Abstract
Cerclage wiring is a simple technique that has been practiced widely since the advent of surgical treatment of fractures. Many studies have reported the use of various cerclage technologies with a wide range of results and clinical applications. The increasing numbers of periprosthetic fractures have led to a revival of interest for this simple technique. When cerclages function as implants, they may be used alone or together with a protecting device such as external or internal splints (such as plates, nails, stems of prosthesis or a combination of thereof). This article presents a review of the available literature relating cerclage-wiring techniques and updates the recommendations for clinical use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerclage wiring is a simple technique that has been practiced widely since the advent of surgical treatment of fractures; in fact, it may have been the first internal fixation technique. The first use of metallic cerclage for fracture fixation has been documented in a 1775 French manuscript [1]. From the mid-nineteenth century, the cerclage fixation has been introduced as an important part of the orthopedic armamentarium, but a cerclage technique using solid wire locked with a twist was described only in 1933 [2]. Since then, many studies have reported the use of various cerclage technologies with a wide range of results and clinical applications. There are four methods of wire usage: tension band, cerclage, hemi-cerclage and interfragmentary wires. The aim of this review is to examine the available literature relating cerclage-wiring techniques and update the recommendations for clinical use.
Indications
When cerclages function as implants, they may be used alone or together with a protecting device such as external or internal splints (such as plates, nails, stems of prosthesis or a combination of thereof). The indications for cerclage as an exclusive implant were limited because other technologies offer a better outcome, while the increasing numbers of periprosthetic fractures has led to a revival of interest for this simple technique. The cerclage may function in two different ways: as a temporarily tool for reduction during surgery and can be used long term as an implant. Many of the current applications are listed in Table 1 [1, 3–8], and the principals have been discussed below.
Primary fixation device for fracture
Cerclages are widely used as primary fixation device for fracture in small animal surgery [9], but as a stand-alone implant for human long bones, they are too weak to fulfill the requirements of functional aftercare [3]. In fact, cerclage itself is not strong enough to withstand forces occurring during functional fracture aftercare and requires some form of augmentation. In 1981, Soeur described a system of fracture fixation that could be considered the predecessor of contemporary methods of cerclage [7]. It consisted of two Steinmann pins placed parallel to the long axis of the bone connected with 90° wire loops used to generate interfragmentary compression and stability. Habernek et al. [6] examined 186 torsional tibial fractures as a result of skiing accidents, treated with cerclage technique. In their series, complications included 13 cases of malalignment, six superficial infection, five cases of peroneal nerve palsy and eight cases of delayed union [6]. In an attempt to minimize malunion, surgeons tried to compensate for the insufficient strength and stability of cerclage by adding an external plaster cast. The conceptual shortcomings in the use of a plaster cast are that (1) cast does not abolish displacements of the fragments due to loose coupling across soft tissues between plaster and bone; (2) cast does not prevent high loads exerted on a comparably stiff cerclage fixation and does not protected from functional load; (3) the additional weight of the plaster may increase the load exerted on the cerclage; and (4) the association of the two procedures combines the disadvantages of surgical and conservative treatments. Even if double wiring techniques or the application of many cerclages reduced the weakness of a single wire cerclage, the unsatisfactory results of cerclages as primary fixation devices (nonunions or bone resorption) have been correlated with an increased instability at the fracture site or with erroneous indications such as spiral fractures with butterfly fragment [3].
Use to prevent inadvertent fractures
A typical application consists of prophylactic placement of cerclage wires when the bone cortex is thin or has been weakened by internal fixation devices or other stress risers. Most of the instrumentation systems for prosthetic stems often include broaches designed to crush and remove cancellous bone from the diaphysis, saving the cortical endosteal bone. Intraoperative fractures can occur during broaching or insertion of prosthetic components, more commonly in cementless implants. In literature it is widely accepted that a cerclage wire could prevent inadvertent fracture if the cortex is thin or if stress risers are present because of previous internal fixation devices or disease [10–12]. Moreover, any intraoperative cracks in the cortex should be protected with cerclage wires or cables [10, 11]. It has been demonstrated that after the femur fissured and cerclage were placed to reestablish the ability to resist hoop stresses required for stem stability, the initial stability of the stems increased [12]. This suggests that the cerclage-stabilized bone is stronger than the intact bone when hoop stress is generated by an applied axial load and that prophylactic cerclage would likely protect apparently low-quality bones from fissuring [12]. Segmental deficits, such as femoral cortical windows, create stress risers in bone that predispose to postoperative fracture. For larger cortical windows, an onlay cortical allograft strut could be used, and it is typically harvested from the proximal or distal femur or from the tibia. In some cases of revision arthroplasty, the endosteal surface of the allograft strut is contoured to match the outer diameter of the host femur and is secured with multiple cerclage wires. In other cases, a massive allograft is part of the implant (the so-called allograft prosthetic composite) and cerclage wires could be used as prophylactic tool for intraoperative fractures [13].
