Abstract
Purpose
To assess skull bone thickness from birth to skeletal maturity at different sites to provide a reference for the correct selection of pin type and pin placement according to age.
Methods
270 children and adolescents (age: 0–17 years) with a normal CT scan obtained at Emergency Department for other medical reasons were included. Skull thickness was measured on the axial plane CT scans at eight different sites of the vault: midline anterior (A) and posterior (P), right and left lateral (L), antero-lateral (AL), postero-lateral (PL).
Results
From birth to skeletal maturity, L thickness was increased significantly less (+ 58%) compared with AL (+ 205%), P (+ 233%), PL (+ 247%), and A (+ 269%) thickness (P < 0.01). At the end of growth, the thickest and thinnest points of the vault (absolute value) were found at the P and L measurement sites, respectively (P < 0.01). Children aged < 4 years exhibited the highest variability in AL and PL skull bone thickness, with thickness < 3 mm observed in 85% (64/75 patients) and 92% (69/75 patients) of cases, respectively.
Conclusion
We recommend that the tip of the pin should not exceed 2–3 mm in children aged < 4, and 4 mm in children aged 4–6 years, to decrease the risk of inner table perforation. After the age of 7 years and 13 years, standard-sized pin tips (5 and 6 mm, respectively) may be safely used. Children aged < 4 years show significant variability in skull thickness, and therefore a CT scan may be required for this particular age group.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Perry and Nickel [1] first introduced the principles of halo fixation of the cervical spine in orthopedics and traumatology. Over time, halo skeletal fixation has become the most commonly used cervical spine-stabilizing method, as it decreases cervical spine motion by 30–96% [2]. Since its introduction, it has been successfully used to manage cervical spine instability of heterogeneous etiologies, including trauma, neoplasms, rheumatoid arthritis, infection, and poliomyelitis. Subsequently, the use of halo gravity traction became a useful adjuvant treatment for children and adolescents with complex spinal deformity [3, 4].
Despite its proven efficacy, the incidence of complications related to the halo fixator remains relatively high. Minor complications include pin loosening, skin breakage, localized infection, periorbital edema, superficial pressure sores, and unaesthetic scars. Major complications include pin penetration, cerebrospinal fluid leakage, osteomyelitis, intradural and extradural abscesses, and nerve palsies [4,5,6,7,8,9,10,11,12,13]. Knowledge of local anatomy, adherence to established guidelines of pin placement, meticulous care of the pin site, and frequent follow-up are needed to decrease the number of pin-related complications [5, 14].
At present, there is no relevant literature investigating the development of skull bone thickness throughout all growth stages (i.e., from birth to skeletal maturity). Furthermore, there is a lack of information concerning the optimal cranial pin placement related to age and bone thickness in skeletally immature patients.
The purpose of this study was to assess skull bone thickness using non-contrast computed tomography (CT) in a large cohort of children and adolescents. In particular, by measuring skull bone thickness from birth to skeletal maturity at different skull bone sites, we aimed to provide a reference to clinicians for the correct selection of pin type and pin placement according to the age of patients.
Methods
This retrospective study was approved by the institutional review board. A total of 270 children and adolescents (131 females; 139 males; age: 0–17 years) underwent non-contrast head CT scan between 2010 and 2015.
All cases were admitted through the Emergency Department (ER) of our institution with a positive history for traumatic brain injury (TBI) associated with lost, decreased, or altered level of consciousness, as well as amnesia, neurologic deficit, or intracranial lesion (20).
The inclusion criteria for this study required the patient to be aged 0–17 years at the time of scanning. Besides TBI, all patients had to be healthy without history of concomitant skull fracture, systemic disease affecting the quality and/or structure of the bone, or any localized skull bone disease (e.g., deformity, tumor, infection, and/or prior head surgery).
Non-contrast head CT scans (i.e., complete images of frontal, temporal, and orbital bones) were obtained with patients in the supine position within 0–24 h after admission to the ER. CT scans should have a spatial resolution > 1.6 mm. CT scans showing motion artifacts were excluded. Selected patients were de-identified and transferred to a secure data repository.
Patients
Patients were stratified in 18 groups (15 patients per group) according to age. Group 1 included patients between birth and 6 months of age; Group 2 included patients aged 7–12 months; Group 3 included patients aged 1–2 years; Group 4 included patients aged 2–3 years, until Group 18 which included patients aged 16–17 years. Table 1 outlines the demographic characteristics of the patients.
