Abstract
In this project, red-backed salamanders, Plethodon cinereus, were collected from the heart of their range (Mountain Lake, Virginia, USA), in an effort to document the typical, or baseline, leukocyte profile of this species in its natural state. From microscopic examination of thin blood films, we determined relative proportions of all leukocyte types and determined the ratio of neutrophils to lymphocytes, which is a useful index of stress levels. In addition, individuals were examined for three naturally occurring endoparasites: two gastro-intestinal (trematodes and a ciliated protozoan in the genus Cepedietta) and one in the blood (a Rickettsial bacteria). This allowed us to conduct statistical comparisons of leukocyte data across parasite infection groups. Of 36 salamanders, 11 (31%) had trematode parasites, 7 (19%) contained Cepedietta parasites, 6 (17%) were parasitized by Rickettsia, and 12 had no parasites. The most common leukocytes were lymphocytes (87.4%), followed by neutrophils (4.2%), monocytes (3.3%), eosinophils (2.6%), and basophils (2.5%). The average neutrophil/lymphocyte ratio for these salamanders was remarkably low (0.05) for an amphibian population. Statistical comparisons of leukocyte profiles among parasite infection groups indicated parasitized salamanders had twice as many circulating leukocytes as nonparasitized individuals, which could be considered an “inflammation response”. There was a slight but significant elevation in relative eosinophil abundance in parasitized salamanders. There were few differences in leukocyte subsets between parasite types, nor was there evidence of any proliferation of phagocytic cells in any parasitized individual.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The physiological health status of a wild animal population can be indirectly gauged by microscopic examination of blood samples, and in particular, by examining the number and proportions of white blood cells. Since the leukocytes are one of the first lines of defense against foreign pathogens in all vertebrates, evaluating their circulating abundance within a sample of animals in a wild population can provide information as to how the entire population is faring (e.g., Barriga-Vallejo et al. 2015; Maceda-Veiga et al. 2015).
There are a variety of indicators of potential health issues that can be obtained by examining leukocyte abundance. For example, one of the hallmarks of an immune reaction is an increase in leukocyte density in circulation (e.g., Kogut et al. 1994; Latimer et al. 1988; Rose et al. 1979); thus, if animals have unusually high densities of leukocytes, it could signal a current infection. In addition, each of the different leukocyte types (neutrophils, lymphocytes, eosinophils, basophils, and monocytes) performs a different function in the immune response, and alterations in certain cells can be indicative of ill-health or stress. The phagocytic neutrophils (heterophils in birds and reptiles) and/or monocytes tend to increase in circulation during immune responses (Kogut et al. 1994; Latimer et al. 1988). Heightened neutrophil production can usually be detected by the presence of new (recently produced) cells, which appear morphologically distinct (called band cells, Al-Gwaiz and Babay 2007). Also, eosinophils are known to respond to a variety of different endo- and ecto-parasite invasions (Klion and Nutman 2004), and in amphibians, in particular, their function appears vital in defense against trematodes (Kiesecker 2002; LaFonte and Johnson 2013).
Counts of the numbers of leukocytes in animals can not only inform about the immune status of an animal but are also useful for determining the degree of stress an animal is currently experiencing, because of the way certain cell types respond to elevations in stress hormones (Davis and Maney 2018; Davis et al. 2008). When stress hormones spike, due to a stress experience, the number of neutrophils (heterophils in birds and reptiles) tends to increase in circulation, while the number of lymphocytes tends to decrease (Dhabhar et al. 1996). Thus, the relative proportions of both (the ratio of neutrophils to lymphocytes) serve as a useful index of stress levels. Moreover, this index appears to be long-lasting, and therefore is useful for detecting persistent environmental stressors (Goessling et al. 2015).