Use for intraoperative femoral fracture
Conventional press-fit femoral implants are associated with significant peak loads during the use of impactors, and a bone fissure can be provoked intraoperatively. Intraoperative fractures are an absolute indication for the operative management of periprosthetic fractures around the femur and may call for nothing more than a single cerclage wire or cable about the femur [11, 14, 15]. Fitzgerald et al. [11] reported intraoperative fractures of the proximal femur in 3.5 % of cementless primary arthroplasties and in 17.6 % of revision procedures. They observed that most of these fractures involved the calcar and the region of the lesser trochanter and recommended prophylactic cerclage wiring of all such fractures [11]. Mallory et al. [16] considered cerclage wiring as adequate management for type I intraoperative femoral fractures (fractures that include the area of the lesser trochanter and calcar) and type II fractures (fractures extend past the lesser trochanter to a point 4 cm proximal to the tip of the prosthesis) with 100 % healing and no long-term prognostic hazards. Technically, a careful inspection of the femoral neck and greater trochanter is necessary in case of suspicious fractures that may have occurred during stem insertion. The implant should be removed, and then, the fracture should be completely expose to its distal extent. One or more cerclage wires are placed around the femoral shaft with a trial broach one size smaller inserted in the canal to prevent overtightening and potential collapse or overlap of the fracture fragments. After wire tensioning, the final component could be reinserted and further expansion of the fracture should be prevented. The cerclage stabilization of an intraoperative femoral fissure demonstrated higher resistance to pure axial loads with no additional subsidence compared with intact bone [15] and to torsional forces [17] Recently, Zeh et al. [18] demonstrated that after cerclage wiring of intraoperative fractures, no adverse effect in the sense of an impaired osteointegration of the prosthesis or loosening could be detected in the medium term. There is a tendency to underestimate such fractures and to regard them as stable; therefore, an accurate exploration of the bone distally to the visible crack is mandatory and cerclage wiring should be applied in all cases. The number of cerclages is dictated by the principle that, to stabilize a fissure and prevent its propagation, cerclage should be applied along the full length of the visible crack [15, 19].
Use for unstable intertrochanteric fractures
Fixation of unstable intertrochanteric fractures is challenging. The most common unstable intertrochanteric fractures exhibit loss of the posteromedial buttress, followed by fractures with reverse obliquity pattern [20]. The importance of the posteromedial fragment realignment is widely accepted in literature, and axial loading studies of unstable fractures have confirmed that its reduction and fixation becomes progressively more important with increasing fragment size [21]. The posteromedial fragment should be reduced without traction of the lower extremity using a bone hook and provisionally stabilized using a Verbrugge or standard reduction clamp, whereas one or more cerclage wires could be used for definitive fixation (Fig. 1). Once the posteromedial fragment is stabilized, traction is placed on the lower extremity and two main fragments reduced. Cho et al. [21] analyzed retrospectively 30 cases of unstable pertrochanteric femoral fractures, reporting good results with additional fixations such as antirotation screw of cerclage wiring. In a study on 60 patients with unstable trochanteric fractures, the Authors concluded that the application of circumferential wires in addition to intramedullary nails provides good primary reduction that is maintained over time [22].
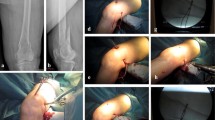
A 87-year-old man with proximal femur fracture (AO 31-A3 multifragmented). a A preoperative AP view radiograph shows the wide dislocation of the fragments. b An AP view radiograph of the implant after reconstruction with epiphysis-metaphyseal Dynamic Internal Fixator (DIF® Intrauma S.r.l., Rivoli (TO), Italy) and low-contact cerclage to stabilize the lesser trochanter. In the box an intraoperative detail of the reduction with cerclage-wiring technique
Use associated with intramedullary nailing
Open nailing of comminuted fractures carries an increased risk of infection because reduction in the multitude of small fragments requires more extensive dissection. Nailing of diaphyseal fractures without exposure of the fracture site was first advocated by Kuntscher in 1940, but only during the past two decades has this become a popular technique. Also cerclage wiring is often used to achieve stability in such a scenario, but few existing clinical studies have shown good results after the use of intramedullary fixation supplied with circumferential wires [23], in the so-called reduce with cerclage cables first, then nail operative plan (Fig. 2). Winquist et al. [24] reported the results of 245 closed intramedullary nailings, some supplemented by cerclage, with only 0.8 % nonunion and 0.4 % infection. Reduction and intramedullary fixation of subtrochanteric fractures is often challenging due to the particular anatomical features including high ratio of cortical and cancellous bone compared to other skeletal regions. Long PFN, long gamma nail or Russell Taylor nail are reliable implants for subtrochanteric fractures, leading to high rates of bone union and minimal soft tissue damage [25] (Fig. 3). However, the intraoperative use of cerclage wires and traction means that the femur is anatomically reduced prior to nailing. This makes intramedullary cannulation with a guidewire straightforward and fast, while the risk of varus malunion is eliminated [23].
A 73-year-old female with left total knee arthroplasty reported a Rorabeck type II femoral shaft fracture with stable implant. a A preoperative AP view radiograph shows the displacement of the fracture. b Through a minimally invasive technique, a cerclage wire has been used in order to align the fracture stumps, facilitating the final fixation with a retrograde nail
a A 80-year-old female reported a subtrochanteric fracture of the right femur (AO 31-A1 spiral). b The fracture has been anatomically reduced with a single low-contact cerclage (Batbridge® Intrauma S.r.l., Rivoli (TO), Italy), prior to definitive stabilization with long intramedullary nail. c An AP view radiograph shows a union without deformity after 70 days of follow-up
Use for acetabular fractures
Displaced acetabular fractures usually occur at various levels along the anterior and posterior column, and sometimes treatment is difficult, requiring an extensile exposure. The use of cerclage wiring has been successfully reported to reduce and fix acetabular fractures [8, 26], even in presence of both-column involvement [27]. Reduction is the most difficult aspect of acetabular surgery even with good exposure, and cerclage wires inserted through the greater or lesser sciatic notch are useful device. Inserting the wire is relatively easy, though, obviously, access must be available to both sides of the ilium. Lin et al. [26] reported their experience on 12 cases of displaced associated anterior column and posterior hemi-transverse acetabular fractures managed with open reduction, internal fixation with cerclage wiring and reconstruction with plates and screws. They supported the use of modified ilioinguinal approach for cerclage wires passage and the placement of clamps for reduction. Moreover, the wire may be retained to also help with fixation [8, 26]. Even if the indications for cerclage as an exclusive implant were limited, in some instances when there is no time for definitive treatment, the wire can be a time-saving device.
