Abstract
Doxorubicin (DOX) is an anthracycline antibiotic with a chemotherapeutic effect and has well-known cardiotoxic side effects. This study aimed to determine potential cardioprotective mechanisms of different doses of resveratrol (RES) in a DOX-induced cardiotoxic rat model. In the study, 42 Sprague–Dawley male rats were randomly divided into six groups (n = 7). The control group (n = 7) was given only ad libitum feed and water; the DOX group (n = 7) was administered DOX intraperitoneally (i.p) at a dose of 15 mg/kg at the beginning of the experiment. The DOX + RES I (n = 7) and DOX + RES II (n = 7) groups were administered RES i.p at doses of 1 mg/kg/day and 5 mg/kg/day, respectively, after administering 15 mg/kg DOX. The RES I (n = 7) and RES II (n = 7) groups were administered RES i.p at doses of 1 mg/kg/day and 5 mg/kg/day, respectively. Cardiac tissues collected upon completion of experimental procedures were used for biochemical, histopathological, and immunohistochemical evaluations. It was determined that RES administration may have an antiapoptotic effect by regulating apoptotic protein activities and an antioxidant effect by regulating oxidative stress parameters in DOX-induced cardiotoxicity. It was also observed that RES may regulate the levels of Meteorin-Like (MTRNL), which plays a role in energy metabolism, and the immunoreactivity of the transient receptor potential melastatin 2 (TRPM2) channel, which has a role in cellular ion homeostasis. RES may improve cardiac tissue damage by showing antiapoptotic and antioxidant effects against DOX-induced cardiotoxicity. Furthermore, MTRNL and TRPM2 ion channels may play a role in DOX-induced cardiotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Doxorubicin or adriamycin (DOX) is a cytotoxic anthracycline antibiotic. It is also an antineoplastic agent used in the treatment of many types of cancer (Alanazi et al. 2020). However, its side effect, cardiotoxicity, limits its clinical use (Adıyaman et al. 2022). Different mechanisms are thought to play a role in the development of DOX cardiotoxicity. Some of these mechanisms are lipid peroxidation and oxidative stress as a result of overproduction of free radicals, increase in intracellular iron, and suppression of natural antioxidants such as glutathione (GSH) (Alanazi et al. 2020). In addition, DOX treatment may cause calcium overload in cardiomyocytes, resulting in inadequate contraction and disruption of mitochondrial calcium homeostasis. This may affect energy metabolism and ROS formation, increase calcium-dependent mitochondrial permeability, and induce apoptosis through cytochrome C release (Xiong et al. 2018). Myocardial apoptosis-induced cardiotoxicity is the main clinical side effect of DOX. Therefore, there is an urgent need to develop new strategies to suppress DOX-induced apoptosis in cardiomyocytes, ameliorate cardiac dysfunction, and alleviate cardiotoxicity. Resveratrol (RES) is a polyphenolic compound found especially in grape and red wine (Kaya and Dönmez 2020). Previous studies showed that RES, which has a strong antioxidant effect, has antifibrotic, anti-inflammatory, and antiapoptotic effects on cardiomyocytes and in vivo cardiotoxicity models (Zordoky et al. 2015; Gu et al. 2018). It was also reported that RES reduces DOX-induced cardiotoxicity through signaling pathways associated with cell apoptosis, oxidative stress, and cell proliferation (Tian et al. 2020). However, the exact molecular mechanisms still require elucidation.
Meteorin-like (MTRNL), which is secreted from adipose and muscle tissue, is an adipomyokine that increases insulin sensitivity and has neuroprotective effects (Kocaman et al. 2022). It plays a role in regulating inflammation and energy expenditure (Baht et al. 2020). MTRNL may play an important role in the pathogenesis of cardiovascular diseases (Feijóo-Bandín et al. 2020). It was also reported that the expression of MTRNL in cardiac tissue increases under physiological conditions that decrease as a result of DOX exposure (Hu et al. 2020). Oxidative stress occurs with the production of oxygen metabolites such as hydrogen peroxide, which increases vascular endothelial permeability. Hydrogen peroxide stimulates the production of ADP-ribose, which opens and activates the transient receptor potential melastatin 2 (TRPM2) channels. Activation of TRPM2 channels leads to the entry of calcium into cells. The increased amount of calcium causes structural and functional changes that may ultimately lead to cell death (Akkoc et al. 2021). TRPM2 is a potential therapeutic target for diseases in which oxidative stress may be involved, including diabetes, inflammation, myocardial infarction, and neurodegenerative diseases (Atilgan et al. 2022).
This study aimed to determine the effect of DOX-induced cardiotoxicity on MTRNL and TRPM2 ion channels and whether RES at different doses exerts a cardioprotective effect by regulating MTRNL and TRPM2 ion channels.
Materials and methods
Ethical approval
This study was carried out with the approval of Fırat University Animal Experiments Ethics Committee dated 05 Jul 2021 and numbered 2830.
Animals and the experimental design
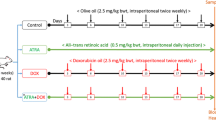
Animal experiments in this study were conducted in accordance with the UK Animals (Scientific Procedures) Act 1986 and related guidelines, EU Directive 2010/63/EU for animal testing. RES was purchased from Sigma-Aldrich, USA. DOX was purchased from Saba Farma Turkey. Lyophilized DOX powder is reconstituted with sterile water for injection to a final concentration of 2 mg/ml. Optimal conditions (12 h light/dark, 22–25 °C temperature, ad libitum water, and feed) were provided for the care of the experimental animals. Forty-two Sprague–Dawley male rats (10 weeks, 200–250 g weight) obtained from Fırat University Experimental Research Unit were randomly divided into six groups (n = 7).
