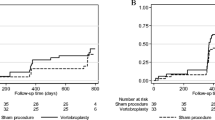
Abstract
Two recent randomised controlled trials (RCT) published by the New England Journal of Medicine (NEJM) in 2009 comparing vertebroplasty to sham procedures have concluded that vertebroplasty is no more effective than injection of local anaesthetic at the pedicle entry point. This finding contradicts previously published clinical series on vertebroplasty which have shown clinical efficacy. The procedural details of the two RCTs are analysed specifically with regard to vertebral levels treated and injected polymethylmethacrylate (PMMA) volumes in an attempt to combine the data for assessment against the available basic science underpinning the effect of vertebral augmentation procedures. Neither investigation provides a breakdown of the vertebral levels treated in the original publication or in supplementary online material. Only one investigation provides information on fill volumes with an overall average fill volume of 2.8 ± 1.2 ml SD. The available basic science indicates a minimum fill volume of 13–16% of the vertebral body volume to be necessary for a relevant biomechanical effect on restoration of vertebral strength. The most commonly treated vertebrae of the thoracolumbar junction have an anatomical vertebral body volume of ~30 ml. An effective fill would require a minimum of ~4 ml PMMA. Anatomical volumes and required fill volumes increase towards the lower lumbar spine. According to the available basic science, only vertebrae of the upper to mid thoracic spine could reasonably have received a biomechanically effective fill with the declared average volume of 2.8 ± 1.2 ml SD. The available data of the NEJM publications strongly indicates that the treatment arm includes patients who were not treated in a reasonably effective manner. The technical information provided by the NEJM publications is insufficient to conclusively prove or disprove the clinical efficacy of vertebroplasty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Randomised controlled trials (RCT) have become established as the Gold Standard of clinical research. When applied to surgical procedures, the highest scientific standard is met when an actual procedure is compared to a placebo or “sham” procedure. Recently two investigations [6, 12] comparing vertebroplasty to “sham” operations were published in the New England Journal of Medicine (NEJM). The findings from these investigations indicate that vertebroplasty is no more effective than injection of local anaesthetic at the pedicle entry site. In view of the widespread use of vertebral augmentation techniques, the implication of inefficacy entails tremendous clinical and medicolegal impact on the surgical community and prospective patients.
As already pointed out by Aebi [1] and Bono [4], these findings are clearly at odds with previously published clinical investigations which indicate a significant clinical benefit of vertebral augmentation procedures, such as vertebro- and kyphoplasty over conservative treatment [3, 18, 20]. This discrepancy can only reasonably have two explanations: the placebo-effect has been severely underestimated in previously published investigations or the study design of the new RCTs is flawed. The very basic foundation of any RCT is to ensure that all patients in the treatment arm have actually received the treatment in a manner that may be presumed to be effective. In other words, the potentially effective pharmaceutical drug has to be given to each treated patient in the presumed effective dose. Likewise, if a procedure is under investigation, the investigators must ensure that all treated patients have undergone the procedure in a fashion that may be assumed to be effective. Vertebroplasty is a complex procedure to investigate with an RCT as there are considerable variables in conducting the actual procedure. Besides the technique of insertion of the delivery needle, the injection of the “active agent”—generally, polymethylmethacrylate (PMMA)—is entirely operator dependent. The instillation of PMMA into the fractured vertebral body improves the mechanical properties and is presumed to provide pain relief by reducing painful loading and abnormal micromotion. Clearly, a minimal amount of PMMA will have no appreciable effect and likewise an excessive amount hardens the vertebral body beyond its original state and may have an adverse effect on adjacent osteoporotic vertebrae. Several biomechanical investigations of high quality have shown that the minimum volume required to achieve an appreciable biomechanical effect of restoration of compressive strength or equalised stress distribution ranges between 13 and 16% of the vertebral body volume [15–17].
The purpose of this review therefore is to analyse the procedural details of the publications by Buchbinder et al. [6] and Kallmes et al. [12] in the context of the standard dictated by the best available basic science.