Use for periprosthetic femoral fractures
Fractures in close proximity to hip arthroplasties pose difficult fixation problems, especially the most common B1-fracture of the Vancouver classification [14]. Surgical management of unstable periprosthetic femoral fractures (PFF) generally is preferable [28], because of unsatisfactory results with conservative treatments [29]. Open cerclage-wiring technique is a well-known procedure for treating PFF [29], but simple cerclage wiring alone is associated with high failures rates, considering that fixation must be rigid [30]. In fact, the mechanical strength of the cerclage is insufficient to allow functional aftercare, whereas it works as a fracture reduction tool for approximation of the fragments and improves initial fixation (Fig. 4). Internal splint using locked plate provides adequate stability of the system [3]. Therefore, at today, a combination of plate and cerclage is the preferable strategy of treatment, eventually with the supplementation with strut allografts [30]. The evolution of percutaneous cerclage wiring and minimally invasive plate osteosynthesis (MIPO) techniques have allowed to reduce the complications associated with extensive exposures (Fig. 5) [31, 32]. Xue et al. [32] reported the results of the locking compress plate with mini open reduction technique with cerclage band and LCP fixation for type B1 PFFs in 12 cases, with good healing and less complications than conventional open techniques. Similar results were reported on ten cases of B1 PFFs treated with percutaneous cerclage wiring and MIPO [31]. Cerclage cables provide more tension and resistant than the twist wire to fragment reduction and can be integrated into the plate: Cables are threaded through a block, which is fitted into a groove in the plate and then crushed over the cables with a special tool. However, the cable crimping instrument is not available at now for percutaneous or minimally invasive techniques. Indications for the use of cerclage for PPFs are related to the type of fracture: It can be used in long oblique fracture, spiral fracture and some wedge fractures whereas should be avoided for transverse or short oblique fractures and multifragmented comminuted fractures.
A 87-year-old female with low general condition reported a a Vancouver B2 periprosthetic fracture with displacement of her left hip prosthesis. b Treatment consisted of removal of the implant and replacement with another long-stemmed associated with multilevel cerclage wiring in order to reduce operative time and blood loss
Use for revision of total hip arthroplasties
When performing a revision total hip arthroplasty, removal of an inaccessible distal cement mantle, infected material or distal fragment of a broken stem is a significant concern. When increased exposure is required in these situations, an osteotomy such as the extended trochanteric osteotomy [33] is usually utilized by most of the surgeons. The goal of fixation after removal of the implant is to secure the osteotomized fragment in place to allow healing. This may be accomplished with protective cerclage cables or wires, useful during insertion of extensively porous-coated revision implants into the femoral shaft. A recent study compared the influence of multifilaments and monofilament cerclages on primary stability of revision hip stems with different fixation principles, founding that both can support the revision hip stem in bridging the extended proximal femoral osteotomy [34]. This report contradicts the results of previous studies that have shown that cables are more effective than wires in enhancing osteotomy union and preventing subsidence [35]. However, technical note study reported that the last cerclage wire should be placed 2 cm distal to the distal portion of the extended osteotomy prior to insertion of any broaches or reamers [33]. Occasionally, bone loss in the proximal femur could be so extensive that the remaining bone cannot support a new prosthesis. The cortex in the proximal 10 cm of the femur is thin and fragile and may be completely absent in several areas, requiring a massive proximal femoral allograft for reconstruction of the femur. In these cases, axial and rotational stability usually can still be achieved by fixing the step-cut with multiple heavy cerclage wires.
Use for complications after total shoulder arthroplasty
The reported prevalence of postoperative periprosthetic humeral shaft fractures ranges from 0.5 to 2.4 % [36]. Intraoperative fractures are estimated to occur in fewer than 1 % of patients, but intraoperative humeral fractures are more frequent than postoperative fractures [36]. Independent classifications for periprosthetic humeral shaft fractures in shoulder or elbow arthroplasties have been reported [36, 37]. After shoulder arthroplasty, periprosthetic fractures are usually categorized into three types: Type-A fractures extend proximally from the tip of the prosthesis, type-B fractures are centered at the tip of the prosthesis without proximal extension, and type-C fractures involve the humeral shaft distal to the prosthesis. Whereas type-C fractures respond favorably to nonsurgical treatments, type-A and type-B fractures usually require surgery [36–38], especially if the humeral component is loose. The role of cerclage wires or cable in these fractures includes revisions in which the stem does not allow the placement of screws and as supplementary fixation of a revision long-stem or structural bone grafts after open reduction [37, 38]. Cameron and Iannotti [39] reported that when treating unstable periprosthetic humeral diaphyseal fractures in the face of well-fixed components, a heavy plate with proximal cerclage wires and distal screws is the treatment of choice. At least four proximal cables (2.0 mm) and four distal screws engaging eight cortices are necessary. For most type-A fractures, simple cerclage wiring of the proximal humerus and implantation of a standard size prosthesis is appropriate [36–38]. A long-stem prosthesis and cerclage wiring can be used to stabilize most intraoperative type-B humeral fractures. Cerclage wire alone, placed circumferentially around the bone, does not provide adequate rigid fixation [14], while clinical studies demonstrate that long-stemmed arthroplasty supplemented with cerclage wiring resulted in a significantly shorter time to union than fractures treated with standard arthroplasty combined with internal fixation [36]. Cameron and Iannotti reported also the use of cerclage wires around a controlled longitudinal osteotomy performed to remove a well-fixed but malpositioned humeral component [39]. Periprosthetic humeral fractures between shoulder and elbow arthroplasties are even more rare, and there is often only a small area of remaining unviolated bone between the two humeral components (Fig. 6) [37]. These fractures are difficult to treat, and related data in literature are lacking. Mavrogenis et al. [40] reported a good result with open reduction in the fracture and internal fixation using a posterior locking plate and a medial strut graft with cables and wires in a 90–90 construct.