I group (control) (n = 7): Rats in this group were given only ad libitum water and feed.
II group (DOX) (n = 7): At the beginning of the experiment, DOX was administered once intraperitoneally (i.p) at a dose of 15 mg/kg (Refaie et al. 2022).
III group (DOX + RES I) (n = 7): After applying DOX i.p at a dose of 15 mg/kg once at the beginning of the experiment, 1 mg/kg resveratrol (Khazaei et al. 2016) i.p was administered every day for 14 days.
IV group (DOX + RES II) (n = 7): After applying DOX i.p at a dose of 15 mg/kg once at the beginning of the experiment, 5 mg/kg resveratrol (Khazaei et al. 2016) i.p was administered every day for 14 days.
V group (RES I) (n = 7): 1 mg/kg resveratrol administered i.p daily for 14 days.
VI group (RES II) (n = 7): 5 mg/kg resveratrol was administered i.p daily for 14 days.
After the completion of all applications (15th day), the experiment was terminated. The rats used in the experiment were decapitated under anesthesia (ketamine and xylazine). Some of the rapidly removed cardiac tissues were used for histopathological and immunohistochemical evaluations. Some of the cardiac tissues were homogenized with 10% phosphate buffer solution (PBS) for biochemical evaluations. Homogenized samples were centrifuged at 5000 rpm + 4 °C for 20 min. Supernatants were kept at − 80 °C until analysis of MTRNL, malondialdehyde (MDA), catalase (CAT), and glutathione (GSH).
Histopathological evaluation
After the cardiac tissues were quickly removed, they were placed in 10% formalin solution for histopathological evaluations. The fixed cardiac tissues were subjected to routine histological follow-up series and embedded in paraffin blocks. Sections of 5-μm thickness were taken from paraffin blocks. After dewaxing the sections with xylene, they were passed through serial dilutions of alcohol. Hematoxylin eosin and Masson trichrome histological staining procedures were applied for histopathological evaluations. Preparations subjected to histological staining methods were examined with a light microscope (DM2500 LED, Leica, Germany) and photographed (MC170 HD, Leica, Germany). Histopathological changes observed in cardiac tissues were myocardial interstitial congestion, myocarditis, myocardiomyopathy, hyaline degeneration of myocardium, and myocardial fibrosis. Histopathological changes were graded as 0: none, 1: mild, 2: moderate, and 3: severe. After scoring all groups, mean values were determined.
Immunohistochemical examination
For detection of B cell lymphoma-2 (BcL2) (SunRed-201r.5304, China), cysteine-aspartic protease 3 (Casp3) (P42574, Bioss Inc., Beijing, China), MTRNL (PA731035LA01HU, Cusabio Diagnostics, Wuhan, China), and TRPM2 (PA2231, Boster, Pleasanton, USA) immunoreactivities in cardiac tissues, Avidin–biotin-peroxidase complex method was applied according to the procedure described previously (Kaya et al. 2022). Briefly, after basic histological procedures, 5-μm-thick sections on polylyzed slides were boiled for 12 min in the microwave (in citrate buffer solution, pH: 6.0) for antigen retrieval. Then, endogenous peroxidase activity was prevented by applying a hydrogen peroxide blocker. Protein block was applied for 5 min to prevent floor staining. Then, the primary antibody applied tissues were incubated for 1 h at room temperature (dark and humid environment). Then, secondary antibody and streptavidin peroxidase were applied to the sections. All sections were counterstained with Mayer’s hematoxylin. Finally, the sections were washed with PBS and distilled water and closed with a capping solution. The prepared preparations were examined under a light microscope (DM 2500, Leica, Germany) and photographed (DFC295, Leica, Germany). Preparations were examined with a light microscope (DM2500 LED, Leica, Germany) and photographed (MC170 HD, Leica, Germany). Immunohistochemical evaluations were calculated based on the intensity of immunoreactivity at 3 levels (0 none, 1 mild, 2 moderate, 3 intense).
TUNEL analysis
Sections of 4-µm thickness from paraffin blocks of cardiac tissue were taken on polylysine-coated slides. The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) test (TUNEL Assay Kit—HRP—DAB, ab206386, Abcam) was performed according to the manufacturer’s instructions. As a result of TUNEL staining, nuclei stained green with methyl green was considered normal, and cells with brown nuclei were considered apoptotic. Percentages of TUNEL-positive cells were quantified by counting 500 cells from ten random microscopic fields and an apoptotic index (AI) was calculated (Gul et al. 2021).
AI: (total number of cells counted − total number of viable cells) × 100 / (total number of cells counted).
Biochemical analyses
Enzyme-linked immunosorbent assay (ELISA) method was used to determine MTRNL, CAT, GSH, and MDA levels in cardiac tissue samples. MTRNL, CAT, GSH, and MDA capable of quantitative measurement in rat-specific tissue samples ELISA kits were obtained from the company (SunRed, Shanghai, China). All analyses were performed in accordance with the kits protocol. Test results were expressed as ng/ml, ng/ml, mg/l, and nmol/ml, respectively. The MTRNL test range was 0.1–30 ng/ml, and the sensitivity was 0.092 ng/ml; the CAT test range 1–300 ng/ml and sensitivity 0.866 ng/ml; GSH test range is 7–1800 mg/l and sensitivity is 6.105 mg/L; the MDA test range was 0.05–10 nmol/ml and the sensitivity was 0.042 nmol/ml.
