Carrothers AD, Rodriguez-Elizalde SR, Rogers BA, Razmjou H, Gollish JD, Murnaghan JJ (2014) Patient-reported compliance with thromboprophylaxis using an oral factor Xa inhibitor (rivaroxaban) following total hip and total knee arthroplasty. J Arthroplasty 29(7):1463–1467. https://doi.org/10.1016/j.arth.2013.02.001
Adam SS, McDuffie JR, Lachiewicz PF, Ortel TL, Williams JW Jr (2013) Comparative effectiveness of new oral anticoagulants and standard thromboprophylaxis in patients having total hip or knee replacement: a systematic review. Ann Intern Med 159(4):275–284. https://doi.org/10.7326/0003-4819-159-4-201308200-00008
Monreal M, Folkerts K, Diamantopoulos A, Imberti D, Brosa M (2013) Cost-effectiveness impact of rivaroxaban versus new and existing prophylaxis for the prevention of venous thromboembolism after total hip or knee replacement surgery in France, Italy and Spain. Thromb Haemost 110(5):987–994. https://doi.org/10.1160/TH12-12-0919
Mahmoudi M, Sobieraj DM (2013) The cost-effectiveness of oral direct factor Xa inhibitors compared with low-molecular-weight heparin for the prevention of venous thromboembolism prophylaxis in total hip or knee replacement surgery. Pharmacotherapy 33(12):1333–1340. https://doi.org/10.1002/phar.1269
Jensen CD, Steval A, Partington PF, Reed MR, Muller SD (2011) Return to theatre following total hip and knee replacement, before and after the introduction of rivaroxaban: a retrospective cohort study. J Bone Joint Surg Br 93(1):91–95. https://doi.org/10.1302/0301-620X.93B1.24987
Galat DD, McGovern SC, Hanssen AD, Larson DR, Harrington JR, Clarke HD (2008) Early return to surgery for evacuation of a postoperative hematoma after primary total knee arthroplasty. J Bone Joint Surg Am 90(11):2331–2336. https://doi.org/10.2106/JBJS.G.01370
Chahal GS, Saithna A, Brewster M, Gilbody J, Lever S, Khan WS, Foguet P (2013) A comparison of complications requiring return to theatre in hip and knee arthroplasty patients taking enoxaparin versus rivaroxaban for thromboprophylaxis. Ortop Traumatol Rehabil 15(2):125–129. https://doi.org/10.5604/15093492.1045953
Bloch BV, Patel V, Best AJ (2014) Thromboprophylaxis with dabigatran leads to an increased incidence of wound leakage and an increased length of stay after total joint replacement. Bone Joint J 96-B(1):122–126. https://doi.org/10.1302/0301-620x.96b1.31569
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Karampinas, P.K., Megaloikonomos, P.D., Lampropoulou-Adamidou, K. et al. Similar thromboprophylaxis with rivaroxaban and low molecular weight heparin but fewer hemorrhagic complications with combined intra-articular and intravenous tranexamic acid in total knee arthroplasty. Eur J Orthop Surg Traumatol 29, 455–460 (2019). https://doi.org/10.1007/s00590-018-2307-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-018-2307-7