Abstract
This study investigated the ameliorative potential of micronized purified flavonoid fraction Daflon on lead-induced alterations in heme-biosynthetic enzyme delta-aminolevulinic acid dehydratase (δ-ALAD) activity and erythrocytic parameters. Thirty-two male adult Wistar rats were divided by simple randomization into four groups of eight rats each. Lead acetate was administered orally at a dose of 190 mg/kg (1/20th of the LD50) body weight for 42 days, either alone or with Daflon at a dose of 100 mg/kg body weight. Blood lead level (BLL) was analyzed using the microwave plasma atomic emission spectrometer (MP-AES), while δ-ALAD activity was quantified using assay kit. Erythrocyte parameters (RBC, packed cell volume, Hb, MCV, and MCHC) were assayed using an automated hematologic analyzer. Erythrocyte osmotic fragility (EOF) was also analyzed using standard procedure. Sub-chronic exposure to lead acetate caused impairment in δ-ALAD activity, erythrocytic parameters, and increased EOF. Conversely, Daflon improved the activity of δ-ALAD and erythrocytic parameters by protecting the integrity of the erythrocyte membrane. The results of our study revealed that sub-chronic exposure of rats to lead acetate induced impairment in δ-ALAD, erythrocytic parameters, and EOF, while pretreatment with Daflon alleviated the ensued changes, and therefore suggested that dietary supplementation with Daflon-enriched foods may be a promising prophylactic agent for individuals living in lead toxicity prone environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An increasing body of knowledge suggests that environmental pollutants contribute to hematopoietic disorders, especially in Africa, which has a substantial share of the world’s environmental pollution (Anakwue and Anakwue 2014; Kasemy et al. 2019).
Globally, lead (Pb) ranks among the most perilous environmental toxicants, implicated in disease burden of animals and humans (Mitra and Sharma 2019). Lead toxicity occurs naturally in situations of environmental contamination and occupational exposures (Sancar 2019). A pathology hazard risk ranking of contaminants identified lead among the five most toxic pollutants implicated in mining-engendered morbidity and mortality in human (Bartrem et al. 2014) and animal (Orisakwe et al. 2017) pollution. In 2010, the World Health Organization (WHO) published a list of 10 chemicals of human health concern, of which lead was chief (WHO 2010). The hematopoietic system is one of the most sensitive systems, functioning not only as a medium of transportation but also as the critical toxicity target for lead (Abadin et al. 2007), and predisposes to anemia by several mechanisms (Flora et al. 2012). The anemic response induced by lead is microcytic hypochromic anemia and it is characterized by a fragility of mature erythrocytes and inhibition of hemoglobin (Hb) synthesis (Mager 2012).
The delta-aminolevulinic acid dehydratase (δ-ALAD) is the second enzyme in the pathway for heme biosynthesis and catalyzes the condensation of two molecules of delta-aminolevulinic acid (δ-ALA) to porphobilinogen (PBG), with the thiol (SH) group essential for its activity. Owing to its affinity for the SH group, lead is known to inhibit δ-ALAD activity (Shraideh et al. 2019), eliciting the accumulation of δ-ALA which has been shown to undergo metal catalyzed auto-oxidation and subsequently, increased generation of reactive oxygen species (ROS) such as superoxide ion (O2+), hydroxyl radical (OH+), and hydrogen peroxide (H2O2). The resultant oxidative stress and tissue damage are the most widely reported pathophysiological mechanisms of lead-related toxicities (Alya et al. 2015).
Daflon® 500 mg, whose active principle is a micronized purified flavonoid, is obtained from plants such as Rutaceae aurantiae (Pitsch 2011), and consists of 90% diosmin and 10% hesperidin. This flavonoid-containing compound belongs to the gamma-benzopyrone class of venoactive drugs, and possesses vasoprotective and venotonic properties (Ramlet 2001).
In the present study, we hypothesized that Daflon may be capable of countering hemotoxicity in male Wistar rats exposed to lead acetate because of its bioprotective effects in the body. Therefore, the current study was undertaken in order to investigate the protective role of Daflon on δ-ALAD and erythrocytic parameters in male Wistar rats exposed to lead acetate.
Experimental animals
A total of thirty-two (32) male Wistar strain albino rats weighing between 200 and 250 g served as subjects. They were procured from the breeding stock of the National Institute of Trypanosomiasis Research (NITR), Kaduna, Nigeria, and maintained in plastic cages under standard laboratory conditions (ambient temperature 24–26 °C, relative humidity 20–80%, 12 h/day light period) in the animal-holding facility of the Department of Veterinary Pharmacology and Toxicology, Ahmadu Bello University, Zaria. Minimal handling was ensured to minimize unwanted stress or discomfort to the animals during experimental procedures. The rats were given access to standard commercially prepared rat chow (TOPFEEDS®, Lagos, Nigeria) and tap water ad libitum. They were acclimatized to their housing environment for 2 weeks prior commencement of the experiment. The study was approved by the Ahmadu Bello University Research Ethics Committee and it was conducted in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals (Garber et al. 2011).