The red-backed salamander, Plethodon cinereus (Fig. 1A), is a stereotypical salamander in the genus Plethodon, being a small (< 3 g), terrestrial, nocturnally active species that lives in cool, moist forest habitats in the eastern USA, and is especially abundant in high-elevation Appalachian Highlands (Milanovich et al. 2010). It has long been the focus of research across a variety of topics, including the significance of its dorsal pigmentation (Davis and Milanovich 2010; Highton 1975; Moreno 1989), habitat preferences and environmental limits (Grover 1998; McKenny et al. 2006; Wyman and Hawksleylescault 1987), and reproductive behavior (Bachmann 1984; Lang and Jaeger 2000). However, there has been little work done to examine the leukocyte subpopulations within this (or related) species, aside from research on captive animals (Davis and Milanovich 2010). Also, unknown is how the immune system of this species (or its relatives) is affected by certain non-lethal and naturally occurring parasites. For example, there are certain species of intestinal nematodes that are found in P. cinereus, including Thelandros magnavulvaris (Oxyuroidea: Oxyuridae), and the trematode, Brachycoelium salamandrae (Muzzall 1990), and very little is known on how these parasites impact their host. An unusual parasite also found in the intestine of plethodontids is a ciliated protozoan in the genus Cepedietta (McAllister and Trauth 1996). This parasite attaches to the intestinal wall and grazes on contents of the lumen, though it is unclear how this affects the host. Plethodon cinereus is also a known host of a little-studied bacterial organism that affects red blood cells and is a member of the order, Rickettsiales (Davis et al. 2009).
(A) Red backed salamander, Plethodon cinereus, photographed by Bill Peterman in Jefferson Co, IN, USA. This terrestrial salamander inhabits cool, moist forests of the eastern United States, and can be found under logs, rocks and in leaf litter. (B) Map of the eastern United States, showing location of study site (inset), the Mountain Lake Biological Station in Giles County, Virginia (blue circle)
The primary goal of this project was to establish baseline leukocyte profiles of a population of P. cinereus that is near the center of their range, in western Virginia (USA), and that is not known to be under any environmental stressors (pollutants or other anthropogenic stressors, etc.). As a secondary goal, given that some individuals sampled harbored certain naturally occurring parasites, this provided a convenient comparison of leukocyte data of the parasitized and nonparasitized individuals.
Methods
Study site
All salamanders were collected in the summers of 2013 and 2018 in and around the Mountain Lake Biological Station (MLBS) in Giles County, VA, USA (Fig. 1B). The collections were made as part of a field course that was taught during those years. The station is situated at 3900 ft in the Appalachian Highlands, and the cool, moist forests at this elevation are ideal habitats for terrestrial salamanders (Milanovich et al. 2010).
Salamander collection and processing
Salamanders were collected by hand by overturning rocks and logs in the forest around the station. They were placed in plastic bags and transported to the station lab (10 min away). There, they were euthanized by exposure to an MS-222 solution and their snout-vent length was measured. The salamanders were then dissected to determine endoparasite status. As part of the dissection, the heads were severed from the body, and we obtained a sample of blood (~ 20 μl) from the exposed hearts; we used this sample to make thin blood smears, which were air-dried and then stained with Giemsa (CAMCO Quik-Stain II). Importantly, the time from collection to blood sampling in all cases was less than 1 h.
The dissection procedure involved examining all organs of the animal in sequence under low-power magnification, and especially the intestine and gall bladder, which are known locations for parasites of Plethodontid salamanders (McAllister et al. 2002, 2008, 1995, Muzzall 1990). Specifically, the lower intestine (under low-power magnification) was removed and opened to search for gastro-intestinal parasites, such as flukes (trematodes; Fig. 2A) and/or the ciliated protozoan in the genus Cepedietta (Fig. 2B). When parasites were found, temporary slide mounts were made to observe them under a higher magnification and to confirm their identity, although we did not attempt to identify them to species. The sex of each salamander was determined based on the presence of reproductive organs. In total, 19 salamanders were examined in 2013, and 17 in 2018 (n = 36 total). Of these, 11 (31%) were found to have trematode parasites, 7 (19%) contained Cepedietta parasites, 6 (17%) were parasitized by Rickettsia, and 12 salamanders had no parasites. Given the small sample size of salamanders, and of each parasite grouping, we did not subcategorize the individuals based on levels of parasite infection.