CT scan assessment
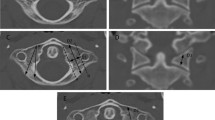
Non-contrast CT scans were processed using the workstation IMPAX ® Client software Version 6.5.2 (Agfa HealthCare, Mortsel, Belgium). The system allowed multiplanar reconstruction at the recommended areas for pin site placement [21, 22]. The plane of measurement was always the same plane where the halo should be placed.
In particular, skull thickness was measured on the axial plane at the standard locations for halo pins: above the eyebrows and lateral to the supraorbital nerves (antero-lateral [AL], right and left) and posterior to the ear above the level of the auditory meatus (postero-lateral [PL], right, and left) using bone windows with the same cut width [21, 22]. All the measurements were obtained using the same CT scan, and bone width cut. The plane of acquisition was adapted to the plane of the halo placement in order to accurately measure the skull width at the site of pin placement. Moreover, skull bone thickness was assessed at four additional sites (i.e., anterior [A], right lateral [Lr], left lateral [Ll], and posterior [P]) using the same axial plane (Fig. 1). A single, senior orthopedic surgeon with expertise in cervical spine surgery (JY) performed all measurements.
Statistical analysis
The categorical variables were described with their absolute values and percentages. The quantitative variables were presented according to their measurements of central tendency (mean and standard deviation). Regression Spearmann correlation was used to study thickness progression between the different pin sites and age. The Mann–Whitney U test was used to compare differences between age groups and thickness of the pin site. A P < 0.05 denoted statistically significant difference. Statistical analysis was conducted using the IBM SPSS Version 24.0 software (IBM Corp., Armonk, NY, USA).
Results
A total of 270 non-contrast CT scans (131 female patients; 139 male patients) were reviewed, and a total of 2,160 measurements were performed (Table 1). There were no significant differences found in the age groups between the right and left side of the AL, PL, and L cranial vault thickness (Table 2).
Skull bone thickness increased with age for all parameters. In particular, A, AL, PL, L, P skull bone thickness was gradually increased with age, demonstrating good to excellent correlation with age (R = 0.718, R = 0.742, R = 0.836, R = 0.569, and R = 0.6, respectively; P < 0.001). From birth to skeletal maturity, L thickness was increased significantly less (only 58%) compared with AL (205%), P (233%), PL (247%), and A (269%) thickness (P < 0.01). At the end of growth, the thickest point of the vault (absolute value) was found at the P site and the thinnest points were found and both L measurement sites (P < 0.01) (Fig. 2).
Children aged < 12 months had an average AL and PL skull thickness of 1.88 ± 0.5 mm and 1.81 ± 0.45 mm, respectively. AL and PL thickness reached 2.74 ± 0.38 mm and 2.29 ± 0.37 mm, 2.67 ± 0.46 mm and 2.42 ± 0.36 mm, and 2.74 ± 0.53 mm and 2.49 ± 0.53 mm in children aged 2, 3, and 4 years, respectively.
Children aged < 4 years exhibited the highest variability in AL and PL skull bone thickness, with thickness < 3 mm observed in 85% (64/75 patients) and 92% (69/75 patients) of cases, respectively (Table 2).
Growth of the vault showed a nonlinear progression. Two periods of accelerated growth could be identified. The first period occurred during the first 2 years of life, with AL and PL thickness increasing by 50%. The second period occurred between the age of 10 and 15 years, with AL and PL thickness increasing by 42% (Fig. 2).
Discussion
This study evaluated skull bone thickness at different sites to provide normative values for the safe placement of pins in skeletally immature patients. Our findings are based on a larger number of CT scans and a higher number of measurements compared with previously published research. In addition, owing to the larger sample size, the results could be stratified according to age, aiming to provide a more precise set of data for all age groups (i.e., from birth to skeletal maturity).
The skull bone consists of the inner table, the outer table, and the middle layer (diploe) of cancellous bone. CT scans provide high-fidelity representations of cranial bones and are the preferred modality for bone imaging. Several investigators performed measurements of bone thickness using CT scans [15,16,17], and found that the measurements were reliable and correlated to similar measurements obtained in human specimens [18, 19]. However, performing CT scans in otherwise healthy children is not justifiable due to concerns regarding the effects of ionizing radiation. All our patients were admitted through the ER of our institution with positive medical history for TBI associated with lost, decreased, or altered level of consciousness, as well as amnesia, neurologic deficit, or intracranial lesion [20]. Therefore, head CT scans were acquired to rule out potentially significant medical conditions.