Chemical acquisition and preparation
Analytical grade of lead acetate (Cat No. 10142) was procured from Sigma-Aldrich (St. Louis, MO, USA). It was reconstituted in deionized water to form a stock solution. Daflon (Daflon, 500® Servier, Neuilly-Surseine, France) (Batch No. MR 110528) was purchased from Liberty pharmaceutical store, Kaduna, Nigeria. Each tablet (500 mg) was reconstituted in 5 mL of deionized water to make 100 mg/mL suspension.
Animal grouping and sample collection
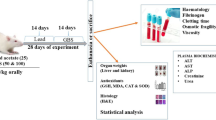
Thirty-two male adult Wistar rats were divided by simple randomization into four groups of eight rats each. Group I was administered with 2 mL/kg body weight of deionized water, group II was administered with lead acetate 190 mg/kg body weight (1/20th of the LD50), group III was administered with Daflon (100 mg/kg body weight) (Kobo et al. 2014), while group IV was given Daflon (100 mg/kg body weight) 30 min before the administration of lead acetate. The regimens were administered by oral gavage, once daily, for a period of 6 weeks (42 days). During this period, the rats were observed for signs of toxicity. At the end of the treatment period, the animals that survived were anesthetized using pentobarbitone and sacrificed by jugular venesection. Blood sample was collected for analyses.
Blood lead level detection
The blood lead level (BLL) was analyzed using a microwave plasma atomic emission spectrometer (MP-AES) after microwave-assisted acid digestion as described by Erick et al. (2016). Briefly, all glassware were cleaned with 10% (v/v) HNO3 solution and rinsed with ultrapure water. A total of 10 μL of each blood sample was digested with 4.0 mL of 65% (v/v) HNO3 and 0.5 mL of 35% (v/v) H2O2 in polytetrafluoroethylene (PTFE) vessels. The vessels were placed into microwave system (MARS 5, CEM). Digestion conditions for the microwave system applied were as follows: up to 120 °C for 15 min and then constant for 10 min; up to 160 °C in 20 min and constant for 15 min; finally, a cooling stage (30 min) was carried out to 22 °C and diluted to 50 mL with deionized ultrapure water. This solution was finally used for elemental analysis using MP-AES (MY15150001) equipped with a concentric nebulizer, a quartz torch with quartz injector tube, and a cyclonic spray chamber. The concentration of lead was determined in blood samples and isotope 208Pb+ was detected. All samples were analyzed in duplicate and each sample was measured in triplicate to increase precision.
Determination of serum δ-aminolevulinic acid dehydratase
Enzyme activity was assayed spectrophotometrically using ZellBio GmbH assay kit (Germany). The enzyme was incubated with excess δ-aminolevulinic acid at 37 °C for 1 h to form porphobilinogen. The porphobilinogen formed was mixed with modified Ehrlich reagent. The color developed was measured spectrophotometrically at 555 nm against a blank. Results were expressed as δ-aminolevulinic acid, μmol/min per milliliter erythrocytes (U/mL).
Determination of hematological parameters
Erythrocytic parameters of red blood cell (RBC) counts, packed cell volume (PCV), hemoglobin concentration (Hb), mean corpuscular volume (MCV), and mean corpuscular hemoglobin concentration (MCHC) were determined using the automated hematologic analyzer (Sysmex, KX-21, Japan).
Hematological counts of monocytes, platelets, absolute leucocytes, and lymphocytes were determined using the automated hematologic analyzer (Sysmex, KX-21, Japan). Blood smears were also prepared, stained with Giemsa and used for determination of differential leucocyte count using the method described by Dacie and Lewis (1991). Briefly, a drop of blood was placed on one end of a glass slide (UNISCOPE, England) and, using a spreader, a blood film was made and allowed to air dry. Giemsa stain was then poured to cover the film, and allowed to stay for 2 min. The film was then rinsed in buffered distilled water and allowed to stand for 10 min. The film was later viewed under a microscope in an oil immersion at × 1000 magnification.