A 71-year-old female. AP (a) and lateral (b) radiographs of the right arm showing a comminuted humeral fracture between a cementless reverse total shoulder arthroplasty and a cemented total elbow arthroplasty. c The fracture was reduced first using a strut allograft and reduction clamps, and then, the construct was neutralized with a 3.5-mm posterolateral locking plate secured with wires and cables
Use for periprosthetic fractures of the elbow
The guidelines for the treatment of intra/postoperative periprosthetic elbow fractures are based predominantly on the degree of displacement, the characteristics of the fracture, the potential for complications and the stability of the implant [41]. Fractures that do not compromise function or the stability of a well-fixed implant may be managed nonsurgically. However, fractures that either occur in association with a loose stem or compromise function because of their location often require fracture fixation or implant revision. The classification of periprosthetic fractures following total elbow arthroplasty is described based on the fracture location, implant fixation and associated bone loss: type I, fracture of the humeral condyles and olecranon; type II, humeral and ulnar shaft fracture along the length of the stem; and type III, fracture past the tip of the prosthesis.
With rare exception, type I fractures should be rigidly fixed to provide a stable surgical construct using plate fixation with or without supplemental autogenous bone grafting, intramedullary techniques and simple cerclage wiring. Cerclage wires should be used to secure the plate to the bone regions that enclose the implant. Type II and type III fractures should be managed with supplemental fixation such as cerclage wires. Occasionally, a long-stem prosthesis that bypasses the fracture site is required.
Wire passer technology
Cerclage wiring performed via an open technique is a well-known secure procedure that has the disadvantage of extensive surgical dissection that alters the fracture zone. The conventional wire passer (so-called Beranger) is a cannulated half-circle that can be guided around the bone, allowing insertion of the cerclage. However, this tool requires a dislodgement of a major part of the soft tissues in order to bring the exit hole of the half-circle into a position where the wire can be caught. Has been described a percutaneous wiring technique performed through medial and lateral thigh incisions and employed an arthroscopic knot pusher with a bent end loaded with a 25-gauge wire. The authors preferred percutaneous technique is performed through two 0.5-cm longitudinal stab incisions at 90° with the fractured bone. Two curved hemostats are advanced dissecting the soft tissues along the bony surface to create path for later wire passage. One hemostat carries a single-lumen catheter on the tip, which can be easily identified and clamped by the other one. Then, the two extremities of the catheter are placed in the same incision, and the wire is inserted manually and then twisted with a battery-driven power tool. Newer devices have been developed with the aims of reducing the invasiveness and maintaining the same efficiency of the open technique, following the concept of minimally invasive osteosynthesis (MIO). The MIO wire passer forms a full circle assembling separately and intraoperatively the two parts of the device (Fig. 7). In this way, it is possible to reduce incision and tissue dislodging, minimize radiation exposure, minimize vascular damage and perform a correct closure of the wire [3, 31].
Mechanical aspects
Material
Cerclage loops may be made of metal or plastic. Plastic will not be considered here because of its lack of strength which led to increasing the dimension of the loop cross section and with it increasing the area of contact impeding blood flow. Stress relaxation with creeping loss of pretension is also a disadvantage of some plastics. A variety of cerclage designs and materials are available, from monofilament wires and braided cables to straps and bands made of metals and synthetics having differing mechanical properties. Cerclage wires made of stainless steel have the best biomechanical properties, are easy to use and are reliable for internal fixation and offer sufficient stability [42, 43]. The Vitallium had the greatest strength and the braided wire the least, whereas the elongation percentages were just the opposite, with that for Vitallium almost double that for the braided steel. Alternative devices such as cable buttons and others made of cobalt-chrome or titanium alloy are insufficient for strength and stability [42, 43]. Even if manufacturers of surgical devices do not recommend combining different metals, putting the complete responsibility and liability of possible complications on the surgeon, some studies did not show any negative effects on fracture healing and complication rate using stainless steel cerclage wire with titanium plates.