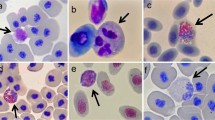
Three parasites typically found in red-backed salamanders: trematodes (A) which can be found in the small intestine; ciliated protozoa (Cepedietta spp.) (B), found in the intestine and gall bladder; and intra-erythrocytic Rickettsial bacteria (C), from the blood. The bacteria are contained within violet- or purple-staining vacuoles in erythrocytes (arrows) of infected animals and can be seen on thin blood smears
Blood Film Assessment
The stained blood films from each salamander were examined with a standard light microscope under 1000× oil immersion for identification and counting of leukocytes, plus determining prevalence of Rickettsial blood parasites (Davis and Cecala 2010; Davis et al. 2009). The Rickettsial bacteria can be readily observed in blood films of infected individuals as purple- or violet-staining inclusions, or vacuoles, within erythrocytes (Fig. 2C). The bacteria live within the vacuoles and cannot be seen themselves.
We obtained an estimate of overall leukocyte abundance for each salamander, to use as an index of immune status, as follows. At each field of view, we counted all leukocyte types, then moved the slide to the next field, and repeated, until at least 100 leukocytes had been tallied. In certain cases, where the blood sample was minimal to begin with, or when leukocyte density was especially low, the counting stopped after 150 fields of view had been examined. Given that each field of view we examined contained similar red cell densities, we estimated the abundance of leukocytes for each individual as the number of cells per field of view. The leukocyte subsets included neutrophils, lymphocytes, eosinophils, basophils, and monocytes (Fig. 3). At the end of counting, we calculated the proportion of each cell type, as well as the ratio of neutrophils to lymphocytes (N/L ratio). While counting the cells, we also took note of the morphological appearance of the neutrophils; young cells can be identified by the lack of nuclear segmentation, and their presence is a sign of heightened cell production, as would occur during an infection (Thrall et al. 2006).
The five different types of leukocytes, or white blood cells, of P. cinereus, as seen on thin blood smears examined at 1000× (oil) magnification. Shown are neutrophil, basophils (A), eosinophils (B), lymphocytes (C), and monocyte (D). Note that the neutrophils shown have segmented nuclei, which indicates cell maturity. All images taken by A. Davis.
Data Analysis
A total of 36 salamanders were included in this investigation across the two sample years (2013 and 2018). Preliminary inspection of the leukocyte data showed there were minimal differences across the years (see Table 1, Results), so these data were pooled for further analyses. For the purposes of this study, we grouped the salamanders according to which parasite was found in them: trematodes (n = 11), Cepedietta (n = 7), Rickettsia (n = 6), or none (n = 12). We then explored possible statistical differences in leukocyte profiles across all groups and specifically tested for differences in estimated WBC abundance (cells/field of view, log transformed), percentage of each leukocyte type, and N/L ratios. Initially, we used ANCOVA to explore these differences and included additional predictors of year, sex, and body size (SVL), though none of these additional predictors were found to be of importance (p > 0.1 for all). We therefore used simple one-way ANOVA to compare the various elements of the leukocyte data across parasite groups. Data analyses was performed using the Statistica 13.3 software package (Tibco Software, Inc.).
Results
Leukocyte profile of P. cinereus
The relative abundance of the different white blood cell types for each sample year is presented in Table 1. The most common cell type observed, by far, was lymphocytes (87%), followed by neutrophils (4%); these are also pictured in Fig. 3. Note that the neutrophils pictured were typical of what was observed, having segmented nuclei, indicating mature cells. The overall average N/L ratio for these salamanders (an index of current stress levels) was 0.05, which was remarkably low, based on multiple prior studies of salamanders and other amphibians (Davis 2012; Davis and Durso 2009; Davis and Maerz 2008a, b, 2009, 2010, 2011; Davis and Milanovich 2010), where “baseline” N/L ratios tend to be closer to 0.3.