Determination of erythrocyte osmotic fragility
Erythrocyte osmotic fragility was determined as described by Faulkner and King (1970) and as modified by Oyewale (1991). Briefly, sodium chloride solution (pH 7.4) was prepared at varying concentrations, 0.0%, 0.1%, 0.3%, 0.5%, 0.7%, and 0.9%. Five milliliters (5 mL) of each sodium chloride (NaCl) concentration was placed in labeled test tubes serially in a rack. Twenty microliters of blood sample from each rat was pipetted into each of the tubes. The contents of the tube were mixed gently by inverting the tubes and allowed to stand at room temperature (24–26 °C) for 30 min. Thereafter, the tubes were centrifuged at 1580×g for 5 min using a centrifuge (IEC HN-SII-Damon/IEC, UK). The supernatant obtained from each tube was transferred to a clean glass cuvette and the absorbance of the supernatant measured using a UV spectrophotometer (Bausch and Lomb, USA) at 540 nm. The percentage hemolysis for each sample was calculated using the following formula:
Data analysis
Values obtained were expressed as mean ± SEM and subjected to statistical analysis using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc multiple comparison test. GraphPad prism version 6.0, San Diego, CA, USA (www.graphpad.com) was used for the analyses. Values of P ≤ 0.05 were considered significant.
Results
Effect of Daflon and lead acetate on blood lead level
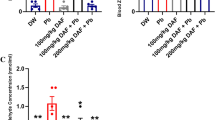
The effect of sub-chronic administration of Daflon and lead acetate on blood lead level (BLL) is shown on Fig. 1. There was a significant increase in BLL in the group treated with lead acetate (10.03 ± 0.43 μg/dL) when compared with the control (DW), Daflon-treated, and Daflon + lead acetate-treated groups (P ≤ 0.01; 0.3 ± 0.05 μg/dL, P ≤ 0.01; 0.32 ± 0.06 μg/dL, and P ≤ 0.05; 2.89 ± 0.10 μg/dL) respectively.
Effect of sub-chronic administration of Daflon and lead acetate on blood lead level of male Wistar rats (means ± SEM, n = 8). Values with different superscript letters (a, b, c) are statistically significant. Keys: DW (deionized water); Pb (lead acetate 190 mg/kg); DAF (Daflon 100 mg/kg); DAF + Pb (Daflon 100 mg/kg + lead acetate 190 mg/kg)
Effect of Daflon and lead acetate on serum delta-aminolevulinic acid dehydratase activity
A significant decrease in the activity of serum δ-ALAD was recorded in the lead acetate-treated group (6.25 ± 0.54 μmol/mL) when compared with the control (P ≤ 0.01; 18.59 ± 0.88 μmol/mL), Daflon (P ≤ 0.01; 20.39 ± 0.88 μmol/mL), and Daflon + lead acetate (P ≤ 0.05; 11.48 ± 0.70 μmol/mL) groups (Fig. 2).
Effect of sub-chronic administration of Daflon and lead acetate on serum delta-aminolevulinic acid dehydratase activity of male Wistar rats (means ± SEM, n = 8). Values with different superscript letters (a, b, c) are statistically significant (P ≤ 0.05). Keys: DW (deionized water); Pb (lead acetate 190 mg/kg); DAF (Daflon 100 mg/kg); DAF + Pb (Daflon 100 mg/kg + lead acetate 190 mg/kg)
Effect of Daflon and lead acetate on packed cell volume
There was a significant decrease (P ≤ 0.05) in PCV of rats in the Pb-treated group (26.58 ± 0.79%), when compared with the groups treated with DW (44.32 ± 1.47%), DAF (46.18 ± 1.70%), and DAF + Pb (38.55 ± 0.99%) Table 1.
Effect of Daflon and lead acetate on hemoglobin concentration
There was a significant decrease (P ≤ 0.05) in hemoglobin concentration of rats in the Pb-treated group (6.05 ± 0.55 g/dL), when compared with the groups treated with DW (14.78 ± 0.71 g/dL), DAF (16.01 ± 0.87 g/dL), and DAF + Pb (12.36 ± 0.63 g/dL) Table 1.
Effect of Daflon and lead acetate on total red blood cells
There was a significant decrease (P ≤ 0.05) in total RBC counts of rats in the Pb-treated group (2.44 ± 0.24 × 1012/L), when compared with the groups treated with DW (8.14 ± 0.48 × 1012/L), DAF (9.91 ± 0.38 × 1012/L), and DAF + Pb (7.99 ± 0.50 × 1012/L) Table 1.
Effect of Daflon and lead acetate on mean corpuscular volume
There was a significant decrease (P ≤ 0.05) in MCV of rats in the Pb-treated group (47.79 ± 3.13 fL), when compared with the groups treated with DW (93.44 ± 3.11 fL), DAF (95.01 ± 3.62 fL), and DAF + Pb (80.19 ± 2.42 fL; Table 1).
Effect of Daflon and lead acetate on mean corpuscular hemoglobin concentration
There was a significant decrease (P ≤ 0.05) in MCHC of rats in the Pb-treated group (18.22 ± 0.80 g/dL), when compared with the groups treated with DW (35.47 ± 1.09 g/dL), DAF (35.22 ± 1.20 g/dL), and DAF + Pb (29.06 ± 1.23 g/dL; Table 1).