Methods
The procedural data of the investigations by Kallmes et al. [12] and Buchbinder et al. [6] (including available supplementary online material) was analysed with respect to the surgical technique (uni- or bipedicular tool placement), vertebral levels treated and polymethylmethacrylate (PMMA) injection volume achieved. Any relevant data available in the description of the INVEST study design [9] as cited by Kallmes et al. [12] was included, as was the description of the study protocol [7] as cited by Buchbinder et al. [6].
Results
The details of tool insertion, whether uni- or bipedicular, are not specified in the study design of the INVEST trial [9]. While Kallmes et al. [12] state that vertebral levels between T4 and L5 were included, no breakdown of the actual number of vertebrae per level is provided. Injection of PMMA “was stopped when the PMMA reached to the posterior aspect of the vertebral body or entered an extraosseous space”, citing the publication by Jensen et al. [11]. There is, however, no record of the injected PMMA volumes or a correlation of injected volumes with vertebral levels. The description of the INVEST trial [9] gives no indication of the PMMA volumes or filling characteristics that are to be achieved. Subsequent personal correspondence with authors of the investigation indicated that volumes were not recorded and therefore cannot be retrieved for further analyses.
The study protocol of Buchbinder et al. [7] published in 2008 is clear on the technique of tool insertion. This is specifically to be transpedicular (left pedicle). The volume of PMMA to be injected is described as “3–4 ml of PMMA will slowly be injected into the vertebral body”. In the NEJM publication of the results by Buchbinder et al. [6], the methods (interventions) section of the paper records the volume injected as “approximately 3 ml”. In the results section of the same paper, the mean volume of cement injected is given as 2.8 ± 1.2 ml SD. No breakdown of the levels treated or a correlation of injected volume per anatomical level or region (i.e. thoracic or lumbar) is provided. The supplementary online data to this publication does not provide additional information in this respect.
It was not possible to combine the procedural data from the two NEJM publications as the volume of PMMA injected was not recorded by Kallmes et al. [12] and the breakdown of PMMA volume per anatomical level is not provided by Buchbinder et al. [6].
Table 1, which lists the average vertebral body volumes determined by Molloy et al. [17] along with the calculated percentage fill (16% for restoration of strength and 30% for restoration of stiffness), indicates that an average fill of 2.8 ml would only reasonably achieve a biomechanically adequate fill cranial of T9 for restoration of vertebral strength. Vertebrae of the thoracolumbar junction possess a volume of ~30 ml, which increase in the lumbar spine to ~40 ml. In these regions filling volumes of 4 ml and more are required for a biomechanically adequate fill of 16%.
Discussion
It is a gross oversimplification to assume that any minimal amount of injected PMMA will have a clinical effect. Keller et al. [14] have shown that the volume and actual distribution of augmentation material has a significant effect on vertebral stress distribution. Homminga et al. [10] have shown that the trabecular core carries 70% of the transmitted load in the vicinity of the vertebral endplates (in the presence of a non-degenerate disc). In the midtransverse area a shift of load transfer occurs towards the cortical shell whereby the trabecular core carries 50% of the transmitted load. In the setting of clinically relevant osteoporosis the percentage of trabecular material at risk of fracture increases from 1.2 (healthy vertebrae) to 16%. Although not strictly comparable, the order of magnitude is interesting in view of ~16% vertebral body volume augmentation being required for equalisation of stress distribution [15–17]. In order to achieve a relevant restoration of the mechanical properties of the fractured vertebral body, a reasonable minimum fill of the vertebral body with PMMA therefore needs to be achieved. In early clinical vertebroplasty series, such as that by Evans et al. [8] and Jensen et al. [11], “injection is performed until hemivertebral or holovertebral filling is achieved, no more material can be pushed into the body, or extravasation into veins or the disk space is noted”, achieving injection volumes from 2.5 to 11 ml (average 7.1 ml). Figure 7 of the same paper shows an L1 vertebral body filled with 10 ml of PMMA. The risk of cement leakage with large injection volumes has, however, led to a significant reduction in injected volumes in current clinical practice. Based on clinical experience, it has since been argued