Associations with parasites
While results here are based on a small sample size (n = 36), there were certain associations between parasite status and leukocyte profiles. Estimated leukocyte abundance (cells/field of view) did not differ significantly between salamanders with trematodes, Cepedietta, Rickettsia, or those with no parasites (F3,32 = 2.25, p = 0.101), although there appeared to be a trend where parasitized individuals from all groups generally had greater WBC densities than nonparasitized individuals. To confirm this, we pooled all parasitized groups and compared these with all unparasitized salamanders, and this revealed a significant difference (F1,34 = 5.05, p = 0.031; Fig. 4). Direct comparison of these means (using the untransformed data) indicated parasitized salamanders had approximately twice as many leukocytes as unparasitized ones (͞x = 1.86 cells vs. 0.96 cells counted per field of view, respectively).
Statistical comparisons of specific cell types across parasite groups did not reveal major differences in the leukocyte profile (p > 0.1 for all; Table 2), except for eosinophils; there was a small, but significant variation across parasite groups in the relative abundance of eosinophils (F3,32 = 3.7, p = 0.021). Here, salamanders with Cepedietta and/or Rickettsia parasites tended to have slightly more eosinophils. Neutrophil/lymphocyte ratios were unaffected by parasitism of any type (F3,32 = 0.09, p = 0.962).
Finally, during the blood cell counting, we endeavored to (subjectively) make note of the cellular morphology, especially of the neutrophils. Young neutrophils are characterized by a lack of nuclear segmentation (called band cells), and their presence in blood films suggests there is heightened production, such as during an infection (Thrall et al. 2006). The majority of neutrophils we observed, even in parasitized salamanders, appeared as those in Fig. 3, with multilobed nuclei, indicating a mature cell. This observation, along with the generally low neutrophil counts of parasitized salamanders, indicates the immune reaction to these parasites did not involve production of phagocytic cells.
Discussion
The baseline leukocyte data obtained in this investigation will help in advancing research into amphibian hematology in general, plus provide valuable comparison data from a wild population of this species. Moreover, these data come from a population that likely faces few, if any, environmental stressors which may alter their leukocyte profiles; this location is in the heart of its range, this area is not under any known anthropogenic stressors, and its forest habitat here could be considered pristine (Davis, pers. obs.). In addition, we were diligent about obtaining blood samples in a timely manner, so as not to alter the leukocyte profiles due to handling stress (Davis et al. 2008).
It is worth noting that the average values of each cell type we obtained differ from those from a prior study of this same species where animals were held in captivity for 1 week prior to sampling (Davis and Milanovich 2010). Specifically, in the two collections of wild salamanders (2013 and 2018), the population-level average N/L ratio (0.05) was remarkably low. Based on prior studies, it is evident that N/L ratios of non-stressed amphibians tend to be close to 0.3–0.5. In stressed situations, this ratio tends to be closer to 0.7–1.0 in salamanders or urodels (Davis and Maerz 2009, 2011; DuRant et al. 2015). Thus, the remarkably low N/L ratio of these red-backed salamanders at the Mountain Lake Biological Station in Virginia, over two disparate sampling years, confirms this population is not subjected to any population-level stressors. Moreover, the lower N/L ratios of wild red-backed salamanders over captive individuals are consistent with recent work from other salamander species; long-term captive tiger salamanders, Ambystoma tigrinum, had higher N/L ratios than recently captured individuals (Waye et al. 2019).
A secondary goal of this project was to identify and/or document how the immune cells of P. cinereus respond to certain naturally occurring parasites, including one hemoparasite and two gastro-intestinal parasites. One of the primary findings of this effort was that, on average, parasitized salamanders had twice as many circulating leukocytes as nonparasitized individuals (Fig. 4). This could be considered an “inflammation response” and suggests that the cellular immune system is activated (or perhaps “heightened”) in parasitized individuals. It is unclear if this response is directly targeting the parasites themselves or if it is an indirect response to tissue damage caused by the parasites (i.e., damage to intestinal walls and/or erythrocyte damage). Regardless, given the energy cost associated with maintaining an immune reaction (Otalora-Ardila et al. 2016), this is something that could be further examined, since it may lead to reductions in energy-intensive activities or behaviors.