Effect of Daflon and or lead acetate on absolute and differential leucocyte counts
The total leucocyte count obtained in the Pb-treated group (4.90 ± 0.06 × 109/L) was significantly lower (P ˂ 0.05) when compared with the counts recorded in the groups treated with DW (6.29 ± 0.04 × 109/L), DAF (7.89 ± 0.02 × 109/L), and DAF + Pb (5.81 ± 0.09 × 109/L) (Table 2).
The number of neutrophil count obtained in the Pb-treated group (1.88 ± 0.02 × 109/L) was significantly higher (P ˂ 0.05) when compared with the counts recorded in the groups treated with DW (1.11 ± 0.02 × 109/L), DAF (1.41 ± 0.05 × 109/L), and DAF + Pb (0.95 ± 0.08 × 109/L) (Table 2).
The number of lymphocyte count obtained in the Pb-treated group (2.50 ± 0.01 × 109/L) was significantly lower (P ˂ 0.05) when compared with the counts recorded in the groups treated with DW (4.95 ± 0.01 × 109/L), DAF (5.66 ± 0.01 × 109/L), and DAF + Pb (4.44 ± 0.02 × 109/L) (Table 2).
The number of monocyte count obtained in the Pb-treated group (0.45 ± 0.02 × 109/L) was significantly lower (P ˂ 0.05) when compared with the counts recorded in the groups treated with DW (0.92 ± 0.01 × 109/L), DAF (0.81 ± 0.03 × 109/L), and DAF + Pb (0.68 ± 0.04 × 109/L) (Table 2).
The neutrophil/lymphocyte ratio obtained in the Pb-treated group (0.75 ± 0.03 × 109/L) was significantly higher (P ˂ 0.05) when compared with groups treated with DW (0.23 ± 0.01 × 109/L), DAF (0.39 ± 0.05 × 109/L), and DAF + Pb (0.25 ± 0.01 × 109/L) (Table 2).
Effect of Daflon and lead acetate on platelet counts
The number of platelet count obtained in the Pb-treated group (439.80 ± 7.91 × 109/L) was significantly lower (P ˂ 0.05) when compared with the counts recorded in the groups treated with DW (637.30 ± 9.26 × 109/L), DAF (532.70 ± 9.12 × 109/L), and DAF + Pb (609.20 ± 6.90 × 109/L) (Table 2).
Effect of Daflon and lead acetate on erythrocyte osmotic fragility
The effect of sub-chronic administration of lead acetate and Daflon on erythrocyte osmotic fragility (EOF) is presented in Fig. 3. The percentage erythrocyte osmotic fragility decreased with increasing sodium chloride (NaCl) concentration (0–0.9%) in all the treatment groups, with complete hemolysis (100%) in the control solvent (deionized water). There was no significant (P ≥ 0.05) difference in the group treated with Daflon and lead acetate (96.25 ± 1.49%, 92.13 ± 1.80%) when compared with the control (92.25 ± 2.54%, 92.25 ± 2.31%), lead acetate (95.25 ± 1.40%, 91.63 ± 2.53%), and Daflon groups (91.38 ± 2.67%, 86.88 ± 2.06%) at 0.1% and 0.3% NaCl concentration respectively. However, there was a consistent significant decrease (P ≤ 0.05) in percentage hemolysis of rats in the group treated with Daflon + lead acetate (19.63 ± 1.27%, 17.88 ± 1.20%, 16.75 ± 1.32%) when compared with the lead acetate-treated group (66.63 ± 2.19%, 45.50 ± 1.68%, 38.69 ± 2.19%) at 0.5%, 0.7%, and 0.9% NaCl concentration respectively.
Effect of sub-chronic administration of Daflon and lead acetate on erythrocyte osmotic fragility (EOF) of male Wistar rats (means ± SEM, n = 8). Values with different superscript letters (a, b) are statistically significant (P ≤ 0.05). Keys: DW (deionized water); Pb (lead acetate 190 mg/kg); DAF (Daflon 100 mg/kg); DAF + Pb (Daflon 100 mg/kg + lead acetate 190 mg/kg)
Discussion
Blood and/or its constituents are reliable indicators of systemic exposure to lead. After oral ingestion, lead undergoes intestinal absorption and is transported via blood. In the blood, it may be distributed via red blood cells or plasma proteins, mainly albumin (Timchalk et al. 2006). As a whole, the uptake, distribution, and accumulation of heavy metals in tissues and organs depend on several factors including the characteristics and forms of the metal, its route, dose, exposure duration, ability to bind cellular ligands, and species sensitivity (Andjelkovic et al. 2019). The result of this study demonstrates a rise in blood lead concentrations, and agrees with the findings of Okediran et al. (2017) and Shraideh et al. (2019) which revealed that absorbed lead following oral ingestion is circulated via blood.