Cerclage configuration and number of twists
The metal loop may consist of a solid wire or of a multi-stranded cable, used with different configurations (Table 2). The wire has a monofilament structure, whereas the cable has a multifilament structure consisting of a central bundle and other outer bundles. Solid wire is very sensitive to notches or scratches, and some studies demonstrated that notches as small as 1 % of the wire diameter can reduce its fatigue life by 63 %. For this reason, cables that have significantly better fatigue performance compared to wires have been introduced for cerclage applications. The cerclage cable consists today of a multitude of finest metal fibers braided to form subassemblies which in turn are braided and twisted to form the final cable. Lenz et al. [44] compared the biomechanical performance of different cable and wire cerclage configurations, concluding that cable cerclages provide an increased fixation strength compared to single wire cerclages, but similar to double-looped wire cerclage, as reported in previous studies [45]. However, they observed that the major surgical disadvantage of double-looped cerclage application is its double looping around the bone, so that the surgical procedure necessary for a single-looped cerclage has to be performed twice, irrespective of a minimally invasive or conventional open technique, including the risk of vascular or nerve damage [44]. Nowadays, considering that cerclage wires are similar to cables in their effectiveness, but are less expensive, they may be the preferred cerclage technique [45].
The procedure of closing the loop is believed to be a major factor influencing the outcome of a cerclage fixation. Twisting the wire ends is the clinically most applied method to tighten the loop and maintain the tension of the solid wire. It has been reported that the use of wire knots as the “knot twist” yields higher ultimate failure strength than other knots and to the twist, while the hairpin cerclage knot generated significantly higher initial compressive force (p < 0.001) and had significantly higher tensile strength (p < 0.02) compared with other five techniques [5]. Differently, most recent papers reported that it is not necessary to perform complicated knotting techniques to ensure high load-to-failure of the wire junction, but a simple symmetrical twist secured against untwisting will be sufficient [42, 43]. Even if more than two twists added no additional strength, the optimal number of turns of surgical wire when twisted is between four and eight. A recent study analyzed systematically all different aspects of the twisting procedure such as wire diameter, deformation of the twist, applied traction, cutting procedure and bending direction [46]. About wire diameter, an increase by 50 % resulted in an increase in load-to-failure of up to 169 % and doubling the wire diameter from 0.45 to 0.98 mm increased the load-to-failure more than 300 % [42]. The cable cerclage (1 mm) provided a longer lasting pretension even under higher tension applied compared to 1-, 1.25- and 1.5-mm wire cerclages, maintaining the degree of plastic and elastic deformation [42–46]. Other significant factors were as follows: twisting the cerclage with applied traction, cutting the twist with protrusion and forward or perpendicular bending direction of the twist flat onto the bone surface [46]. Obviously, the twist consists of symmetrically deformed wires obtained applying at the same time torque and traction, and attention is required to avoid that one wire stays straight and the second wire turns around it resulting in an asymmetric twist. Good results have been reported with a new technique using a modified ASIF wire-tightener, in which the wires were always perfectly symmetrical and may be tightened and secured to maximal pretension without weakening of the wire [44]. The best wire tension for the construction of a twist knot cerclage wire has been reported to be 200 N. Biomechanical studies reported that the use of braided wires are not recommended due to the minor quality of the twist explained as loss of pretension at lower load and early plastic deformation [44].
Stability
Biomechanical studies demonstrated that cerclages are well suited to fix radially displaced fracture fragments around a prosthesis stem and do not require intracortical fixation [3, 46]. However, although providing a good fixation against lateral load, even well-tightened cable cerclages are susceptible to axial compression and torsion [4]. There are three different types of stability: absolute stability, relative stability and “loose-lock stability.” Absolute stability is characterized by absence of displacement locally within the fracture and requires that sufficient tension of the cerclage is maintained until the fracture is solidly united [3]. Relative stability consists of a reversible displacement of the fragments within the fracture during functional loading, with a return of the fragments to their original position at unloading. The concept of “loose-lock stability” is characterized by two distinct phases and has been recently introduced in order to describe a frequent situation after application of a cerclage: In the first phase, the wire is not under tension and the fragments displace freely, but when the wire is tensioned, the situation changes abruptly into an elastically (or plastically) resisted displacement, mostly in some degree of malalignment [3]. The stability of a cerclage as a circumferential and extraosseous fixation device depends on its lasting tension and could be further enhanced by application of a larger cerclage diameter [4] and double-looping techniques [4]. Some studies reported that wire cerclages are more susceptible to loss of pretension than cable cerclages closed by a crimp [46], whereas other described significant loosening of the cables during surgery and in the postoperative period. Comparing the capacity to maintain tension of different types of orthopedic cable systems, significant tension loss was observed with crimping for all cables and removing the tensioner led to an additional unexpected tension loss.
Another relevant factor that influence stability of the implant is the positioning of the wire loops in relation to the type of fracture and the tip of bony fragments. The outermost cerclage wire should be placed at least 1 cm from the tip of a single fragment, especially in long oblique fractures where the lever arm between the outermost cerclages in the same fragment is long [3]. In fractures with butterfly fragment, it is not possible to obtain long lever arm between the outermost cerclages, and therefore, cerclage wiring should be avoided [3]. Finally, cerclage-bone contact area is dependent on bone geometry [4]. Lenz et al. [4] investigated the influence of bone geometry on cerclage application (cerclage wire and cable) and studied the interface contact area. They found that both cerclages exhibited an inhomogeneous interface pressure distribution depending on the bone surface geometry and that histologically, cortical bone was intact without affection after loading of cerclage.
