Abstract
Purpose
To clarify the significance of Modic changes, bony endplate lesions, and disc degeneration as predictors of chronic low back pain (LBP) during 1-year follow-up.
Methods
49 patients with severe, non-specific, chronic LBP, and Modic 1 lesion (M1) were prospectively studied with MRI and questionnaire. Changes in grade of disc degeneration, severity of Modic changes, Schmorl lesions, and bony endplate irregularities were evaluated and changes assessed in LBP intensity on numeric rating scale 0–10 and severity with Oswestry disability index 0–100 (ODI). Association between change in MRI findings and symptoms was computed using generalized estimating equations analysis.
Results
Although pain decreased in most patients during 1-year follow-up, it increased or persisted in 36 %. Change in M1, M2, bony endplate lesions, and signal intensity (SI) and height of the disc associated with change of pain intensity, while change in M1, bony endplate lesions, and disc height associated with change of ODI. Not only persistent M1s, increasing bony endplate lesions, decreasing disc height, and M2s, but also new M2s predicted persistence of pain, while decrease of M1s and SI of the disc and increase of size of M2s predicted decrease of pain. Changes in disc bulges did not associate with pain.
Conclusions
In patients with chronic non-specific LBP, persisting M1, decreasing disc height, and increasing bony endplate lesions associated with persisting pain while decrease of SI of the disc with decrease of pain. Such changing MRI findings in the same disc space have earlier been shown to progress abnormally fast. They may be signs or biomarkers of a prolonged pain causing, deforming degenerative process, and should lead to considering early intervention or specific treatments to affect that process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The association between non-specific low back pain (LBP) and degenerative disc disease has remained controversial despite positive findings in many studies [1–3]. Discogenic pain has been suggested to be rather a symptom in patients with different stages of spinal impairment than one entity or diagnosis [4]. In the previous MRI studies, different gradings for degenerative findings have been used, linear associations with LBP have been assumed, and single MRI findings have been studied instead of clusters of findings together to find predictors for LBP [3]. More sensitive diagnostic methods and specific analyses may be needed to understand discogenic pain.
Modic-type subchondral signal abnormalities on MRI in the bone marrow adjacent to degenerated discs [5] have been found to have an association with LBP [6–9], Modic type 1 (M1) stronger than Modic type 2 (M2) [10, 11]. M2 develops from M1 [11]. Prevalence of M2s increases with age [7]. Therefore, Modic changes have also been suggested to represent normal age-dependent findings. Role of Modic change as a predictor for LBP has been evaluated in only few prospective studies, and other degenerative findings have not been commonly included in the analyses.
Modic changes associate with histologically proven fissures and defects in the cartilaginous endplate of the disc [3]. In a recent cadaver study, bony endplate lesions were shown to associate with the history of LBP [12]. In chronic LBP patients with M1, Schmorl lesion-like defects and irregularities were found to appear and progress rapidly in adjacent bony endplates on MRI [13]. An accelerated process of disc degeneration (DD) with decreased disc height and increased signal intensity (SI) of nucleus pulposus was found in the same disc spaces [14, 15]. The significance of such a fast progressing, degenerative process in causing LBP is not well understood. Both Modic changes and bony endplate defects have been found to be rich in nociceptive receptors [16]. Thus, either one could well be a source of non-specific LBP.
In this study, the association of chronic non-specific LBP with Modic changes, degenerative disc changes, and bony endplate lesions is studied prospectively during a 1-year follow-up. The effect of a change in degenerative grade of each MRI variable on change in severity of LBP (NRS or ODI) is separately studied. In addition, the effect of all MRI variables together is evaluated. First, hypothesis is that M1-type Modic change is not an age-dependent degenerative finding, but associated with LBP. Second, hypothesis is that M1 is not an independent predictor of LBP, but associated specific degenerative MRI findings in the adjacent disc and bony endplate play a role, too. Third, hypothesis is that this fast degenerative process is painful, as long as M1 has not changed into M2 type.
Materials and methods
From 3811 consecutive chronic LBP patients, who had been scanned for LBP (lasting >3 months) with MRI (1.5 or 1.0 T), 18–65-year-old patients with a large M1 were considered to be included in this prospective MRI study. All patients with a specific back disease like malignancy, trauma, infectious or inflammatory back disease, posterior disc extrusion, or any sign of nerve root compression were excluded. An informed consent was obtained from the 54 included subjects. The study protocol, the inclusion and exclusion criteria, and grading of MRI findings have been presented earlier, in detail [15, 17]. It was approved by the local ethical committee.