The aminolevulinic acid dehydratase (ALAD) enzyme is sensitive to lead, with inhibition prominent from concentrations of 5 μg/dL blood lead level (BLL) (Sakai and Morita 1996). Lead has been proven to bind to the –SH group of ALAD enzyme in erythrocytes, thereby preventing conversion of aminolevulinic acid (ALA) to porphobilinogen and consequently blocking the synthesis of heme group (Onalaja and Claudio 2000; Supabhorn et al. 2019). It is expected, therefore, that the higher the concentration of lead in blood, the greater will be the percentage inhibition of ALAD and therefore the lower will be the activity of this enzyme, which was likewise observed in our study.
It is worthy to note that Daflon attenuated lead-induced alteration in serum ALAD activity in the Daflon pretreated group, and this may be attributed to the ability of Daflon to form a complex with lead. The result of the present study is in line with that of Martins et al. (2015) and Shraideh et al. (2019) who reported decreased activity of blood ALAD following lead exposure.
A significantly lower packed cell volume, hemoglobin concentration, and red blood cell count alongside erythrocytic indices of mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC) were observed in the lead acetate-exposed group, substantiating the presence of anemia. This finding corroborates the report of Akande et al. (2014) and Warang et al. (2017).
We believe that the lead-induced anemia may have been prompted by the interference of lead with heme biosynthesis through inhibition of δ-aminolevulinic acid dehydratase activity, a key enzyme of heme synthesis. Moreover, lead may inhibit the conversion of coproporphyrinogen III to protoporphyrin IX leading to reduction in hemoglobin production and shortened life span of erythrocytes (Suradkar et al. 2009). Similarly, the progressive destruction of red blood cells due to binding of lead with red blood cells leading to increased fragility and destruction could be another reason for the derangement seen in erythrocytes and erythrocytic indices (Rous 2000).
It has been documented that lead damages the erythrocyte membrane leading to hemolysis or reduced blood iron level and subsequent decrease in hemoglobin concentration and packed cell volume (Ibrahim et al. 2012).
Analysis of total leucocyte counts revealed leucopenia in the lead acetate-exposed rats, and this might be due to direct toxic action of lead on leucopoiesis in lymphoid organs or oxidative damage to the leukocytes. Decrease in total leucocyte count is directly related with either decreased production from germinal center of lymphoid organs or increased lysis due to presence of lead in the body (Suradkar et al. 2009). Repeated exposure to lead has been demonstrated to induce lymphopenic leucopenia (Hashem and El-Sharkawy 2009).
Additionally, neutrophilia has been reported following repeated exposure to lead (Khan et al. 2016) and this report corroborated the neutrophilia observed in the lead acetate-exposed group in the present study.
The apparent increase in neutrophil/lymphocyte ratio in the lead acetate-exposed group, which also indicates neutrophilia, shows the level of stress experienced by rats in this group and this is in agreement with the findings of Minka and Ayo (2007) that the ratio increases in stress situations, especially in those induced via free-radical mechanism. Furthermore, the increased neutrophil/lymphocyte ratio (NLR) in the lead acetate-treated group is a strong indication that the leukopenia recorded in this group was due to lymphopenia. It is known that NLR provides an indication of the inflammatory status and it can be used for the prediction of the outcome of diseases (Halazun et al. 2008). It is probable that Daflon protected the leukocytes from destruction by inhibiting lipid peroxidation and neutrophil activation (Andrew 2003; John 2005), and by modulating immune function through the inhibition of the respiratory burst enzyme, myeloperoxidase (Unlu et al. 2003).
The thrombocytopenia recorded in the lead acetate-exposed group could be due to oxidative damage to the platelet membranes, which apparently results in the formation of lipid peroxides within the platelet membranes thereby provoking cellular lysis (Ohyashiki et al. 1991). The improvement in platelet count in the group pretreated with Daflon shows the ability of the antioxidant to protect the platelet from oxidative damage by reducing the formation of lipid peroxides within the platelet membranes, and hence, improvement of cellular integrity and reduction of cellular destruction.
The hematotoxic effects of lead are largely ascribed to inhibition of the enzyme, ALAD, which catalyzes a key step in the heme synthesis pathway, by condensing two ALA molecules to form a single porphobilinogen molecule (Magyar et al. 2005). Consequently, reduction in heme synthesis, accumulation of aminolevulinic acid (ALA), anemia, modification of erythrocyte indices, release of free radicals, and oxidative stress ensue (Rendon-Ramirez et al. 2007; Keleiny et al. 2019).
In contrast, Daflon attenuated the anemic response instigated by the sub-chronic administration of lead acetate in the Daflon pretreated rats in the present study. This is partly due to the ability of Daflon to improve the integrity of erythrocyte membrane by mitigating oxidative damage.
EOF measures the extent of resistance of erythrocytes to intracellular pressure and is known to be a biomarker of lipid peroxidation (Asala et al. 2011). Exposure of rats to lead acetate induced a significant increase in EOF in the present study. This increase may be ascribed to the increase in the generation of reactive oxygen species (ROS) following oxidative damage, as triggered by the heavy metal.