Statistical analysis
The data obtained within the scope of the research were analyzed using the Statistical Package for Social Sciences (SPSS) 22.0 package program. The conformity of the data to the normal distribution was examined using the Shapiro–Wilk test. One-way ANOVA and post hoc TUKEY tests were used for statistical analysis of normally distributed results. Data were presented as mean ± standard error. Kruskal–Wallis and Dunn’s pairwise comparisons tests were used in the analysis of results that did not show normal distribution. Data were presented as median value (minimum–maximum). A p value of < 0.05 was considered statistically significant. Graphs were drawn using GraphPad Prism 9.3.1 software. G*Power version 3.1 (University Kiel, Germany) was used to determine the number of animals in the groups in the experiment. The sample size in each group was determined by power analysis, with a type 1 error (α) of 0.05 and a power of 0.90, where n = 7 animals should be present (Faul et al. 2009).
Results
Effects of DOX and/or RES administration on histopathological changes in cardiac tissue
Cardiomyocytes and myofibrils had a normal histological appearance in the control, RES I and RES II groups in the myocardium of the heart tissue (Fig. 1A, B (a, e, f)). DOX administration caused histopathological changes such as myocardial interstitial congestion, myocarditis, myocardiomyopathy, hyaline degeneration of myocardium, and myocardial fibrosis in cardiac tissue (Fig. 1A, B (b, c, d)). RES treatment (1 mg/kg/day) (DOX + RES I group) given to DOX-administered rats failed to alleviate histopathological changes (Fig. 1A, B (c)). However, 5 mg/kg/day RES treatment (DOX + RES II group) significantly reduced DOX-induced histopathological changes (Fig. 1A, B (d); Table 1).
Photomicrographs of the histopathological effects of DOX and/or RES administration in cardiac tissue: In the myocardial layer of the control, RES I and RES II groups cardiomyocyte and myofibril structures had normal histological structure (A, B; (a, e, f)). DOX administration caused myocardial interstitial congestion (star), myocardiomyopathy (arrowhead), myocarditis (thick arrow), hyaline degeneration of myocardium (notched arrow), and hemorrhagic areas (thin arrow) in the myocardial layer (A; (b, c, d)). In addition, DOX administration increased myocardial fibrosis (arrow, B; (d)). Especially in the DOX + RES II group, the alleviation of DOX-induced histopathological changes was remarkable (A, B; (d)). A Hematoxylin eosin, B Masson trichrome, scale bar: 100 µm, × 200. DOX doxorubicin; RES resveratrol
Effects of DOX and/or RES administration on BcL2 and Casp3 immunoreactivity in cardiac tissue
BcL2 immunoreactivity in cardiac tissue showed similar in the control, RES I, and RES II groups (Fig. 2a, e, f). Immunoreactivity of BcL2, which has antiapoptotic effect, decreased significantly with DOX administration (p < 0.05) (Fig. 2b, *). The DOX + RES I group showed similar BcL2 immunoreactivity as the DOX group. However, there was a relatively, but not statistically significant, increase in BcL2 immunoreactivity in the DOX + RES II group compared to the DOX group (Fig. 2; Table 2).
Photomicrographs of the effects of DOX and/or RES administration on BcL2 immunoreactivity in cardiac tissue and BcL2 histoscore graph: BcL2 immunoreactivity was similar in the control, RES I, and RES II groups (a, e, f). There was a significant decrease in BcL2 immunoreactivity in the DOX and DOX + RES I groups compared to the control group (b, c, *). There was a relative increase in BcL2 immunoreactivity in the DOX + RES II group compared to the DOX group (p > 0.05) (d). BcL2 positive control (g; testicular tissue), BcL2 histoscore graph (h). BcL2 immunohistochemical images, scale bar: 100 µm, × 200. *Compared to the control group, #compared to the DOX group, DOX doxorubicin, BcL2 B-cell lymphoma 2, RES resveratrol
Casp3 immunoreactivity in cardiac tissue was similar in the control, RES I, and RES II groups. There was a significant increase in Casp3 immunoreactivity in the DOX and DOX + RES I groups (p < 0.05) (Fig. 3b, c, *). The increase in immunoreactivity of the Casp3, which has an DOX-induced proapoptotic effect, was significantly reduced in cardiac tissue with 5 mg/kg/day RES treatment (DOX + RES II group) (p < 0.05) (Fig. 3d, #; Table 2).
Photomicrographs of the effects of DOX and/or RES administration on Casp3 immunoreactivity in cardiac tissue and Casp3 histoscore graph: Casp3 immunoreactivity was similarly low in the control, RES I, and RES II groups (a, e, f). There was a significant increase in Casp3 immunoreactivity in the DOX and DOX + RES I groups compared to the control group (p < 0.05) (b, c, *). There was a significant decrease in Casp3 immunoreactivity in the DOX + RES II group compared to the DOX group (p < 0.05) (d). Casp3-positive control (g; kidney tissue), Casp3 histoscore graph (h). Casp3 immunohistochemical images, scale bar: 100 µm, × 200. *Compared to the control group, #compared to the DOX group, DOX doxorubicin, Casp3 caspase 3, RES resveratrol
Effects of DOX and/or RES administration on TUNEL-positive apoptotic cells and apoptotic index in cardiac tissue
Apoptotic cells detected by TUNEL staining were rare in the control, RES I, and RES II groups (Fig. 4a, e, f). DOX administration increased the number of TUNEL-positive apoptotic cells in cardiac tissue (p < 0.05) (Fig. 4b, *). Similar to the DOX group, a higher rate of apoptotic cells was detected in the DOX + RES I group than in the control group (p < 0.05) (Fig. 4c, *). On the other hand, 5 mg/kg/day RES treatment (DOX + RES II group) reduced the increased DOX-induced apoptotic cells in cardiac tissue (p < 0.05) (Fig. 4d, #; Table 3).