When specific differences within the five leukocyte types were examined, it was determined that the relative abundance of one cell, the eosinophil, was slightly elevated in salamanders infected with certain parasites, at least compared with unparasitized individuals (Table 2). However, while this elevation was statistically significant, it should be cautioned that the overall abundance of the eosinophils in these cases was not remarkable (in the clinical sense). Nevertheless, this result is consistent with the idea that its function is to defend against parasites, including trematodes in amphibians (Kiesecker 2002; LaFonte and Johnson 2013). Interestingly, in the salamanders infected with trematodes, we noted their, eosinophil population was low (Table 2), but they were more elevated in salamanders parasitized with Rickettsia and Cepedietta. Regarding other cell types, we found no evidence of a proliferation of any phagocytic cells (neutrophils or monocytes) in any of the parasitized salamanders; proportions of these cells were low across all groups (Table 2), and most of the neutrophils observed were mature (Fig. 3).
Based on the leukocyte data collected, there was little evidence that one parasite is more consequential than the other, in terms of an immunological cost, and/or in terms of elevated N/L ratios (i.e., higher host stress), although we reiterate that our sample size was small in this investigation. Nevertheless, we saw no evidence that parasitism of any kind, at least in the parasites we detected, caused elevations in baseline stress levels of the host, since the N/L ratios we observed were unaffected by parasitism (and were themselves remarkably low). This would suggest that these naturally occurring parasites do not influence the life of the animals in such a way as to cause stress (such as by causing reductions in foraging ability, Davis et al. 2004, 2010). This result would appear to be consistent with recent work with anurans (Koprivnikar et al. 2019), where experimental exposure to trematodes did not result in heightened stress levels and produced only minor alterations in leukocyte profiles. Similarly, larval bullfrogs (Lithobates catesbeianus) parasitized by copepods did not have higher N/L ratios (Salinas et al. 2019).
In summary, the results and observations from this project provide two points of interest that can be considered in future hematological investigations of amphibians and other wildlife. First, we determined that leukocyte profiles of wild-caught red-backed salamanders appear to differ from those of captive collections. Specifically, the ratio of neutrophils to lymphocytes (an index of current stress) is much lower in wild samples. Second, by comparing leukocyte profiles among individuals with natural parasites, we found that parasitized salamanders show evidence of heightened leukocyte levels, but only minor differences among leukocyte subsets. Collectively, these results can add to the small but growing literature based around amphibian hematology.
References
Al-Gwaiz LA, Babay HH (2007) The diagnostic value of absolute neutrophil count, band count and morphologic changes of neutrophils in predicting bacterial infections. Med Princ Pract 16:344–347
Bachmann MD (1984) Defensive behavior of brooding female red-backed salamanders (Plethodon cinereus). Herpetologica 40:436–443
Barriga-Vallejo C et al (2015) Assessing population health of the Toluca axolotl Ambystoma rivulare (Taylor, 1940) from Mexico, using leukocyte profiles. Herpetol Conserv Biol 10:592–601
Davis AK (2012) Investigating the optimal rearing strategy for Ambystoma salamanders using a hematological stress index. Herpetol Conserv Biol 7:95–100
Davis AK, Cecala K (2010) Intraerythrocytic rickettsial inclusions in Ocoee salamanders (Desmognathus ocoee): prevalence, morphology and comparisons with inclusions of Plethodon cinereus. Parasitol Res 107:363–367