Flora (2002) reported that exposure to heavy metals, such as lead, may undermine the antioxidant defense system against free radicals culminating in the over production of ROS or depletion of endogenous antioxidants. Furthermore, lead triggers oxidation of hemoglobin, and induces erythrocyte hemolysis following inhibition of aminolevulinic acid dehydratase (ALAD) and subsequently, increases concentration of the substrate aminolevulinic acid (ALA) in both blood and urine. These elevated ALA levels engender the formation of hydrogen peroxide and superoxide radicals and also interact with oxyhemoglobin, resulting in the generation of hydroxyl radicals (Patrick 2006).
Sequel to the aforementioned mechanisms, the erythrocytes become particularly vulnerable to oxidative stress, resulting in loss of membrane integrity and subsequent lysis. Our study shows that erythrocytes of the lead acetate-exposed group were more susceptible to hemolysis, suggesting that the erythrocytes have been subjected to some deleterious changes. The results of this study is consistent with the findings of Debosree et al. (2016), Jalali et al. (2017), and Yusuf et al. (2018) who observed similar changes in lead exposed subjects.
Loss of membrane symmetry and an increase in membrane permeability, inducing morphological changes in the surface of erythrocytes, have been demonstrated to increase EOF (Ambali et al. 2010). Changes in the surface of erythrocyte membranes caused by lead acetate exposure may be responsible for the increased fragility of erythrocytes, as was observed in the lead acetate-exposed group. The erythrocytes of rats pretreated with Daflon and exposed to lead acetate were protected from significant EOF owing to the fact that flavonoids have been reported to be potent antioxidants (Terao 2009) scavenging free radicals and shielding against free radical-induced vicissitudes (Isaac et al. 2017).
Conclusion
The results of this study revealed that sub-chronic exposure of rats to lead acetate induced impairment in δ-ALAD and hematological parameters while pretreatment with Daflon mitigated the ensued changes. Notably, lead decreased PCV, Hb, RBC, MCV, and MCHC by 66.75%, 144.30%, 233.61%, 95.522%, and 94.68% respectively while the administration of Daflon improved PCV, Hb, RBC, MCV, and MCHC by 45.03%, 104.30%, 227.46%, 67.80%, and 59.50% respectively. This therefore suggests that augmented consumption of Daflon-enriched foods may be beneficial to individuals living in lead toxicity prone environments.
References
Abadin H, Ashizawa A, Stevens YW, Llados F, Diamond G, Sage G, Citra M, Quinones A, Bosch SJ, Swarts SG (2007) Toxicological profile for lead; the United States Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, p 582
Akande MG, Aliu YO, Ambali SF, Ayo JO (2014) Protective effect of taurine in chlorpyrifos and lead-induced hematological alterations in Wistar rats. Toxicol Environ Chem 96(1):171–182
Alya A, Ines BD, Montassar L, Najoua GH, Saloua EF (2015) Oxidative stress, biochemical alterations and hyperlipidemia in female rats induced by lead chronic toxicity during puberty and post puberty periods. Iran J Basic Med Sci 18:1034–1043
Ambali SF, Abubakar M, Shittu M, Yaqub LS, Anafi SB, Abdullahi A (2010) Chlorpyrifos-induced alteration of haematological parameters in Wistar rats: ameriolative effect of zinc. Res J Environ Toxicol 4:55–66
Anakwue RC, Anakwue AC (2014) Cardiovascular disease profiling in Africa: environmental pollutants are not on the agenda. Cardiovasc Toxicol 14:193–207
Andjelkovic M, Djordjevic AB, Antonijevic E, Antonijevic B, Stanic M, Kotur-Stevuljevic J, Spasojevic-Kalimanovska V, Jovanovic M, Boricic N, Wallace D, Bulat Z (2019) Toxic effect of acute cadmium and Lead exposure in rat blood, liver, and kidney. Int J Environ Res Public Health 16:274–295
Andrew NN (2003) From symptoms to leg edema: efficacy of Daflon 500 mg. Angiology 54(1):33–44
Asala OO, Ayo JO, Rekwot PI, Minka NS, Omoniwa DO, Adenkola AY (2011) Effect of ascorbic acid administration on erythrocyte osmotic fragility of pigs transported by road during the hot-dry season. Vet Res Commun 35:245–254
Bartrem C, Tirima S, Von Lindern I, Von Braun M, Worrell MC, Mohammed AS, Abdullahi A, Moller G (2014) Unknown risk: co-exposure to lead and other heavy metals among children living in small-scale mining communities in Zamfara state, Nigeria. Int J Environ Health Res 24:304–319