TUNEL test photomicrographs of cardiac tissues of the experimental groups and apoptotic index graph: TUNEL-positive cells and apoptotic index were similar in the control, RES I and RES II groups (a, e, f). There was a significant increase in the number of apoptotic cells in the DOX and DOX + RES I groups compared to the control group (p < 0.05) (b, c, *). There was a significant decrease in the number of apoptotic cells in the DOX + RES II group compared to the DOX group (p < 0.05) (d). TUNEL staining, scale bar: 100 µm, × 200 (a, b, c, d, e, f). *Compared to the control group, #compared to the DOX group, arrows TUNEL-positive apoptotic cells, DOX doxorubicin, RES resveratrol
Effects of DOX and/or RES administration on TRPM2 and MTRNL immunoreactivity in cardiac tissue
TRPM2 immunoreactivity in cardiac tissue was similar in the control, RES I, and RES II groups. TRPM2 immunoreactivity significantly increased in the DOX and DOX + RES I groups (p < 0.05) (Fig. 5b, c, *). The increase in TRPM2 immunoreactivity, which was increased by DOX, was significantly reduced in cardiac tissue with 5 mg/kg/day RES treatment (DOX + RES II group) (p < 0.05) (Fig. 5d, #; Table 2).
Photomicrographs of the effects of DOX and/or RES administration on TRPM2 immunoreactivity in cardiac tissue and TRPM2 histoscore graph: TRPM2 immunoreactivity was similar in the control, RES I, and RES II groups (a, e, f). There was a significant increase in TRPM2 immunoreactivity in the DOX and DOX + RES I groups compared to the control group (p < 0.05) (b, c, *). There was a significant decrease in TRPM2 immunoreactivity in the DOX + RES II group compared to the DOX group (p < 0.05) (d). TRPM2-positive control (g; cerebral cortex), TRPM2 histoscore graph (h). TRPM2 immunohistochemical images, scale bar: 100 µm, × 200. *Compared to the control group, #compared to the DOX group, DOX doxorubicin, TRPM2 transient receptor potential melastatin 2, RES resveratrol
MTRNL immunoreactivity in cardiac tissue was similar in the control, RES I, and RES II groups (Fig. 6a, e, f). MTRNL immunoreactivity significantly increased with DOX administration (p < 0.05) (Fig. 6b, *). There was a gradual but not significant decrease in the DOX + RES I and DOX + RES II groups (Fig. 6; Table 2).
Photomicrographs of the effects of DOX and/or RES administration on MTRNL immunoreactivity in cardiac tissue and MTRNL histoscore graph: MTRNL immunoreactivity was similar in the control, RES I, and RES II groups (a, e, f). There was a significant increase in MTRNL immunoreactivity in the DOX group compared to the control group (p < 0.05) (b, *). There was a relative decrease in MTRNL immunoreactivity in the DOX + RES I and DOX + RES II groups compared to the DOX group (p > 0.05) (c, d). MTRNL-positive control (g; gastrocnemius muscle tissue), MTRNL histoscore graph (h). MTRNL immunohistochemical images, scale bar: 100 µm, × 200. *Compared to the control group, DOX doxorubicin, MTRNL meteorin-like, RES resveratrol
Effects of DOX and/or RES administration on MTRNL levels in cardiac tissue
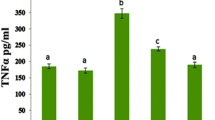
The level of MTRNL measured by the ELISA method in cardiac tissue homogenate was similar in the control, RES I, and RES II groups (Fig. 7; Table 3). There was a significant increase in MTRNL levels in the DOX and DOX + RES I groups (p < 0.05) (Fig. 7; *). The MTRNL level, which increased due to DOX, decreased relatively, but not significantly, in cardiac tissue with 5 mg/kg/day RES treatment (DOX + RES II group) (p < 0.05) (Fig. 7d, #; Table 3).
Effects of DOX and/or RES administration on MTRNL levels in cardiac tissue: Cardiac tissue MTRNL levels were at similar levels in the control, RES I, and RES II groups. MTRNL levels significantly increased in the DOX and DOX + RES I groups compared to the control group (*p < 0.05). In the DOX + RES II group, MTRNL levels significantly decreased compared to the DOX group (#p < 0.05). *Compared to the control group, #compared to the DOX group, DOX doxorubicin, MTRNL meteorin-like, RES resveratrol
Effects of DOX and/or RES administration on oxidative stress parameters in cardiac tissue
Levels of MDA, GSH, and CAT levels measured by the ELISA method in cardiac tissue homogenate were similar in the control, RES I, and RES II groups (Fig. 8; Table 3). While MDA levels increased in the DOX and DOX + RES I groups, GSH and CAT levels decreased significantly (p < 0.05) (Fig. 8 *; Table 3). DOX-induced irregular oxidative stress parameters were regulated in cardiac tissue with 5 mg/kg/day RES treatment (DOX + RES II group) (p < 0.05) (Fig. 8 #; Table 3).