In the current study, skull thickness was gradually increased during growth. Children aged < 3 years had the thinnest cortical bone. Caird et al. investigated 13 infants (aged 16–43 months) treated with halo fixation. They found an overall complication rate of 53.8% (six infections at the site of the pin; one respiratory difficulty), and concluded that the complication rate was similar to that reported in older children. Moreover, there was no skull deformity observed in any of the patients at the end of treatment [6]. Arkader et al. [7] conducted a similar study involving 10 infants (aged 10–34 months) and reported a 20% lower complication rate (one infection at the site of the pin; one pin loosening). Mubarak et al. reported three additional cases of infants (aged 10–24 months) treated with halo fixations [21]. All investigators recommended performing a preoperative head CT scan to evaluate skull bone thickness prior to halo application. They suggested that a higher number of pins, meticulous care of the pin site, frequent follow-ups, and limited ambulation (toddlers may be more prone to falling than older children) are needed to decrease the number of pin-related complications [6, 7, 21].
Garfin et al. reviewed 18 CT scans of pediatric skulls. The skulls were divided into three age groups (i.e., 1–2 years, 2–5 years, and 5–12 years). They found that the mean total thickness at the level of the AL and PL portions of the calvaria was increased from 3.7 and 3.9 mm, respectively, in infants (aged 1–2 years) to 6.1 and 5.9 mm, respectively, in children and preadolescents (aged 5–12 years). The thickness was the lowest at the temporal fossa, increasing from 3 mm in infants to 4.1 mm in the oldest children [22]. However, the number of patients was low and did not include patients aged < 1 year and > 12 years. Most importantly, the selected age range was very broad, not allowing to clearly evaluate changes occurring over time. Lett et al. reviewed 68 pediatric skulls (35 males and 33 females). They found that skull thickness varied considerably in the first 6 years of age regardless of age and sex. In addition, they reported a trend toward increasing skull thickness in adolescence. This trend was initiated at approximately 10 years of age, and adult thickness was attained by the age of 16 years [23].
Loder reviewed 82 non-contrast head CT scans and did not find statistically significant differences according to race or gender. He reported that the average skull bone thickness increased with age and was not influenced by race [24].
Haas et al. showed that the external skull length and width reach 94% and 89% of its adult measurements, respectively, by the age of 3–5 years [25]. There was also a significant increase in skull bone thickness, in particular during the first 3 years of life. During this period of rapid skull growth, the size of the halo ring must be carefully selected. When possible, pin selection should be based on knowledge of the skull thickness. Stone et al. reported that adults have similar skull bone thickness compared with children aged > 12 years, and found that the inner table was thicker at the AL site [26]. Our findings show that by the age of 4 years, the AL thickness has reached 3 mm in 20% of children. This percentage reaches 60% by the age of 9 years, > 80% by the age of 12 years, and 100% between puberty and skeletal maturity. On the other hand, only 7% of patients reached a PL thickness of 3 mm by the age 4 years, 40% by the age of 6 years, 80% by the age of 9 years, and 100% between puberty and skeletal maturity.
One of major concerns is the occurrence of cerebrospinal fluid (CSF) leakage at the time of pin placement, which a lot of times is more related with the pressure applied to a point than skull thickness. Torque forces were not considered in this study. The literature recommends significant less torque infants than in older children, not exceeding 2 in/lb [21].
Therefore, caution during the application of a halo fixator must be exercised equally for all children. We recommend that the tip of the pin should not exceed 2–3 mm in children aged < 4, and 4 mm in children aged 4–6 years, to decrease the risk of inner table perforation. After the age of 7 years and 13 years, standard-sized pin tips (5 and 6 mm, respectively) may be safely used. It has been recommended that toddlers and children should undergo head CT scans prior to the placement of pins. Considering that skull bone thickness varies between the AL and PL pin sites, this approach assists in assessing cranial bone thickness and facilitating the selection of pin length and placement of the pin [3, 5]. The findings of this study provided normative data regarding cranial bone thickness that may be used in skeletally immature patients requiring halo fixation. The present data may help spine surgeons to avoid performing systematic preoperative CT scan of the head in skeletally immature patients requiring halo fixation. This is especially important in infants and toddlers who are particularly sensitive to exposure to ionizing radiation.
Particular attention should be paid to children aged < 4 years who show significant variability in skull thickness compared with other age groups; a CT scan may still be required for this particular age group. Moreover, specific pins used for halo fixation should be placed antero-laterally and postero-laterally (highest bone thickness), thus avoiding the temporal fossa (lowest bone thickness).