Biological aspects
Blood supply to bone is an essential prerequisite for uneventful bone healing. The belief of a strangled blood supply owing to cerclage application is still present, and it is supported by three mechanisms: (1) the effect of the contact between cerclage and bone surface; (2) the strangulation of periosteal blood vessels and (3) the effect like “Gigli saw” during passage of the cerclage around the bone. (1) Any contact of an implant with the bone surface covers by necessity a surface area and impedes blood inflow and outflow. As previously reported, bone geometry influences interface contact area as well as type of cerclage. When the cerclage consists of a solid wire or a cable, the area of contact is a rather narrow line and its effect on blood supply to bone is minimal, whereas plastic straps cause larger avascular areas. Plates with reduced bone contact surface show less early temporary bone porosity due to less damage to the blood supply [3]. Analogical to the low-contact plates, different strategies were designed in order to minimize the contact area of cerclage devices such as small transverse undercuts in plastic straps to produce a surface that reminds a cogwheel, or the elevation of wires from the bone surface with the use of protrusions or spheres (Fig. 8). Braided configuration of cerclage wires reduces the contact area with an interrupted dotted pattern at the pressure test. (2) The blood flow through the cortex is centrifugal, in radial direction from the medulla to the periosteum where venous blood escapes from the bone. In addition, large longitudinal vessels may be found in the vascular layer of the periosteum and in the medullary canal. Therefore, one can expect that the strangulation would have a small effect. Apivatthakakul et al. [31] demonstrated that a percutaneous cerclage wire technique does not strangulate the femoral blood supply. Several studies have reported that the cerclage wire used for fracture fixation does not restrict cortical vascularity and that the wire can be found to have been incorporated within the growing bone with time [3]. Also devitalized cortical bone segments, fixed by cerclages, showed a complete revascularization. In 2005, Nather et al. concluded that “the old taboo that applying a cerclage wire strangulates the periosteal blood supply to a bone no longer holds true” and recent studies confirmed this statement [3, 31]. During the healing of nondisplaced fractures, the endosteal circulation remains intact and provides a majority of the blood supply around the fracture zone. In most of the cases of periprosthetic fractures, the intramedullary blood supply is affected by an implant and a possible impairment of the periosteum by the cerclage is considered of importance for the fracture healing. Some authors found that extraperiosteal placement of the wires had less effect on the bone and that it is preferable to subperiosteal placement, but interestingly, in vivo experiments published 2 years later exhibited no difference in fracture healing, irrespective of whether the cerclage was placed below or above the periosteum [47]. (3) The sliding of the cerclage on the bone surface (called by the authors “Gigli saw effect”) can cause damage to the periosteal blood supply, but there are no studies in the literature designed to analyze this phenomenon. In order to avoid this possible effect, we prefer to use wires elevated from the bone surface with protrusions or spheres.
Complications
Strangulation of large blood vessels
The inadvertent strangulation of a major vessels has been observed in clinic [48] and in a cadaveric experiment [31]. Some cases of strangulation of the superficial femoral artery during revision total hip arthroplasty have been reported in literature [48]. Apivatthakakul et al. [31] reported an incidence of perforating artery interruption after femoral wiring of 23.6 %, even if in all cases, the integrity of the blood supply was maintained by an anastomotic system around the femur. They found also that when a pair of wire loops was placed with a 5-cm space between them, at least one perforator was injured in all cases [31]. This is a major potentially devastating complication which should be avoided by guiding the tip of the wire passer in contact with the periosteum [3], and when using grafts, it is best to place the cerclage wires first around the femur and then pass them around the graft [48].
Bone surface resorption
Bone surface resorption can be evoked by the so-called loose-lock stability [3], which is typically observed in loosened cerclages. In vivo, no cortical bone resorption was seen under well-tightened cerclages; in contrast, a bony ingrowth in terms of callus formation was found [3]. In vivo experiments with a spring-locked cerclage providing a constant tension revealed no shortening travel of the cerclage during fracture healing, which might have occurred during cortical bone resorption and grade cutting. Analyzing the radiographs from the case reports on cerclage pressure-induced bone necrosis, in all cases fracture instability due to insufficient reduction or fixation could be detected, supporting the motion-induced bone necrosis theory [3]. The concept of point contact fixation was successfully introduced in plate osteosynthesis to ameliorate the blood supply of the underlying bone. Lenz et al. [4] studied the contact area, the bone pressure along the interface and the critical resistance underneath loaded cerclages in fresh-frozen human femoral diaphyseal bones. They found that cortical bone withstands static concentric pressure produced by the cerclages (wire Ø 1.5 mm and cable Ø 1.7 mm) and that cortical damage and bone resorption have to be attributed to micromotion. In fact, living bone can tolerate static compression up to but not reaching the limit of mechanical strength [3], and the histologic examination revealed no bone surface affection by the cerclage, indicating no groove formation on the bone as reported by other authors [4]. An open question remains the tension level of the wire that may imply the risk of local mechanical overload exceeding the strength of bone.
Broken wire migration
Most breakages of cerclage wires are asymptomatic, and the migration of hardware is rarely reported in literature, even if well documented [49, 50]. In most of the cases has been reported a remote migration of a broken wire fragment into the heart, supposedly through the venous circulation [49, 50], with potential critical complications. The location, relationship with vessels or organs, size, shape and nature of the wire are all relevant parameters when deciding with the patient upon surgical removal of the broken hardware. When hardware removal will be deemed too risky or impractical, biodegradable materials could be considered a good alternative option.
Conclusions
The indications for cerclage as an exclusive implant were limited, while this simple technique is frequently used to secure femoral fractures, allografts and plates, especially in periprosthetic fractures. The cerclage may function in two different ways: as a temporarily tool for reduction during surgery or can be used long term as an implant. The advantages consist in minimally invasive reduction and fixation technique, low cost and early holding. The improvement of passer technology allowed to reduce incision and tissue dislodging, minimize radiation exposure, minimize vascular damage and perform a correct closure of the wire. Moreover, recent studies demonstrated that cerclage damages blood supply to bone less than expected, supporting good clinical outcomes. As for all minimally invasive techniques, the authors caution that cerclage must be done carefully to avoid serious complications, e.g., vascular injury.