Questions about the severity of the LBP symptoms were presented 1–3 weeks after the baseline MRI and at the time of the follow-up MRI. The average LBP intensity during the preceding week was reported by the patient on a 0–10 numerical rating scale (NRS): 0 = no pain, 10 = worst possible pain both at the baseline and follow-up and the disability caused by the symptoms using the Oswestry Disability Index (ODI) on a scale 0–100 %; 0 % = no experienced disability at all, 100 % = worst experienced disability, accordingly. Five subjects were excluded, because complete data about the symptoms either at baseline or follow-up were not eligible. The subgroup of the remaining 49/54 patients formed the subjects of this study. 42 (86 %) of them were females. The mean age was 43.7 years (SD 10.5). Eight patients had earlier had a spine operation. They were included, because they had been recovered and painless before this new, long-lasting LBP period. No specific treatment besides pain medication and consulting was given to the subjects before or after MRI. The painful time period before MRI varied between the patients, but it had lasted at least 3 months.
MRI of the lumbar spine
Baseline MRI studies of the lumbar spine were obtained with five MRI devices in the university hospital area, operating at 1.5 and 1.0 T, using a standard protocol, TSE or FSE sequences with sagittal, and axial T1- and T2-weighted images [15]. All follow-up MRI studies were obtained with the same 1.0 T device and imaging protocol, with sagittal T1 and T2 images, on the average 13.2 months (SD 2.0) after the baseline MRI.
MR image analysis
All MRI findings were visually analyzed in consensus by two experienced radiologists who were blinded to the patients’ clinical data, using a detailed grading method [15, 17]. Intra- and inter-observer agreement of the MRI variables was acceptable (from moderate to excellent) [13, 14], and that of the size of M1 was 0.88 and 0.82 (ICC) and that of the presence of an endplate lesion (kappa) was 0.89 and 0.80 [13]. The size of each Modic lesion was graded on the sagittal slice as percentage of the area of the vertebra. Baseline bony endplate lesions were subclassified as focal subchondral hypointensity, small defect or larger Schmorl lesion like bony defect, multifocal lesions, diffuse irregularity, and combined (Fig. 1) [17].
T1-weighted MR image of a lumbar spine with one degenerated disc after 1-year follow-up. Disc space L4/L5 has decreased disc height and Schmorl lesion-type defects (thin arrows) and irregularities (white arrows) at the endplate region (border between disc and subchondral bone marrow). Modic 2-type abnormal (hyperintense) signal change in the subchondral bone marrow surrounds bony irregularities and endplate defects adjacent to both the upper and lower endplates of the L4/L5 disc
The signal intensity (SI) of nucleus pulposus of the intervertebral disc was classified on a five-point scale. If the SI of both nucleus pulposus and annulus fibrosus was very dark like that of cortical bone, the disc SI was classified as severely decreased. If SI in nucleus pulposus region was bright (normal or increased signal) but that of annulus very dark in a disc with a decreased height, the disc SI was classified as increased (Fig. 2). Height of the disc was classified on a four-point scale and anterior and posterior disc bulge (or protrusion) as none or present. When height and SI of the L5/S1 disc were evaluated the presence of a transitional vertebra L5 was considered, since low height but high (normal) SI is common in non-degenerated discs below such a developmental anomaly [18]. That finding was determined using criteria presented earlier [19].
T2-weighted MR image of a lumbar spine with one severely degenerated disc after 1-year follow-up. In disc space L4/L5 with hyperintense Modic 2-type subchondral changes (thin arrows) and slight bony endplate irregularities (white arrows), the disc height is severely decreased, but the signal intensity of the nucleus pulposus is increased. In the disc space L3/L4 without Modic changes, the disc is degenerated, has an annular fissure, decreased signal intensity of nucleus pulposus, but normal height
Follow-up MRI variables
The change in the size of each M1 and M2 between baseline and follow-up images was visually evaluated and graded, as no (abnormal) finding, disappearance, decrease, persistence, increase (or enlargement), or appearance of a (new) finding. The change in the adjacent bony endplate lesion and of each degenerative disc finding was graded accordingly. The decrease of disc height and SI was graded as “finding decreased”, the increase of SI in a bright nucleus pulposus of a degenerated disc as “finding increased” and the change of SI in nucleus pulposus of a degenerated disc from dark to bright as “new finding appeared”.