that the injected volume of PMMA has no influence on clinical outcome [2, 13]. In a retrospective review of 158 patients by Kaufman et al. [13] the mean cement volume injected was 3.4 ml (range 0.5–10.3 ml). Thoracic vertebrae received a median volume of 2.5 ml (range 0.8–6.1 ml) and lumbar vertebrae 3.5 ml (range 0.5–10.3 ml). In a prospective study on 403 successive patients by Al-Ali et al. [2], no statistical correlation could be found between the injected cement volume and clinical outcome. The mean injected volume for all levels was 5.1 ml (±2.2 ml SD). A breakdown of the volumes by anatomical regions reveals filling volumes of 3.5 ml (±1.2 ml SD) for T3–T8; 5.0 ml (±2.0 ml SD) for T9–T12 and 6.0 ml (±2.3 ml SD) for L1–L5. These studies indicate that there is no obvious advantage in large filling volumes. Kaufman et al. [13] recorded clinical pain score improvement at 2 years of close to 6 points average. A mean VAS improvement of 6.2 (±3.5 SD) was achieved by Ali et al. [2]. Both of these studies record a higher pain score reduction than the RCTs of Kallmes et al. [12] and Buchbinder et al. [6] which achieved an average reduction of 3.0 and 2.6, respectively. This raises the question whether the treatment methods employed in these different investigations are comparable. The obvious difference is in the filling volumes achieved by Buchbinder et al. [6]. The average filling volume of 2.8 ml (±1.2 ml SD) falls significantly short of the volumes achieved by Kaufman et al. and Al-Ali et al. [2, 13], especially when considering thoracolumbar and lumbar vertebrae which have vertebral body volumes of ~30–40 ml (Table 1). Table 1 reveals that a biomechanically effective fill of 16% would only reasonably have been achieved at thoracic vertebral levels ranging between T6 and T9 by Buchbinder et al. [6]. Unfortunately no breakdown of treated anatomical levels is provided, but taking the standard deviation of 1.2 ml into account, there were clearly vertebrae in the treatment group that received only 1.6 ml or less. This volume must be considered as biomechanically inadequate at any level between T6 and L5. In contrast, it is of note that the regional average values of Ali et al. [2] are compatible with the biomechanically required fill percentage of 13–16% of vertebral body volume [15–17] (Table 1). A further source of concern is the stipulation of the unilateral transpedicular approach to all vertebrae regardless of level by Buchbinder et al. [6, 7]. A unilateral approach is not unreasonable in principle and the biomechanical results of Tohmeh et al. [19] indicate bi- and unipedicular vertebroplasty to be equally effective. However, in the investigation by Tohmeh et al. [19] 6 ml was injected in the unipedicular group and 10 ml in the bipedicular group. Personal experience has shown that central positioning of the delivery tool can be restricted in cases of slender thoracic pedicles and that a transcostovertebral approach [5] yields central placement more reliably. With the injection of such small volumes as by Buchbinder et al. [6] via a unilateral transpedicular route, it cannot be excluded that cement may not have adequately reached the area of vertebral damage in all cases. Kallmes et al. [12] surprisingly provide no record of injected volumes and personal communication with authors of the study indicates that this was not recorded and therefore cannot be retrieved for future analysis. It is disappointing that the NEJM has not insisted on obtaining and publishing the basic data pertinent to the actual procedure in either publication [6, 12]. Even more so in view of the violation of the study protocol by Buchbinder et al. [6] which clearly indicates that 3–4 ml is to be injected per treated vertebra.
It therefore appears reasonable to conclude that the treatment arm of the study by Buchbinder et al. [6] includes an unknown number of patients who received an inadequate “dose” of PMMA. These patients should have been excluded from the treatment arm as they arguably have been treated with a placebo “dose” of PMMA. Given the small number of treated patients (N = 38), it is not surprising that no statistically beneficial effect was recorded between the groups, both of which in all likelihood included “placebo” patients. The injection volumes of Kallmes et al. [12] will never be known, however, if the trend to very low injection volumes demonstrated by Buchbinder et al. [6] has become common amongst interventional radiologists, it is possible that the investigation by Kallmes et al. [12] is flawed in a similar way. As pointed out by Bono et al. [4] the authors are to be commended for having undertaken the daunting task of a RCT in vertebroplasty; however, the data unfortunately does not conclusively prove or disprove the efficacy of vertebroplasty.