References
Evans PE (1983) Cerclage fixation of a fractured humerus in 1775. Fact or fiction? Clin Orthop 174:138–142
Götze O (1933) Subcutane Drahnaht bei Tibiaschrägbrüchen. Arch Klin Chir 177:145
Perren SM, Fernandez Dell’Oca A, Lenz M, Windolf M (2011) Cerclage, evolution and potential of a Cinderella technology. An overview with reference to periprosthetic fractures. Acta Chir Orthop Traumatol Cech 78(3):190–199
Lenz M, Perren SM, Gueorguiev B, Richards RG, Krause F, Fernandez Dell’Oca A, Höntzsch D, Windolf M (2012) Underneath the cerclage: an ex vivo study on the cerclage–bone interface mechanics. Arch Orthop Trauma Surg 132(10):1467–1472
Cheng SL, Smith TJ, Davey JR (1993) A comparison of the strength and stability of six techniques of cerclage wire fixation for fractures. J Orthop Trauma 7(3):222
Habernek H, Walch G, Dengg C (1989) Cerclage for torsional fractures of the tibia. J Bone Joint Surg 71B:311–313
Soeur R (1981) Fractures of the limbs: the relationship between mechanism and treatment. Brussels: La. Clinique Orthopedique:542–3. 7
Schopfer A, Willett K, Powell J et al (1993) Cerclage wiring in internal fixation of acetabular fractures. J Orthop Trauma 7(3):236–241
Stiffler KS (2004) Internal fracture fixation. Clin Tech Small Anim Pract 19:105–113
Christensen CM, Seger BM, Schultz RB (1989) Management of intraoperative femur fractures associated with revision hip arthroplasty. Clin Orthop Rel Res 248:177–180
Fitzgerald RH Jr, Brindley GW, Kavanagh BF (1988) The uncemented total hip arthroplasty: intraoperative femoral fractures. Clin Orthop Relat Res 235:61–66
Berend K, Lombardi A, Mallory T et al (2004) Cerclage wires or cables for the management of intraoperative fracture associated with a cementless, tapered femoral prosthesis results at 2 to 16 years. J Arthroplast 19:17–21
Mayle RE Jr, Paprosky WG (2012) Massive bone loss: allograft-prosthetic composites and beyond. J Bone Joint Surg Br 94(11 Suppl A):61–64
Duncan C, Masri B (1995) Fractures of the femur after hip replacement. Instr Course Lect 44:293–304
McCulloch RS, Roe SC, Marcellin-Little DJ, Mente PL (2012) Resistance to subsidence of an uncemented femoral stem after cerclage wiring of a fissure. Vet Surg 41(1):163–167
Mallory TH, Kraus TJ, Vaughn BK (1989) Intraoperative femoral fractures associated with cementless total hip arthroplasty. Orthopedics 12(2):231–239
Otani T, Whiteside LA, White SE, McCarthy DS (1995) Reaming technique of the femoral diaphysis in cementless total hip arthroplasty. Clin Orthop Relat Res 311:210–221
Zeh A, Radetzki F, Diers V, Bach D, Röllinghoff M, Delank KS (2011) Is there an increased stem migration or compromised osteointegration of the Mayo short-stemmed prosthesis following cerclage wiring of an intrasurgical periprosthetic fracture? Arch Orthop Trauma Surg 131(12):1717–1722
Fishkin Z, Han S, Ziv I (1999) Cerclage wiring technique after proximal femoral fracture in total hip arthroplasty. J Arthroplast 14:98–101
Zuckerman JD (1996) Hip fracture. N Engl J Med 334:1519–1525
Cho SH, Lee SH, Cho HL, Ku JH, Choi JH, Lee AJ (2011) Additional fixations for sliding hip screws in treating unstable pertrochanteric femoral fractures (AO Type 31-A2): short-term clinical results. Clin Orthop Surg 3(2):107–113
Ban I, Birkelund L, Palm H, Brix M, Troelsen A (2012) Circumferential wires as a supplement to intramedullary nailing in unstable trochanteric hip fractures: 4 reoperations in 60 patients followed for 1 year. Acta Orthop 83(3):240–243
Kennedy MT, Mitra A, Hierlihy TG, Harty JA, Reidy D, Dolan M (2011) Subtrochanteric hip fractures treated with cerclage cables and long cephalomedullary nails: a review of 17 consecutive cases over 2 years. Injury 42:1317–1321
Winquist RA, Hansen ST Jr, Clawson DK (1984) Closed intramedullary nailing of femoral fractures. A report of five hundred and twenty cases. J Bone Joint Surg Am 66(4):529–539
Roberts CS, Nawab A, Wang M, Voor MJ, Seligson D (2002) Second generation intramedullary nailing of subtrochanteric femur fractures: a biomechanical study of fracture site motion. J Orthop Trauma 16(4):231–238
Lin HH, Hung SH, Su YP, Chiu FY, Liu CL (2012) Cerclage wiring in displaced associated anterior column and posterior hemi-transverse acetabular fractures. Injury 43(6):917–920
Chen CM, Chiu FY, Lo WH, Chung TY (2001) Cerclage wiring in displaced both-column fractures of the acetabulum. Injury 32(5):391–394
Pike J, Davidson D, Garbuz D, Duncan CP, O’Brien PJ, Masri BA (2009) Principles of treatment for periprosthetic femoral shaft fractures around well-fixed total hip arthroplasty. J Am Acad Orthop Surg 17(11):677–688