Davis AK, Durso AM (2009) Leukocyte differentials of northern cricket frogs (Acris c. crepitans) with a compilation of published values from other amphibians. Herpetologica 65:260–267
Davis AK, Maerz JC (2008a) Comparison of hematological stress indicators in recently captured and captive paedomorphic mole salamanders, Ambystoma talpoideum. Copeia 2008:613–617
Davis AK, Maerz JC (2008b) Sex-related differences in hematological stress indices of breeding, paedomorphic mole salamanders. J Herpetol 42:197–201
Davis AK, Maerz JC (2009) Effects of larval density on hematological stress indices in salamanders. J Exp Zool A Ecol Genet Physiol 311A:697–704
Davis AK, Maerz JC (2010) Effects of exogenous corticosterone on circulating leukocytes of a salamander (Ambystoma talpoideum) with unusually abundant eosinophils. Int J Zool 2010:1–8. https://doi.org/10.1155/2010/735937
Davis AK, Maerz JC (2011) Assessing stress levels of captive-reared amphibians with hematological data: implications for conservation initiatives. J Herpetol 45:40–44
Davis AK, Maney DL (2018) The use of glucocorticoid hormones or leucocyte profiles to measure stress in vertebrates: What’s the difference? Methods Ecol Evol 9:1556–1568
Davis AK, Milanovich JR (2010) Lead-phase and red-stripe color morphs of red-backed salamanders (Plethodon cinereus) differ in hematological stress indices: a consequence of differential predation pressure? Curr Zool 56:238–243
Davis AK, Cook KC, Altizer S (2004) Leukocyte profiles of house finches with and without mycoplasmal conjunctivitis, a recently emerged bacterial disease. Ecohealth 1:362–373
Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22:760–772
Davis AK, DeVore JL, Milanovich JR, Cecala K, Maerz JC, Yabsley MJ (2009) New findings from an old pathogen: intraerythrocytic bacteria (family Anaplasmatacea) in red-backed salamanders Plethodon cinereus. Ecohealth 6:219–228
Davis AK, Keel MK, Ferreira A, Maerz JC (2010) Effects of chytridiomycosis on circulating white blood cell distributions of bullfrog larvae (Rana catesbeiana). Comp Clin Pathol 19:49–55
Dhabhar FS, Miller AH, McEwen BS, Spencer RL (1996) Stress-induced changes in blood leukocyte distribution: role of adrenal steroid hormones. J Immunol 157:1638–1644
DuRant SE, Romero LM, Davis AK, Hopkins WA (2015) Evidence of ectoparasite-induced endocrine disruption in an imperiled giant salamander, the eastern hellbender (Cryptobranchus alleganiensis). J Exp Biol 218:2297–2304
Goessling JM, Kennedy H, Mendonca T, Wilson AE (2015) A meta-analysis of plasma corticosterone and heterophil:lymphocyte ratios - is there conservation of physiological stress responses over time? Funct Ecol 29:1189–1196
Grover MC (1998) Influence of cover and moisture on abundances of the terrestrial salamanders Plethodon cinereus and Plethodon glutinosus. J Herpetol 32:489–497
Highton R (1975) Geographic variation in genetic dominance of color morphs of red-backed salamander, Plethodon cinereus. Genetics 80:363–374
Kiesecker JM (2002) Synergism between trematode infection and pesticide exposure: a link to amphibian deformities in nature? Proc Natl Acad Sci 99:9900–9904
Klion AD, Nutman TB (2004) The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol 113:30–37
Kogut MH, McGruder ED, Hargis BM, Corrier DE, DeLoach JR (1994) Dynamics of avian inflammatory response to Salmonella-immune lymphokines. Changes in avian blood leukocyte populations. Inflammation 18:373–388
Koprivnikar J, Hoye BJ, Urichuk TMY, Johnson PTJ (2019) Endocrine and immune responses of larval amphibians to trematode exposure. Parasitol Res 118:275–288
LaFonte BE, Johnson PTJ (2013) Experimental infection dynamics: using immunosuppression and in vivo parasite tracking to understand host resistance in an amphibian-trematode system. J Exp Biol 216:3700–3708
Lang C, Jaeger RG (2000) Defense of territories by male-female pairs in the Red-Backed Salamander (Plethodon cinereus). Copeia 200:169–177