Change history
15 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00586-020-06432-5
References
Perry J, Nickel VL (1959) Total cervical-spine fusion for neck paralysis. J Bone Joint Surg Am 41:37–60
Bono CM (2007) The halo fixator. J Am Acd Orthop Surg 15(12):728–737
Bogunovic L, Lenke LG, Bridwell KH, Luhmann SJ (2013) Pre-operative halo-gravity traction for severe pediatric spinal deformity: complications, radiographic correction and changes in pulmonary function. Spine Deform 1:33–39
Sponseller PD, Takenage RK, Newton P et al (2008) The use of traction in the treatment of severe spinal deformity. Spine 33:2305–2309
Botte MJ, Byrne TP, Abrams RA, Garfin SR (1996) Halo skeletal fixation: techniques of application and prevention of complications. J Am Acad Orthop Surg 4(1):44–53
Caird M, Hesinger RN, Weiss N, Farley FA (2006) Complications and problems in halo treatment of toddlers: limited ambulation is recommended. J Pediatr Orthop 26(6):750–752
Arkader A, Hosalkar HS, Drummond DS, Dormans JP (2007) Analysis of halo-orthoses application in children less than three years old. J Child Orthop 1(6):337–344
Hayes VM, Silber JS, Siddiqi FN, Kondrachov D, Lipetz JS, Lonner B (2005) Complications of halo fixation of the cervical spine. Am J Orthop 34(6):271–276
Dormans JP, Criscitiello AA, Drummond DS et al (1995) Complications in children managed with immobilization in a halo vest. J Bone Joint Surg Am 77:1370–1373
Garfin SR, Butt MJ, Waters RL, Nickel VL (1986) Complications in the use of the halo fixation device. J Bone Joint Surg Am 68:320–325
Saeed MU, Dacuycuy MA, Kennedy DJ (2007) Halo pin insertion-associated bran abscess: case report and review of literature. Spine 32(8):E271–274
Goodman ML, Nelson PB (1987) Brain abscess complicating the use of halo orthosis. Neurosurgery 20:27–30
Tindall GT, Flanagan JF, Nashold BS (1959) Brain abscess and osteomyelitis following skull traction. Arch Surg 79:638–641
Garfin SR, Botte MI, Centeno RS, Nickel VL (1985) Osteology of the skull as it affects halo pin placement. Spine 10:696–698
Gregory BA, Snow RD, Brogdon BG, Williams JP (1997) Value of bone window images in routine brain CT: EXAMINATION beyond trauma. Appl Radiol 26:26–42
Pillai P, Sammet S, Ammirati M (2008) Application accuracy of computed tomography-based, image-guided navigation of temporal bone. Neurosurgery 63:326–332
Moreira-Gonzalez A, Papai FE, Zins JE (2006) Calvarial thickness and its relation to cranial bone harvest. Plast Reconstr Surg 117:1964–1971
Adeloye A, Katten KR, Silverman FN (1976) Thickness of the normal skull in the American blacks and whites. Am J Phys Anthropol 43:23–30
Ross MD, Lee KA, Castle WM (1976) Skull thickness of Black and White races. S Afr Med J 50:635–638
Blyth BJ, Bazarian JJ (2010) Traumatic alterations in consciousness: traumatic brain injury. Emerg Med Clin North Am 28(3):571–594
Mubarak SJ, Camp JF, Vuletich W, Wenger D, Garfin SR (1989) Halo application in the infant. J Pediatr Orthop 9(5):612–614
Garfin SR, Roux R, Botte MJ, Centeno R, Woo SL (1986) Skull osteology as it affects halo pin placement in children. J Pediatr Orthop 6(4):434–436
Letts M, Kaylor D, Gouw G (1988) A biomechanical analysis of halo fixation in children. J Bone Joint Surg Br 70:277–279
Loder RT (1996) Skull thickness and halo-pin placement in children: the effects of race, gender, and laterality. J Pediatr Orthop 16(3):340–343
Haas LL (1952) Roentgenological skull measurements and their diagnostic application. AJR 67:197–209
Stone JL, Gulabani A, Gorelick G, Vennemreddy S, Vannemreddy P (2013) Frontolateral pins for halo ring placement: reassessment of a common neurosurgical procedure with CT scan measurements of skull bone thickness. J Neurosurg Spine 19:744–749
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Domenech-Fernandez, P., Yamane, J., Domenech, J. et al. Analysis of skull bone thickness during growth: an anatomical guide for safe pin placement in halo fixation. Eur Spine J 30, 410–415 (2021). https://doi.org/10.1007/s00586-020-06367-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-020-06367-x