Effects of DOX and/or RES administration on oxidative stress parameters in cardiac tissue: MDA, GSH, and CAT levels were similar in the control, RES I, and RES II groups. MDA increased significantly (*p < 0.05) whereas GSH and CAT levels decreased significantly (*p < 0.05) in the DOX and DOX + RES I groups compared to the control group. There was a significant increase in CAT levels in the DOX + RES II group compared to the DOX group (#p < 0.05). The MDA level relatively decreased, and the GSH level relatively increased in the DOX + RES II group compared to the DOX group. *Compared to the control group, #compared to the DOX group, DOX doxorubicin, MDA malondialdehyde, GSH glutathione, CAT catalase, RES resveratrol
Discussion
DOX is a cytotoxic chemotherapeutic drug widely used in cancer treatment. However, its clinical use is limited due to its oxidative damage–based cardiotoxicity (Adıyaman et al. 2022). Previous studies reported that RES administration alleviates DOX-induced histopathological changes in cardiac tissue (Shoukry et al. 2017; Zhang et al. 2019). Consistent with these data, the current study results showed that DOX administration caused myocardial interstitial congestion, myocarditis, myocardiomyopathy, hyaline degeneration of myocardium, and myocardial fibrosis in cardiac tissue, but RES (5 mg/kg) administration alleviated these histopathological changes.
It was reported that RES administration reduces the level of malondialdehyde (MDA), which increases with DOX administration (Alanazi et al. 2020). Studies conducted on rats reported that DOX-induced decreased GSH, superoxide dismutase (SOD), and CAT activities increased significantly with RES treatment (Ekinci Akdemir et al. 2021; Saleh Ahmed 2022). In this study, similar to these results, it was determined that the MDA level increased with DOX administration and decreased with RES treatment. The decreased GSH and CAT levels caused by DOX administration also increased with RES treatment (Fig. 8). It was reported that RES alleviates DOX-induced cardiotoxicity by regulating signaling pathways related to cell proliferation, oxidative stress, and cell apoptosis (Gu et al. 2018). However, in the current study, it was determined that DOX caused a decrease in the level of BcL2, which has an antiapoptotic effect, and an increase in the level of Casp3, which has a proapoptotic effect. However, RES treatment reduced the rate of apoptotic cells by showing a regulatory effect on antiapoptotic/proapoptotic protein activities. Previous studies reported that BcL2 decreases with DOX administration in cardiac tissue; however, RES treatment increases BcL2 (Gu et al. 2018; Liu et al. 2016). Furthermore, studies demonstrated that DOX increases Casp3 in cardiac tissue and apoptosis in cardiomyocytes; however, Casp3 and apoptosis are suppressed by RES administration (Gu et al. 2018; Tian et al. 2020; Sin et al. 2015).
MTRNL is known to play a role in various physiological and pathophysiological events (Alizadeh 2021). Recently, MTRNL has been shown to promote muscle regeneration in response to local muscle injury (Baht et al. 2020). However, MTRNL is also abundantly expressed in the heart and plays a critical role in the pathogenesis of cardiovascular diseases (Miao et al. 2020). A recent study reported that mice lacking MTRNL are prone to develop cardiac hypertrophy and that MTRNL may directly act to protect against hypertrophic processes in cardiomyocytes (Rupérez et al. 2021). It was reported that MTRNL administration significantly improved DOX-induced apoptosis, oxidative stress, survival, and cardiac dysfunction in heart tissue. In contrast, endogenous MTRNL deficiency was reported to exacerbate DOX-induced cardiotoxicity. Targeting MTRNL was suggested as a new potential therapeutic strategy in the prevention of DOX-induced cardiotoxicity (Hu et al. 2020). It was reported that MTRNL has a reducing effect on DOX-induced apoptosis in cardiomyocytes (Hu et al. 2020; Xu et al. 2020). A recent study showed that plasma MTRNL levels are high in patients with heart failure and correlated with an increased risk of death (Rupérez et al. 2021). In the current study, it was determined that the level of MTRNL in cardiac tissue increased in DOX-induced cardiotoxicity. These results suggest that MTRNL may play an important role in cardiac tissue repair in response to various injuries (Cai et al. 2022).
Disruption of calcium homeostasis is another mechanism involved in DOX-induced cardiotoxicity (Alanazi et al. 2020). The calcium ion is the second main intracellular messenger signaling cation that regulates many physiological functions, including cell migration and apoptosis (Nazıroğlu 2017). TRPM2 is activated by ROS in cancer cells (Öz and Çelik 2016; Uslusoy et al. 2017; Kang et al. 2018). It was reported that mitochondrial ROS activates TRPM2, causing increased Casp3 activation and apoptosis and decreased cell viability. This will contribute to cancer cell death by increasing the calcium flow to the cell through the activation of TRPM2 channels (Öztürk et al. 2019). In this current study, it was determined that DOX cardiotoxicity increased TRPM2 immunoreactivity in cardiac tissue. However, a study reported that RES treatment decreased calcium flux and intracellular calcium overload via TRPM2, leading to increased cell survival (Sun et al. 2018). In the current study, it was observed that RES treatment suppressed TRPM2 immunoreactivity, which increased with DOX administration. RES can improve the cardiomyocyte calcium cycle by upregulating SIRT1-mediated deacetylation. Thus, it can inhibit ROS production to protect the myocardium and improve DOX-induced cardiotoxicity (Gu et al. 2016).