Statistical analysis
The frequencies of the graded follow-up MRI variables were computed. Differences between baseline and follow-up NRS were tested with the paired t test and those for ODI accordingly. Then, the association of change in each degenerative MRI finding (at all disc levels) with change in NRS and ODI was explored. Graded follow-up MRI variables were used as predictors and difference between baseline and follow-up NRS and corresponding value for ODI as outcomes.
Recording several findings in the same individuals causes cluster bias that has to be treated by special statistical methods. Many patients had degenerative findings (e.g., decreased SI of the disc) at several disc levels. We, therefore, used generalized estimating equations (GEE), a type of general linear model to evaluate the association of the graded follow-up MRI variables with the differences in NRS and ODI. The SPSS 20.0 (IBM, USA) software was used. The disk level (L1/L2–L5/S1) and the location of endplate (upper or lower in that disc space) were used as within patient factors. Age, sex, interval between the MRI studies, and history of a previous spine operation (yes/no) were considered as possible confounders. Only main effects were studied.
First, the crude association of each graded follow-up MRI variable and age, sex, interval between the MRI studies, and history of spine operation was evaluated with the difference in NRS and then with that in ODI. Reference category was MRI-grade 0 (normal finding). Then, the corresponding adjusted associations of follow-up MRI variables with difference in NRS and ODI were computed by including age, sex, interval between the MRI studies, and history of a spine operation as possible confounders in the model. Relation of individual degenerative follow-up grades with change in LBP was evaluated by calculating the estimated marginal means for each follow-up MRI variable.
Results
Baseline MRI findings
42/49 subjects had M1s located in one single disc space, 5 in two, and 2 in three disc spaces. In 46 disc spaces, both adjacent endplates had an M1 and in 8 cases either one. Most (56) of the 100 M1s located at L5/S1 disc level, 34 at L4/L5, 2 at L3/L4, 6 at L2/L3, and 2 at L1/L2 level. There were 63 M2s and 174 bony endplate lesions in 490 endplates. 206 of the 245 discs had a decreased SI, 104 a decreased height, 84 an anterior, and 95 a posterior bulge.
Follow-up of MRI findings
Frequencies of the follow-up MRI variables (presence and graded change in M1, M2 and each degenerative disc finding) are presented in Table 1. No focal or irregular bony endplate lesions were found in 308/490 endplates even after the follow-up. 2/177 bony endplate lesions decreased, 113/177 remained unchanged, and 62/177 enlarged or increased. A new focal hypointensity, defect, erosion, or Schmorl-type lesion appeared on five smooth and 16 irregular endplates. In 30 bony endplates, the irregularities increased, in eight, the focal defect enlarged, and in eight endplates, both the focal defect enlarged and the irregularities increased. The association of changing M1s with adjacent degenerative disc changes and bony endplate lesions in 54 patients during the 1-year follow-up has been reported earlier [15].
Prevalence and follow-up of LBP symptoms
The mean intensity of pain (NRS) was 6.3 (SD 1.9) at baseline and 5.0 (SD 2.7) after follow-up (p = 0.001, paired t test). Mean ODI was 29.2 (SD 14.4) and 23.8 (SD 19.0) (p = 0.01, paired t test), accordingly. NRS decreased in 31 (63 %), persisted in 9 (18 %), and increased in 9 (18 %) patients.
Change of LBP in relation to that of MRI findings
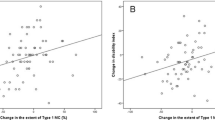
Results of the analyses using generalized estimating equations are presented in Table 2. The change in NRS associated with the change in M1, M2, bony endplate lesions, SI of nucleus pulposus, and disc height as studied crude. The change in ODI associated with that of M1, bony endplate lesions, disc height, and anterior bulge, accordingly. In the fully adjusted GEE models, the change in NRS associated weaker with change in M1, but stronger with that in SI of nucleus pulposus and significantly with the change in M2, bony endplate lesions, and disc height, too. The change in ODI associated weaker with that of M1 and disc height, but the association with bony endplate lesions remained strong after adjustment. No significant association with anterior bulge was found after adjustment (p = 0.488). The change in pain was not associated with age or sex of the patient and not with the interval between MRI studies or history of spine operation.