Johannsson JE, McBroom R, Barrington TW, Hunter GA (1981) Fracture of the ipsilateral femur in patients with total hip replacement. J Bone Joint Surg Am 63(9):1435–1442
Dennis MG, Simon JA, Kummer FJ, Koval KJ, DiCesare PE (2000) Fixation of periprosthetic femoral shaft fractures occurring at the tip of the stem: a biomechanical study of five techniques. J Arthroplast 15(4):523–528
Apivatthakakul T, Phaliphot J, Leuvitoonvechkit S (2013) Percutaneous cerclage wiring, does it disrupt femoral blood supply? A cadaveric injection study. Injury 44(2):168–174
Xue H, Tu Y, Cai M, Yang A (2011) Locking compression plate and cerclage band for type B1 periprosthetic femoral fractures preliminary results at average 30-month follow-up. J Arthroplast 26(3):467–471
Younger TI, Bradford MS, Magnus RE et al (1995) Extended proximal femoral osteotomy. A new technique for femoral revision arthroplasty. J Arthroplast 10(3):329–338
Jakubowitz E, Kinkel S, Nadorf J, Heisel C, Kretzer JP, Thomsen MN (2011) The effect of multifilaments and monofilaments on cementless femoral revision hip components: an experimental study. Clin Biomech (Bristol, Avon) 26(3):257–261
Schwab JH, Camacho J, Kaufman K, Chen Q, Berry DJ, Trousdale RT (2008) Optimal fixation for the extended trochanteric osteotomy: a pilot study comparing 3 cables vs 2 cables. J Arthroplast 23(4):534–538
Campbell JT, Moore RS, Iannotti JP, Norris TR, Williams GR (1998) Periprosthetic humeral fractures: mechanisms of fracture and treatment options. J Shoulder Elbow Surg 7:406–413
Mavrogenis AF, Angelini A, Guerra E, Papagelopoulos PJ, Ruggieri P, Rotini R (2009) Periprosthetic fractures of the humerus. J Long Term Eff Med Implants 19(4):305–311
Wutzler S, Laurer HL, Huhnstock S, Geiger EV, Buehren V, Marzi I (2009) Periprosthetic humeral fractures after shoulder arthroplasty: operative management and functional outcome. Arch Orthop Trauma Surg 129:237–243
Cameron B, Iannotti JP (1999) Periprosthetic fractures of the humerus and scapula: management and prevention. Orthop Clin North Am 30:305–318
Mavrogenis AF, Angelini A, Guerra E, Rotini R (2011) Humeral fracture between a total elbow and total shoulder arthroplasty. Orthopedics. 34(4)
Choo A, Ramsey ML (2013) Total elbow arthroplasty: current options. J Am Acad Orthop Surg 21(7):427–437
Bostrom MP, Asnis SE, Ernberg JJ, Wright TM, Giddings VL, Berberian WS et al (1994) Fatigue testing of cerclage stainless steel wire fixation. J Orthop Trauma 8:422–428
Shaw JA, Daubert HB (1988) Compression capability of cerclage fixation systems: a biomechanical study. Orthopedics 11:1169–1177
Lenz M, Perren SM, Richards RG, Mückley T, Hofmann GO, Gueorguiev B, Windolf M (2013) Biomechanical performance of different cable and wire cerclage configurations. Int Orthop 37(1):125–130
Ritter MA, Lutgring JD, Davis KE, Berend ME, Meding JB (2006) A clinical, radiographic, and cost comparison of cerclage techniques: wires vs cables. J Arthroplast 21(7):1064–1067
Wähnert D, Lenz M, Schlegel U, Perren S, Windolf M (2011) Cerclage handling for improved fracture treatment. A biomechanical study on the twisting procedure. Acta Chir Orthop Traumatol Cech 78(3):208–214
Wilson JW (1987) Effect of cerclage wires on periosteal bone in growing dogs. Vet Surg 16(4):299–302
Aleto T, Ritter M, Berend ME (2008) Case report: superficial femoral artery injury resulting from cerclage wiring during revision THA. Clin Orthop Relat Res 466(3):749–753
Biddau F, Fioriti M, Benelli G (2006) Migration of a broken cerclage wire from the patella into the heart. A case report. JBone Jt Surg Am 88(9):2057–2059
Wirth MA, Lakoski SG, Rockwood CA Jr (2000) Migration of broken cerclage wire from the shoulder girdle into the heart: a case report. J Shoulder Elbow Surg 9:543–544
Conflict of interest
None of the authors have any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Angelini, A., Battiato, C. Past and present of the use of cerclage wires in orthopedics. Eur J Orthop Surg Traumatol 25, 623–635 (2015). https://doi.org/10.1007/s00590-014-1520-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-014-1520-2
Keywords
- Cerclage
- Cerclage-wiring techniques
- Wire
- Femoral fracture
- Periprosthetic fracture
- Internal fixation
- Intraoperative fracture
- Unstable intertrochanteric fracture
- Intramedullary nailing
- Reduction
- Cable
- Acetabular fracture
- Minimally invasive techniques
- Wire passer technology
- Twist
- Loop
- Periosteal blood vessels
- Vascular injury