DOX-induced cardiotoxicity is associated with increased oxidative stress in cardiomyocytes. Evidence showed that DOX treatment excessively increases the production of ROS in cardiac tissue, causes mitochondrial dysfunction, affects energy metabolism, and leads to the death of cardiomyocytes (Hu et al. 2019). Therefore, it was reported that antioxidative protection is important in the prevention of DOX-induced cardiotoxicity (Xiao et al. 2022; Gong et al. 2022). The results obtained from the current study showed that DOX administration may cause oxidative stress by increasing the MDA level and decreasing GSH and CAT levels in cardiac tissue. However, cardiac tissue immunoreactivity of TRPM2 ion channels sensitive to oxidative stress was determined to increase in DOX-induced cardiotoxicity. In this process, the level of MTRNL, which plays a role in the regulation of energy metabolism, was found to increase. It was also determined that DOX administration triggered cardiomyocyte apoptosis with the decrease of BcL2, which has an antiapoptotic effect, and the increase in the level of Casp3, which has a proapoptotic effect. It was observed that the rate of apoptotic cardiomyocytes detected by the TUNEL method was significantly higher in the DOX group. It was noticed that the DOX-induced irregularities in the antioxidant/oxidant parameters and apoptotic protein activities were eliminated with the administration of 5 mg/kg RES and as a result, the rate of apoptotic cells significantly decreased. MTRNL levels and TRPM2 immunoreactivity, which increased with DOX administration in cardiac tissue, also decreased with 5 mg/kg RES treatment. However, it was determined that 1 mg/kg RES administration was not as effective as 5 mg/kg RES in improving DOX-induced cardiotoxicity. These results revealed that RES treatment may have a protective effect on cardiac tissue depending on the dose applied. A recent study reported that low-dose (2–2.5 mg/kg) RES is beneficial in cardiac tissue only in chronic treatment conditions and that higher doses of RES are needed for acute treatment (Arinno et al. 2021). The fact that 1 mg/kg RES treatment did not result in as expected in this study may be related to the duration of the study. This is very important for determining the most ideal dose of RES required to benefit from RES’s potential antiapoptotic, antioxidant, anti-cancer, and anti-diabetes effects. In a study conducted on diabetic rats, the antidiabetic effects of 1 mg/kg, 5 mg/kg, and 10 mg/kg RES treatment were compared, and it was reported that the best results were reached with 5 mg/kg RES treatment (Khazaei et al. 2016). Likewise, considering the current study results, we think that 5 mg/kg RES may be beneficial against DOX-induced cardiotoxicity, taking into account the possible side effects of high-dose RES treatment.
Conclusion
The findings obtained in the study showed that RES administration has a curative effect against DOX-induced cardiotoxicity in rats, but this effect may vary depending on the dose. Moreover, the results support the idea that RES may have antiapoptotic and antioxidant effects and may have curative effects against cardiac tissue damage by reducing ROS formation. In addition, the increase in MTRNL levels and the reducing effect of RES in DOX-induced cardiotoxicity suggest that energy metabolism may be affected by the process. It was also determined that RES treatment may regulate cellular ion homeostasis by reducing the increased TRPM2 ion channel immunoreactivity in cardiac tissue caused by DOX administration. Based on these data, we think that RES may exert a cardioprotective effect with MTRNL and TRPM2 channel modulation against DOX-induced cardiotoxicity, and supplementing DOX administration with RES may be beneficial.
Data availability
Data obtained and/or analyzed in the present study are available from the corresponding author upon reasonable request.
References
Adıyaman MS, Adıyaman ÖA, Dağlı AF, Karahan MZ, Dağlı MN (2022) Prevention of doxorubicin-induced experimental cardiotoxicity by Nigella sativa in rats. Rev Port Cardiol 41(2):99–105. https://doi.org/10.1016/j.repc.2020.12.015
Akkoc RF, Ogeturk M, Aydin S, Kuloglu T, Aydin S (2021) Effects of carnosine on apoptosis, transient receptor potential melastatin 2, and betatrophin in rats exposed to formaldehyde. Biotech Histochem 96(3):223–229. https://doi.org/10.1080/10520295.2020
Alanazi AM, Fadda L, Alhusaini A, Ahmad R, Hasan IH, Mahmoud AM (2020) Liposomal resveratrol and/or carvedilol attenuate doxorubicin-induced cardiotoxicity by modulating inflammation, oxidative stress and S100A1 in rats. Antioxidants (basel, Switzerland) 9(2):159. https://doi.org/10.3390/antiox9020159
Alizadeh H (2021) Myokine-mediated exercise effects: the role of myokine meteorin-like hormone (Metrnl). Growth Factors 39(1–6):71–78
Arinno A, Apaijai N, Chattipakorn SC, Chattipakorn N (2021) The roles of resveratrol on cardiac mitochondrial function in cardiac diseases. Eur J Nutr 60(1):29–44