If severity of pain changed, it did not decrease in all patients, but increased in some. The same type of change was found in graded follow-up MRI variables M1, M2, disc bulges or signal intensity of the nucleus pulposus. Disc height decreased in most cases of change and most changing endplate lesions enlarged or increased.
To show as examples, how the different follow-up grades of degeneration associated with graded change in pain, numbers, and frequencies of graded change in M1, endplate lesion and SI of the nucleus pulposus are cross tabulated with the graded (by strength and direction) change in NRS (Tables 3, 4, 5). Significant associations were found between some grades of follow-up MRI variables and graded change in pain, as estimated marginal means were calculated.
Decrease of M1 predicted decrease of pain (p = 0.011), but persistence of M1 increase of pain (p = 0.001). Enlargement (p = 0.062) of M1 was more common among those with persisting or increasing pain. In contrast, enlargement of M2 predicted decreasing of pain (p = 0.025), while decrease of M2 size (p = 0.000), new M2 (p = 0.000) and M2 developing from M1s (p = 0.050) predicted increase of pain (Table 3). Increase of bony endplate irregularities (p = 0.023) and associated Schmorl lesions or endplate defects (p = 0.041) predicted increase in pain, while the decrease of a lesion predicted decrease in pain (p = 0.000) (Table 4). The decrease of SI in nucleus pulposus predicted decrease of pain (p = 0.001), while discs with normal signal intensity (p = 0.048) and degenerated discs with increase of SI in nucleus pulposus (p = 0.099) were more common among those with persisting or increasing pain (Table 5). The decrease of disc height predicted increasing or persisting pain (p = 0.008).
The predictive effect of the change in M1, disc height, and endplate lesions on change of ODI was similar. Associations calculated for grades of change in M1 and disc height were even stronger than those with NRS. Those for bony endplate lesions were weaker. Decrease of SI of nucleus pulposus predicted decrease in ODI (p = 0.012), but the overall association between change in disc SI and that in ODI was not significant (p = 0.078). Other degenerative variables did not have significant association with ODI.
Discussion
In this prospective MRI study of chronic LBP patients with large Modic 1 type change, the intensity of LBP (NRS) decreased in most patients during 1 year, but increased or persisted in 36 %. The poor outcome was associated with increasing or persisting subchondral oedema (M1), decrease of disc height, and a deforming process in the bony endplates, while decrease of M1 and of signal intensity in nucleus pulposus predicted decrease of pain. Association between M2 and LBP was not consistent.
Our results are in accordance with the earlier studies, which suggested that M1 is associated with LBP [3] and has a stronger association with pain than M2 [10, 11], and with follow-up studies, in which an association was found between the persistence of M1 and LBP [8, 20] and between a change of M1 and that of LBP [21]. Increasing M2 associated with decrease in pain, which supports the hypothesis of pain decreasing as M1 changes into M2 [7]. M2s developed from M1s [15]. Many M2s were of mixed type (M1/M2).
Our results regarding the association of LBP with bony endplate lesions are in accordance with those of a recent cadaver study [12]. Schmorl lesions have different etiologies, and may have various MRI appearances [22]. Those adjacent to changing M1s have been found to enlarge or change rapidly along with progressing disc degeneration, decreasing disc height, and increasing and decreasing SI of nucleus pulposus [13, 14]. Thus, they may be one feature of an accelerated degenerative process. Schmorl lesions associated with unspecified Modic changes and disc degeneration also in a recent cross-sectional population study [23]. In another one, Schmorl lesions, Modic changes, and disc degeneration together were associated with LBP, but none of them alone [24]. Modic changes and bony endplates have nociceptive receptors, and in bony endplate defects, their number is increased [16]. An increased number of tumor necrosis factor-immunoreactive nerve cells and fibres have been found in endplates with Modic change [25]. Enlarging Schmorl lesions could thus cause pain.
Increase of SI in nucleus pulposus was more common in patients with increase or persistence of pain than in those with decrease of pain, although the association was not significant. The small size of our material may decrease possibilities to detect significant associations. An increased disc SI is detected in trauma, spondylodiscitis, and inflammatory spine disease. It is not a specific sign of discitis, however. The clearly increased SI, but decreased height in a disc may be explained by advanced degenerative changes with fissures and collapse of the inner structure of the disc as found in a cadaver study [26]. An increased SI of nucleus pulposus was observed to be associated with an accelerated degenerative process in disc spaces with a large M1 [13, 14].