Dacie JV, Lewis SM (1991) Practical Haematology, 7th edn. Churchill Livingstone, London, p 659–661
Debosree G, Sudeshna P, Shamreen N, Debajit B, Mousumi D, Arnab KG, Syed BF, Aindrila C, Russel JR, Debasish B (2016) Melatonin protects against lead acetate induced oxidative stress-mediated changes in morphology and metabolic status in rat red blood cells: a flow cytometric and biochemical analysis. J Pharm Res 10(6):381–402
Erick H, Yong W, Ian DB (2016) A novel methodology for rapid digestion of rare earth element ores and determination by microwave plasma-atomic emission spectrometry and dynamic reaction cell-inductively coupled plasma-mass spectrometry. Talanta 160:521–527
Faulkner WR, King JW (1970) Manual of clinical laboratory procedures. Chemical Rubber Company, Cleveland, p 345
Flora SJS (2002) Nutritional components modify metal absorption, toxic response and chelation therapy. J Nutr Environ Med 12:53–67
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5:47–58
Garber JC, Barbee RW, Bielitzki JT, Clayton LA, Donovan JC, Kohn DF, Lipman NS et al (2011) Guide for the care and use of laboratory animals, 8th edn. National Academies Press, Washington, DC
Halazun KJ, Aldoori A, Malik HZ (2008) Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol 34(1):55–60
Hashem MA, El-Sharkawy NI (2009) Haematobiochemical and immunotoxicological effects of low electromagnetic field and its interaction with lead in mice. Iraqi J Vet Sci 23:105–114
Ibrahim NM, Eweis EA, El-Bertagi HS, Abdel-Mobdy YE (2012) Effect of lead acetate toxicity on experimental male albino rat. Asian Pac J Trop Biomed 2(1):41–46
Isaac AO, Joseph AO, Victor SO, Lamidi YI, Andrew AM (2017) Ameliorative effects of kaempferol and zinc gluconate on erythrocyte osmotic fragility and haematological parameters in Wistar rats exposed to noise stress. Insights Biomed 2(3):1–7
Jalali MS, Seyedeh NH, Mousavi M (2017) Comparative effect of silymarin and D-penicillamine on lead induced hemotoxicity and oxidative stress in rat. Iran J Toxicol 11(3):12–18
John JB (2005) Chronic venous insufficiency and the therapeutic effects of Daflon 500 mg. Angiology 56(6):21–24
Kasemy ZA, Kamel GM, Abdel-Rasoul GM, Ismail AA (2019) Environmental and health effects of benzene exposure among Egyptian taxi drivers. J Environ Public Health 4(10):707–802
Keleiny EG, Ibrahim Laila IM, Ibrahim SR (2019) The effect of ferulic acid against lead induced oxidative stress and DNA damage in kidney and testes of rats. Environ Sci Pollut Res 26:31675–31684
Khan SY, Arshad M, Arshad N, Shaafat S, Tahir HM (2016) A probable role of blood lead levels on some haematological parameters in traffic police, Lahore Pakistan. Toxicol Ind Health 32:795–800
Kobo PI, Ayo JO, Aluwong T, Zezi AU, Maikai V, Ambali SF (2014) Flavonoid mixture ameliorates increase in erythrocyte osmotic fragility and malondialdehyde concentration induced by Trypanosoma brucei brucei-infection in Wistar rats. Res Vet Sci 96:139–142
Mager EM (2012) Homeostasis and toxicology of non-essential metals. In: Wood CM, Anthony P, Farrell, Brauner CJ (eds) Fish physiology, notably, lead decreased PCV, Hb, RBC, MCV, and MCHC by 66.75%, 144.30%, 233.61%, 95.522%, and 94.68% respectively while the administration of DAF improved PCV, Hb, RBC, MCV, and MCHC by 45.03%, 104.30%, 227.46%, 67.80%, and 59.50% respectively. Elsevier Incorporation, New York, pp 85–236
Magyar JS, Weng TC, Stern CM, Dye DF, Rous BW, Payne JC, Bridgewater BM (2005) Re-examination of lead (II) coordination preferences in sulphur-rich sites: implications for a critical mechanism of lead poisoning. J Am Chem Soc 127(6):9495–9505
Martins JA, Gustavo RB, Anna JB, Marilesia FS (2015) Effects of lead exposure and genetic polymorphisms on ALAD and GPx activities in Brazillian battery workers. J Toxicol Environ Health 78(16):1–9
Minka NS, Ayo JO (2007) Physiological responses of transported goats treated with ascorbic acid during the hot-dry season. Anim Sci J 78(2):164–172
Mitra P, Sharma P (2019) Novel direction in mechanisms underlying lead toxicity: evidence and prospective. Indian J Clin Biochem 34:121–122
Ohyashiki T, Kobayashi M, Mashi K (1991) Antioxidants in lead toxicity. Arch Biochem Biophys 228:282–286