Atilgan FA, Atescelik M, Yilmaz M, Turk A, Gurger M, Goktekin MC, Kuloglu T (2022) Effects of N-acetyl cysteine on TRPM2 expression in kidney and liver tissues following malathion intoxication. Biotech Histochem 97(5):340–346. https://doi.org/10.1080/10520295.2021
Baht GS, Bareja A, Lee DE, Rao RR, Huang R, Huebner JL, Bartlett DB, Hart CR, Gibson JR, Lanza IR, Kraus VB, Gregory SG, Spiegelman BM, White JP (2020) Meteorin-like facilitates skeletal muscle repair through a Stat3/IGF-1 mechanism. Nat Metab 2(3):278–289. https://doi.org/10.1038/s42255-020-0184-y
Cai J, Wang QM, Li JW, Xu F, Bu YL, Wang M, Lu X, Gao W (2022) Serum meteorin-like is associated with weight loss in the elderly patients with chronic heart failure. J Cachexia Sarcopenia Muscle 13(1):409–417. https://doi.org/10.1002/jcsm.12865
Ekinci Akdemir FN, Yildirim S, Kandemir FM, Tanyeli A, Küçükler S, Bahaeddin Dortbudak M (2021) Protective effects of gallic acid on doxorubicin-induced cardiotoxicity; an experimantal study. Arch Physiol Biochem 127(3):258–265. https://doi.org/10.1080/13813455.2019
Faul F, Erdfelder E, Buchner A, Lang AG (2009) Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 41(4):1149–60. https://doi.org/10.3758/BRM.41.4.1149
Feijóo-Bandín S, Aragón-Herrera A, Moraña-Fernández S, Anido-Varela L, Tarazón E, Roselló-Lletí E, Portolés M, Moscoso I, Gualillo O, González-Juanatey JR, Lago F (2020) Adipokines and inflammation: focus on cardiovascular diseases. Int J Mol Sci 21(20):7711. https://doi.org/10.3390/ijms21207711
Gong F, Jin J, Li H, Mao H (2022) Alpha-lipoic acid protects against doxorubicin-induced cardiotoxicity by regulating pyruvate dehydrogenase kinase 4. Cardiovasc Toxicol 1–13. https://doi.org/10.1007/s12012-022-09766-2
Gu J, Fan YQ, Zhang HL, Pan JA, Yu JY, Zhang JF, Wang CQ (2018) Resveratrol suppresses doxorubicin-induced cardiotoxicity by disrupting E2F1 mediated autophagy inhibition and apoptosis promotion. Biochem Pharmacol 150:202–213. https://doi.org/10.1016/j.bcp.2018.02.025
Gu J, Hu W, Song ZP, Chen YG, Zhang DD, Wang CQ (2016) Resveratrol-induced autophagy promotes survival and attenuates doxorubicin-induced cardiotoxicity. Int Immunopharmacol 32:1–7. https://doi.org/10.1016/j.intimp.2016.01.002
Gul HF, Ilhan N, Ilhan N, Ozercan IH, Kuloglu T (2021) The combined effect of pomegranate extract and tangeretin on the DMBA-induced breast cancer model. J Nutr Biochem 89:108566. https://doi.org/10.1016/j.jnutbio.2020
Hu C, Zhang X, Song P, Yuan YP, Kong CY, Wu HM, Xu SC, Ma ZG, Tang QZ (2020) Meteorin-like protein attenuates doxorubicin-induced cardiotoxicity via activating cAMP/PKA/SIRT1 pathway. Redox Biol 37:101747. https://doi.org/10.1016/j.redox.2020.101747
Hu X, Liu H, Wang Z, Hu Z, Li L (2019) miR-200a attenuated doxorubicin-induced cardiotoxicity through upregulation of Nrf2 in mice. Oxid Med Cell Longev 2019. https://doi.org/10.1155/2019/1512326
Kang P, Zhang W, Chen X, Yi X, Song P, Chang Y, Zhang S, Gao T, Li C, Li S (2018) TRPM2 mediates mitochondria-dependent apoptosis of melanocytes under oxidative stress. Free Radical Biol Med 126:259–268. https://doi.org/10.1016/j.freeradbiomed.2018.08.022
Kaya S, Dönmez HH (2020) Effects of paclitaxel and resveratrol on blood characteristics in rabbits. Biotech Histochem 95(3):198–202. https://doi.org/10.1080/10520295.2019
Kaya S, Yalçın T, Boydak M, Dönmez HH (2022) Protective effect of N-acetylcysteine against aluminum-induced kidney tissue damage in rats. Biol Trace Elem Res 1–10. https://doi.org/10.1007/s12011-022-03276-6
Khazaei M, Karimi J, Sheikh N, Goodarzi MT, Saidijam M, Khodadadi I, Moridi H (2016) Effects of resveratrol on receptor for advanced glycation end products (RAGE) expression and oxidative stress in the liver of rats with type 2 diabetes. Phytother Res 30(1):66–71. https://doi.org/10.1002/ptr.5501
Kocaman N, Yuksel EI, Demir B, Calik I, Cicek D (2022) Two novel biomarker candidates for differentiating basal cell carcinoma from trichoblastoma; asprosin and meteorine like peptide. Tissue and Cell 76:101752. https://doi.org/10.1016/j.tice.2022.101752
Liu MH, Lin XL, Guo DM, Zhang Y, Yuan C, Tan TP, Chen YD, Wu SJ, Ye ZF, He J (2016) Resveratrol protects cardiomyocytes from doxorubicin-induced apoptosis through the AMPK/P53 pathway. Mol Med Rep 13(2):1281–1286. https://doi.org/10.3892/mmr.2015.4665
Miao ZW, Hu WJ, Li ZY, Miao CY (2020) Involvement of the secreted protein Metrnl in human diseases. Acta Pharmacol Sin 41(12):1525–1530
Nazıroğlu M (2017) Activation of TRPM2 and TRPV1 channels in dorsal root ganglion by NADPH oxidase and protein kinase C molecular pathways: a patch clamp study. J Mol Neurosci 61(3):425–435. https://doi.org/10.1007/s12031-017-0882-4
Öz A, Çelik Ö (2016) Curcumin inhibits oxidative stress-induced TRPM2 channel activation, calcium ion entry and apoptosis values in SH-SY5Y neuroblastoma cells: involvement of transfection procedure. Mol Membr Biol 33(3–5):76–88. https://doi.org/10.1080/09687688.2017