Antibiotic treatment was effective on chronic LBP symptoms in patients with M1 after disc herniation, according to a recent double-blind randomized clinical controlled trial [27]. Large M1s were found to decrease, too, with that treatment, but not without. M1s were smaller in the placebo group. In our study, NRS decreased in 31/49 (63 %) of patients, without antibiotic treatment. 50/100 baseline M1s decreased, too. Differences in inclusion criteria and grading of changes may explain differences between the natural courses in these two studies. Our M1s were relatively large (>15 % of the sagittal area of the vertebra) in contrast to the placebo group, and disc herniations were excluded from our material. Infectious disease was clinically excluded in our patients, but disc biopsies were not obtained. Thus, we cannot totally exclude the possibility of that disease. In clinical patients, the possibility of an infectious or inflammatory spine disease needs to be considered and excluded when evaluating the cause of LBP with MRI.
There could be other explanations to the pain, however. The association of prolonged or increasing LBP with decreasing disc height was expected. It may indicate that deterioration and collapse of the disc lead to painful changes in the disc. Decreasing disc height is known to affect the size of the nerve root channels and loading on the facet joints. Morphologic degenerative changes in those structures could lead to pain provoking changes outside the disc, too, and explain the prolonged LBP. We did not evaluate facet joints, nerve root channels, spondylolisthesis or instability, because only sagittal slices were obtained for the follow-up MRI.
In this study, a more detailed MRI classification was used instead of the original Modic classification for subchondral signal changes [5] or the Pfirrman grading for DD [28], to cover the variety of degenerative MRI findings. It allows a separate assessment of each type of MRI finding, different subchondral signal abnormalities and bony endplate lesions, discs adjacent to a transitional vertebra, and a follow-up of even the common mixed (M1-/M2-/M3-type) Modic-lesions [17]. We studied the predictive effect of many different degenerative findings on LBP in addition to M1s, separately and together, unlike in many previous studies.
The highly selected material in this study may decrease the possibilities to generalize the results. We wanted to exclude patients with any known, possible cause for their pain except degenerative changes. On the other hand, this material represents the clinically relevant population of chronic LBP patients. M1s are known to change into M2 type lesions which are far more common than M1s in LBP patients and in the general adult population. Our sample can be considered to represent LBP patients with Modic changes, in a certain phase (M1). Posterior disc extrusions were common in the original LBP patient population (N = 3811). Since patients without disc extrusion but with a large M1 were rare, the final material (N = 49) was small. The age of the included subjects was relatively high, because Modic changes are rare among the young. Most included patients were females for an unknown reason. Differences in the prevalence of disc degeneration and LBP symptoms between sexes have been reported [29]. Android pattern of fat distribution has been found to increase the risk of Modic change [30]. M2 may have a metabolic component in its aetiology [30]. Bone structure is known to depend on metabolic factors. It might have a role in development of subchondral signal changes and associated bony endplate defects. The gender or age was not found to affect our results. Part of the M2-type lesions (fatty degeneration) detectable on MRI is not associated with endplate lesions and disc degeneration or even located adjacent to endplates and, thus, may have a different effect (or no effect) on LBP.
In studying the causes of LBP, the number, diversity, and multilevel location of MRI findings in each patient bring problems in determining, which is the cause of pain. We, therefore, chose to use generalized estimating equations, even if this material was relatively small (N = 49). Possible predictors could be controlled for in the analysis. By this method, we could use the full power of the material without the need to stratify our data. The use of pain medication was not questioned. Neither was any effect of genes, psychosocial factors, or other possible confounders considered. Differences in the timely course of changes within each degenerative variable may affect possibilities to detect significant associations with pain and to find most relevant MRI signs.
This study brings new information about the significance of different degenerative changes in explaining the development of symptoms in chronic LBP patients. The effects of enlarging and decreasing M1 and M2, increasing Schmorl lesions and other bony endplate lesions, decreasing disc height, and changing SI in the nucleus pulposus on LBP were studied, and each variable was found to be associated with the change in pain. Changing M1s have been found to associate closely with those other, fast changing degenerative findings. Decrease in M1 and increase in M2 associated with decrease in pain while persistence of M1 with that of pain which strengthens the view that Modic changes are painful, as long as they have not changed into M2 type. This study shows that M1 and adjacent, increasing bony endplate lesions, and decreasing disc height play an important role in chronic LBP. Increasing and decreasing SI in the nucleus pulposus also seem to have a role, although the association found with decreasing pain was unexpected. Adjacent bony endplate lesions and changes in signal intensity of nucleus pulposus may need attention in addition to M1-type subchondral signal changes, as they all may be signs of a fast progressing degenerative process associated with decreasing disc height and prolonged LBP symptoms.