Okediran BS, Biobaku KT, Olaifa FH, Atata AJ (2017) Haematological and antioxidant enzyme response to lead toxicity in male Wistar rats. Ceylon J Sci 46(2):31–37
Onalaja AO, Claudio L (2000) Genetic susceptibility to lead poisoning. Environ Health Perspect 108:23–28
Orisakwe OE, Oladipo OO, Ajaezi GC, Udowelle NA (2017) Horizontal and vertical distribution of heavy metals in farm produce and livestock around lead-contaminated goldmine in Dareta and Abare, Zamfara state, Northern Nigeria Journal of Environmental and Public.Health
Oyewale JO (1991) Osmotic fragility of erythrocytes of guinea fowls at 21 and 156 weeks of age. Veterinariski Arhiv 61(1):49–56
Patrick L (2006) Lead toxicity, a review of the literature. Part 1: exposure, evaluation, and treatment. Altern Med Rev 11:2–22
Pitsch A (2011) The place of Daflon-500mg in recent guidelines on the management of chronic venous disease. Medicograhia 33(3):306–314
Ramlet AA (2001) Clinical benefits of Daflon 500 mg in the most severe stages of chronic venous insufficiency. Angiology 52:49–56
Rendon-Ramirez A, Cerbon-Solorzano J, Maldonado-Vega M, Quintanar-Escorza MA, Calderon-Salinas JV (2007) Vitamin E reduces the oxidative damage on d-aminolevulinic dehydratase induced by lead intoxication in rat erythrocytes. Toxicol in Vitro 21(6):1121–1126
Rous P (2000) The effect of heavy metals boundary contaminated soil on haematological and selected biochemical parameters in blood plasma of rabbits. Acta-Universitatis-Agriculturae-et-SilviculturaeMendelianae-Brunensis 48(3):93–99
Sakai T, Morita Y (1996) Delta aminolevulinic acid in plasma or whole blood as a sensitive indicator of lead effects, and its relation to the other heme-related parameters. Int Arch Occup Environ Health 68:126–132
Sancar F (2019) Childhood lead exposure may affect personality, mental health in adulthood. JAMA 32(15):1445–1446
Shraideh AZ, Badran HD, Hunaiti AA, Battahn A (2019) Delta-aminolevulinic acid dehydratase inhibition and RBC abnormalities in relation to blood lead among selected Jordanian workers. Jordan J Biol Sci 12(2):237–241
Supabhorn Y, Donrawee W, Saruda K (2019) Screening for elevated blood lead levels and related risk factors among Thai children residing in a fishing community. Toxics 7:54
Suradkar SG, Ghodasara DJ, Vihol P, Patel J, Jaiswal V, Prajapati KS (2009) Haematobiochemical alterations induced by lead acetate toxicity in wistar rats. Vet World 2:429–439
Terao J (2009) Dietary flavonoids as antioxidants. Forum Nutr 61:87–94
Timchalk C, Lin Y, Witz KK, Wu H, Gies RA, Moore DA, Yantasee W (2006) Disposition of lead (Pb) in saliva and blood of Sprague-Dawley rats following single or repeated oral exposure to Pb-acetate. Toxicology 222(1–2):86–94
Unlu BZ, Singletary KW, Murphy LL, Venema RC, Young AJ (2003) Phytonutrients differentially stimulate NAD(P)H: quinone oxidoreductase, inhibit proliferation, and trigger mitotic catastrophe in Hepa1c1c7 cells. J Med Food 19(1):47–53
Warang P, Colah R, Kedar P (2017) Lead poisoning induced severe hemolytic anaemia, basophilic stippling, mimicking erythrocyte pyrimidine 5′-nucleotidase deficiency in beta thalassemia minor. J Clin Toxicol 7(2):346
WHO (2010) Action is needed on chemicals of major public health concern. Public Health Environmental pp 1–4. Available online: https://www.who.int/ipcs/assessment/public_health/chemicals_phc/en/
Yusuf IL, Akefe IO, Tijjani MB, Yusuf H, Salihu SI, Muhammad Z (2018) Lead-induced increase in erythrocyte osmotic fragility and malondialdehyde concentration in Wistar rats: chemo-protective effects of flavonoid mixture. Biochem Biotechnol Res 6(1):9–14
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed concept
All authors were duly notified before submitting this manuscript.
Ethical approval
This research was approved by the Ahmadu Bello University Committee on Animal Use and Care (ABUCAUC) with ethical approval number ABU/2018/7162.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lamidi, I.Y., Hudu, M.G., Akefe, I.O. et al. Sub-chronic administration of flavonoid fraction Daflon improve lead-induced alterations in delta-aminolevulinic acid dehydratase activity, erythrocytic parameters, and erythrocyte osmotic fragility in Wistar rats. Comp Clin Pathol 29, 955–963 (2020). https://doi.org/10.1007/s00580-020-03144-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-020-03144-6