Latimer KS, Tang KN, Goodwin MA, Steffens WL, Brown J (1988) Leukocyte changes associated with acute inflammation in chickens. Avian Dis 32:760–772
Maceda-Veiga A, Figuerola J, Martinez-Silvestre A, Viscor G, Ferrari N, Pacheco M (2015) Inside the Redbox: applications of haematology in wildlife monitoring and ecosystem health assessment. Sci Total Environ 514:322–332
McAllister CT, Trauth SE (1996) Ultrastructure of Cepedietta virginiensis (Protista: Haptophryidae) from the gallbladder of the pickerel frog, Rana palustris, in Arkansas. Proc Ark Acad Sci 50:133–136
McAllister CT, Bursey CR, Upton SJ, Trauth SE, Conn DB (1995) Parasites of Desmognathus brimleyorum (Caudata, Plethodontidae) from the Ouachita mountains of Arkansas and Oklahoma. J Helminthol Soc Wash 62:150–156
McAllister CT, Bursey CR, Trauth SE (2002) Parasites of four species of endemic Plethodon from Arkansas and Oklamhoma. J Ark Acad Sci 56:239–242
McAllister CT, Bursey CR, Trauth SE (2008) New host and geographic distribution records for some endoparasites (Myxosporea, Trematoda, Cestoidea, Nematoda) of amphibians and reptiles from Arkansas and Texas, USA. Comp Parasitol 75:241–254
McKenny HC, Keeton WS, Donovan TM (2006) Effects of structural complexity enhancement on eastern red-backed salamander (Plethodon cinereus) populations in northern hardwood forests. For Ecol Manag 230:186–196
Milanovich JR, Peterman WE, Nibbelink NP, Maerz JC (2010) Projected loss of a salamander diversity hotspot as a consequence of projected global climate change. Plos One 5:e12189. https://doi.org/10.11371/journal.pone.0012189
Moreno G (1989) Behavioral and physiological differentiation between the color morphs of the salamander, Plethodon cinereus. J Herpetol 23:335–341
Muzzall PM (1990) Endoparasites of the red-backed salamander, Plethodon c. cinereus, from southwestern Michigan. J Helminthol Soc Wash 57:165–167
Otalora-Ardila A, Herrera LG, Flores-Martinez JJ, Welch KC (2016) Metabolic cost of the activation of immune response in the fish-eating myotis (Myotis vivesi): the effects of inflammation and the acute phase response. PLoS One 11:14
Rose ME, Hesketh P, Ogilvie BM (1979) Peripheral blood leukocyte response to coccidial infection: a comparison of the response in rats and chickens and its correlation with resistance to reinfection. Immunology 36:71–79
Salinas ZA, Babini MS, Grenat PR, Biolé FG, Martino AL, Salasa NE (2019) Effect of parasitism of Lernaea cyprinacea on tadpoles of the invasive species Lithobates catesbeianus. Heliyon 5:e01834
Thrall MA et al (2006) Veterinary hematology and clinical chemistry. Blackwell Publishing, Ames
Waye HL, Dolan PC, Hernandez A (2019) White blood cell profiles in long-term captive and recently captured eastern tiger salamanders (Ambystoma tigrinum). Copeia 107:138–143
Wyman RL, Hawksleylescault DS (1987) Soil acidity affects distribution, behavior, and physiology of the salamander Plethodon cinereus. Ecology 68:1819–1827
Acknowledgements
We thank the MLBS Disease Ecology classes of 2013 and 2018 for their assistance with salamander collecting and dissections. We are also grateful to Sonia Altizer, Amy Pedersen, and Dana Hawley, for logistical assistance with the class.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Davis declares that he has no conflict of interest. C. Golladay declares that she has no conflict of interest.
Ethical approval
All animals in this study were handled and sacrificed following approval of the University of Virginia Animal Care and Use committee. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Davis, A.K., Golladay, C. A survey of leukocyte profiles of red-backed salamanders from Mountain Lake, Virginia, and associations with host parasite types. Comp Clin Pathol 28, 1743–1750 (2019). https://doi.org/10.1007/s00580-019-03015-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-019-03015-9