Conclusion
Modic 1 change and associated bony endplate lesions and disc height decrease are suggested to be signs of a degenerative process that predicts long-lasting LBP. Such degenerative MRI findings and associated changes in SI of nucleus pulposus have been earlier shown to progress during 1 year, faster than the age-dependent degeneration. They should alert considering early intervention and possibilities to slow down the further deterioration of the discovertebral unit and to affect the associated prolonged LBP with available treatment methods.
References
Luoma K, Riihimäki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A (2000) Low back pain in relation to lumbar disc degeneration. Spine 25(4):487–492
Endean A, Palmer KT, Coggon D (2011) Potential of magnetic resonance imaging findings to refine case definition for mechanical low back pain in epidemiological studies: a systematic review. Spine 36(2):160–169. doi:10.1097/BRS.0b013e3181cd9adb
Steffens D, Hancock MJ, Maher CG et al (2014) Does magnetic resonance imaging predict future low back pain? A systematic review. Eur J Pain (England) 18(6):755–765. doi:10.1002/j.1532-2149.2013.00427
Hebelka H, Brisby H, Hansson T (2014) Comparison between pain at discography and morphological disc changes at axial loaded MRI in patients with low back pain. Eur Spine J (Germany) 23(10):2075–2082. doi:10.1007/s00586-014-3408-6
Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR (1988) Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 166:193–199
Kjaer P, Leboeuf-Yde C, Korsholm L, Sørensen JS, Bendix T (2005) Magnetic resonance imaging and low back pain in adults: a diagnostic imaging study of 40-year-old men and women. Spine 30:1173–1180
Jensen TS, Karppinen J, Sørensen JS, Niinimäki J, Leboeuf-Yde C (2008) Vertebral endplate signal changes (Modic change): a systematic literature review of prevalence and association with non-specific low back pain. Eur Spine J 17:1407–1422. doi:10.1007/s00586-008-0770-2
Määttä JH, Wadge S, MacGregor A, Karppinen J, Williams FM (2015) ISSLS prize winner: vertebral endplate (Modic) change is an independent risk factor for episodes of severe and disabling low back pain. Spine 40(15):1187–1193. doi:10.1097/BRS.0000000000000937
Mok FP, Samartzis D, Karppinen J, Fong DY, Luk KD, Cheung KM (2016) Modic changes of the lumbar spine: prevalence, risk factors, and association with disc degeneration and low back pain in a large-scale population-based cohort. Spine 16(1):32–41. doi:10.1016/j.spinee.2015.09.060
Kääpä E, Luoma K, Pitkäniemi J, Kerttula L, Grönblad M (2012) Correlation of size and type of modic type 1 and 2 lesion with clinical symptoms—a descriptive study in a subgroup of chronic low back pain patients based on a university hospital patient sample. Spine 37:134–139. doi:10.1097/BRS.0b013e3182188a90
Mitra D, Cassar-Pullicino VN, McCall IW (2004) Longitudinal study of vertebral type-1 end-plate changes on MR of the lumbar spine. Eur Radiol 14:1574–1581
Wang Y, Videman T, Battié MC (2012) ISSLS prize winner: lumbar vertebral endplate lesions: associations with disc degeneration and back pain history. Spine 37(17):1490–1496. doi:10.1097/BRS.0b013e3182608ac4
Luoma K, Vehmas T, Grönblad M, Kerttula L, Kääpä E (2008) MRI follow-up of subchondral signal abnormalities in a selected group of chronic low back pain patients. Eur Spine J 17:1300–1308. doi:10.1007/s00586-008-0716-8
Luoma K, Vehmas T, Grönblad M, Kerttula L, Kääpä E (2009) Relationship of Modic type 1change with disc degeneration: a prospective MRI study. Skelet Radiol 38:237–244. doi:10.1007/s00256-008-0611-8
Kerttula L, Luoma K, Vehmas T, Grönblad M, Kääpä E (2012) Modic type I change may predict rapid progressive, deforming disc degeneration: a prospective 1-year follow-up study. Eur Spine J 21:1135–1142. doi:10.1007/s00586-012-2147-9