References
Aebi M (2009) Vertebroplasty: about sense and nonsense of uncontrolled “controlled randomized prospective trials”. Eur Spine J 18:1247–1248
Al-Ali F, Barrow T, Luke K (2009) Vertebroplasty: what is important and what is not. Am J Neuroradiol 30:1835–1839
Álvarez L, Alcarez M, Pérez-Higueras A et al (2006) Percutaneous vertebroplasty: functional improvement in patients with osteoporotic compression fractures. Spine 31:1113–1118
Bono C, Heggeness M, Mick C, Resnick D, Watters W (2009) Newly released vertebroplasty randomized controlled trials: a tale of two trials. Spine J. doi:10.1016/j.spinee.2009.09.007
Boszczyk BM, Bierschneider M, Hauck S et al (2005) Transcostovertebral kyphoplasty of the mid and high thoracic spine. Eur Spine J 14:992–999
Buchbinder R, Osborne RH, Ebeling PR et al (2009) A randomized controlled trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 361:557–568
Buchbinder R, Osborne RH, Ebeling PR et al (2008) Efficacy and safety of vertebroplasty for treatment of painful osteoporotic vertebral fractures: a randomised controlled trial. BMC Musculoskelet Disord 9:156
Evans AJ, Jensen ME, Kip KE et al (2003) Vertebral compression fractures: pain reduction and improvement in functional mobility after percutaneous polymethylmethacrylate vertebroplasty—retrospective report of 245 cases. Radiology 226:366–372
Gray LA, Jarvik JG, Heagerty PJ et al (2007) Investigational vertebroplasty efficacy and safety trial (invest): a randomized controlled trial of percutaneous vertebroplasty. BMC Musculoskelet Disord 8:126
Homminga J, Weinans H, Gowin W, Felsenberg D, Huiskes R (2001) Osteoporosis changes the amount of vertebral trabecular bone at risk of fracture but not the vertebral load distribution. Spine 26:1555–1561
Jensen ME, Evans AJ, Mathis JM et al (1997) Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. Am J Neuroradiol 18:1897–1904
Kallmes DF, Comstock BA, Heagerty PJ et al (2009) A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 361:569–579
Kaufmann TJ, Trout AT, Kallmes DF (2006) The effects of cement volume on clinical outcomes of percutaneous vertebroplasty. Am J Neuroradiol 27:1933–1937
Keller TS, Kosmopoulos V, Lieberman IH (2005) Vertebroplasty and kyphoplasty affect vertebral motion segment stiffness and stress distributions—a microstructural finite-element study. Spine 30:1258–1265
Liebschner LA, Rosenberg WS, Keaveny TM (2001) Effects of bone cement volume and distribution on vertebral stiffness after vertebroplasty. Spine 26:1547–1554
Luo J, Daines L, Charalambous A, Adams MA, Annesley-Williams DJ, Dolan P (2009) Vertebroplasty: only small cement volumes are required to normalize stress distributions on the vertebral bodies. Spine 34:2865–2873
Molloy S, Mathis JM, Belkoff SM (2003) The effect of vertebral body percentage fill on mechanical behaviour during percutaneous vertebroplasty. Spine 28:1549–1554
Taylor RS, Fritzell P, Taylor RJ (2007) Balloon kyphoplasty in the management of vertebral compression fractures: an updated systematic review and meta-analysis. Eur Spine J 16:1085–1100
Tohmeh AG, Mathis JM, Fenton DC, Levine AM, Belkoff SM (1999) Biomechanical efficacy of unipedicular versus bipedicular vertebroplasty for the management of osteoporotic compression fractures. Spine 24:1772–1776
Wardlaw D, Cummings SR, Van Meirhaeghe J, Bastian L, Tillman JB, Ranstam J, Eastell R, Shabe P, Talmadge K, Boonen S (2009) Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet 373:1016–1024
Acknowledgments
No grants were received in support of this manuscript.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boszczyk, B. Volume matters: a review of procedural details of two randomised controlled vertebroplasty trials of 2009. Eur Spine J 19, 1837–1840 (2010). https://doi.org/10.1007/s00586-010-1525-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-010-1525-4