Öztürk Y, Günaydın C, Yalçın F, Nazıroğlu M, Braidy N (2019) Resveratrol enhances apoptotic and oxidant effects of paclitaxel through TRPM2 channel activation in DBTRG glioblastoma cells. Oxid Med Cell Longev 7(2019):4619865. https://doi.org/10.1155/2019/4619865
Refaie MMM, El-Hussieny M, Abdel-Hakeem EA, Fawzy MA, Mahmoud Abd El Rahman ES, Shehata S (2022) Phosphodiesterase inhibitor, vinpocetine, guards against doxorubicin induced cardiotoxicity via modulation of HIF/VEGF and cGMP/cAMP/SIRT signaling pathways. Hum Exp Toxicol 41. https://doi.org/10.1177/09603271221136209
Rupérez C, Ferrer-Curriu G, Cervera-Barea A, Florit L, Guitart-Mampel M, Garrabou G, Zamora M, Crispi F, Fernandez-Solà J, Lupón J, Bayes-Genis A, Villarroya F, Planavila A (2021) Meteorin-like/meteorin-β protects heart against cardiac dysfunction. J Expl Med 218(5). https://doi.org/10.1084/jem.20201206
Saleh Ahmed AS (2022) Potential protective effect of catechin on doxorubicin-induced cardiotoxicity in adult male albino rats. Toxicol Mech Methods 32(2):97–105. https://doi.org/10.1080/15376516.2021
Sin TK, Tam BT, Yung BY, Yip SP, Chan LW, Wong CS, Ying M, Rudd JA, Siu PM (2015) Resveratrol protects against doxorubicin-induced cardiotoxicity in aged hearts through the SIRT1-USP7 axis. J Physiol 593(8):1887–1899. https://doi.org/10.1113/jphysiol.2014.270101
Shoukry HS, Ammar HI, Rashed LA, Zikri MB, Shamaa AA, Abou Elfadl SG, Rub EA, Saravanan S, Dhingra S (2017) Prophylactic supplementation of resveratrol is more effective than its therapeutic use against doxorubicin induced cardiotoxicity. PLoS One 20;12(7):e0181535. https://doi.org/10.1371/journal.pone.0181535
Sun Y, Sukumaran P, Selvaraj S et al (2018) TRPM2 promotes neurotoxin MPP+/MPTP-induced cell death. Mol Neurobiol 55(1):409–420. https://doi.org/10.1007/s12035-016-0338-9
Tian W, Yang L, Liu Y, He J, Yang L, Zhang Q et al (2020) Resveratrol attenuates doxorubicin-induced cardiotoxicity in rats by up-regulation of vascular endothelial growth factor B. J Nutr Biochem 79:108132. https://doi.org/10.1016/j.jnutbio.2019.01.018
Uslusoy F, Nazıroğlu M, Çiğ B (2017) Inhibition of the TRPM2 and TRPV1 channels through Hypericum perforatum in sciatic nerve injury-induced rats demonstrates their key role in apoptosis and mitochondrial oxidative stress of sciatic nerve and dorsal root ganglion. Front Physiol 8:335. https://doi.org/10.3389/fphys.2017.00335
Xiao M, Tang Y, Wang J (a), Lu G, Niu J, Wang J (b), Li J, Liu Q, Wang Z, Huang Z, Guo Y, Gao T, Zhang X, Yue S, Gu J (2022) A new FGF1 variant protects against adriamycin-induced cardiotoxicity via modulating p53 activity. Redox Biol 49:102219. https://doi.org/10.1016/j.redox.2021
Xiong C, Wu YZ, Zhang Y, Wu ZX, Chen XY, Jiang P, Su SW (2018) Protective effect of berberine on acute cardiomyopathy associated with doxorubicin treatment. Oncol Lett 15(4):5721–5729. https://doi.org/10.3892/ol.2018.8020
Xu L, Cai Y, Wang Y, Xu C (2020) Meteorin-like (METRNL) attenuates myocardial ischemia/reperfusion injury-induced cardiomyocytes apoptosis by alleviating endoplasmic reticulum stress via activation of AMPK-PAK2 signaling in H9C2 cells. Med Sci Monit Int Med J Exp Clin Res 26:e924564–e924571. https://doi.org/10.12659/MSM.924564
Zhang L, Zhu K, Zeng H, Zhang J, Pu Y, Wang Z, Zhang T, Wang B (2019) Resveratrol solid lipid nanoparticles to trigger credible inhibition of doxorubicin cardiotoxicity. Int J Nanomedicine 31(14):6061–6071. https://doi.org/10.2147/IJN.S211130
Zordoky BN, Robertson IM, Dyck JR (2015) Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim Biophys Acta Mol Basis Dis 1852(6):1155–1177. https://doi.org/10.1016/j.bbadis.2014.10.016
Author information
Authors and Affiliations
Contributions
SK and TY took part in the study plan, animal experiment design, data analysis, laboratory studies, and manuscript writing. TK was involved in interpreting the data and editing the manuscript. All researchers have read and approved the final article.
Corresponding author
Ethics declarations
Funding
This study was not supported by any funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was carried out with the approval of Fırat University Animal Experiments Ethics Committee dated 05.07.2021 and numbered 2830. All procedures performed in studies involving animals comply with the ethical standards of the institution or practice in which the studies are conducted.
Informed consent
For this type of study, informed consent is not required.
Consent for publication
For this type of study, consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaya, S., Yalçın, T. & Kuloğlu, T. Resveratrol may reduce oxidative stress and apoptosis in doxorubicin-induced cardiotoxicity by regulating meteorin-like and TRPM2 levels. Comp Clin Pathol 32, 393–404 (2023). https://doi.org/10.1007/s00580-023-03449-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-023-03449-2