Fields AJ, Liebenberg EC, Lotz JC (2014) Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. Spine J 14(3):513–521. doi:10.1016/j.spinee.2013.06.075
Luoma K, Kerttula L, Vehmas T, Grönblad M (2014) Association of low-back pain with modic type endplate changes and disc degeneration using a detailed MRI-classification. Insights. Imaging 5(Suppl 1):S135–S136. doi:10.1007/s13244-014-0317-5 (Scientific paper) doi:10.1594/ecr2014/B-0458
Luoma K, Vehmas T, Raininko R, Luukkonen R, Riihimäki H (2004) Lumbosacral transitional vertebra: relation to disc degeneration and low back pain. Spine 29(2):200–205
Desmond PM, Buirski G (1993) Magnetic resonance appearances of developmental disc anomalies in the lumbar spine. Australas Radiol 37(1):26–29
Jensen RK, Leboeuf-Yde C, Wedderkopp N, Sørensen JS, Jensen TS, Manniche C (2012) Is the development of modic changes associated with clinical symptoms? A 14-month cohort study with MRI. Eur Spine J 21(11):2271–2279. doi:10.1007/s00586-012-2309-9
Järvinen J, Karppinen J, Niinimäki J, Haapea M, Grönblad M, Luoma K, Kääpä E (2015) Association between changes in lumbar Modic changes and low back symptoms over a 2-year period. BMC Musculoskelet Disord 16:98. doi:10.1186/s12891-015-0540-3
Peters CA, Vande Berg BC, Galand C, Lecouvet FE, Malghem J (2009) Fracture-associated and idiopathic subchondral vertebral lesions: a magnetic resonance study in autopsy specimens with histologic correlation. Skelet Radiol 38(3):245–253. doi:10.1007/s00256-008-0614-5
Määttä JH, Karppinen JI, Luk KD, Cheung KM, Samartzis D (2015) Phenotype profiling of Modic changes of the lumbar spine and its association with other MRI phenotypes: a large-scale population-based study. Spine J 15(9):1933–1942. doi:10.1016/j.spinee.2015.06.056
Teraguchi M, Yoshimura N, Hashizume H, Muraki S, Yamada H, Oka H, Minamide A, Nakagawa H, Ishimoto Y, Nagata K, Kagotani R, Tanaka S, Kawaguchi H, Nakamura K, Akune T, Yoshida M (2015) The association of combination of disc degeneration, end plate signal change, and Schmorl node with low back pain in a large population study: the Wakayama Spine Study. Spine J 15(4):622–628. doi:10.1016/j.spinee.2014.11.012
Ohtori S, Inoue G, Ito T, Koshi T, Ozawa T, Doya H, Saito T, Moriya H, Takahashi K (2006) Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back pain and Modic type 1 or type 2 changes on MRI. Spine 31:1026–1031
Yu SW, Haughton VM, Ho PS, Sether LA, Wagner M, Ho KC (1988) Progressive and regressive changes in the nucleus pulposus. Part II. The adult. Radiology 169(1):93–97
Albert HB, Sorensen JS, Christensen BS, Manniche C (2013) Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double-blind randomized clinical controlled trial of efficacy. Eur Spine J (Germany) 22(4):697–707. doi:10.1007/s00586-013-2675-y
Pfirmann C, Metzdorf A, Zanetti M, Hodler J, Boos N (2001) Magnetic resonance classification of lumbar intervertabral disc degeneration. Spine 26(17):1873–1878
De Schepper EI, Damen J, van Meurs JB, Ginai AZ, Popham M, Hofman A, Koes BW, Bierma-Zeinstra SM (2010) The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine 35(5):531–536. doi:10.1097/BRS.0b013e3181aa5b33
Teichtahl AJ, Urquhart DM, Wang Y, Wluka AE, O’Sullivan R, Jones G, Cicuttini FM (2016) Modic changes in the lumbar spine and their association with body composition, fat distribution and intervertebral disc height—a 3.0 T-MRI study. BMC Musculoskelet Disord (England) 17(1):92. doi:10.1186/s12891-016-0934-x
Acknowledgments
Supported by Finska Läkaresällskapet.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Luoma, K., Vehmas, T., Kerttula, L. et al. Chronic low back pain in relation to Modic changes, bony endplate lesions, and disc degeneration in a prospective MRI study. Eur Spine J 25, 2873–2881 (2016). https://doi.org/10.1007/s00586-016-4715-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-016-4715-x