Abstract
Bone marrow responses have been identified as key determinants of trypanotolerance in cattle as they determine the ability for hemopoietic cell regeneration and control of anemia. However, not much is known about such responses in Nigerian breed of goats known to show different susceptibilities to trypanosome infections. Early bone marrow events associated with pathogenesis of Sokoto Red goats to Trypanosoma vivax were investigated in six goats to assess their role in susceptibility of this goat breed to trypanosomiasis. A total of six Sokoto Red goats of mixed sexes were used. While four of the goats randomly selected were infected with Trypanosoma vivax, the remaining two served as control. The parameters examined included packed cell volume (PCV) and differential bone marrow cytology. Cytological changes in the bone marrow of goats with acute trypanosomosis were compared to the controls. T. vivax caused an acute disease course in the goats resulting to mild drop in PCV and death 2–3 weeks post infection (PI). Associated bone marrow (BM) cytological changes were characterized by moderate erythroid hyperplasia with a resultant lower myeloid:erythroid (M:E) ratio, while the granulocytic maturation rate was 3.15 ± 0.6 and 2.42 ± 0.0 for infected and control animals, respectively. However, significant macrophage (MC) numbers (hyperplasia) were detected in the BM of the infected group. Most of the MCs phagocytized mature red blood cells (RBC) and band or mature white blood cells (WBC) only while no MC phagocytized immature cells. The MCs in BM of control goats phagocytized no blood cells. The study confirmed that mature blood cells form the first cell types to be phagocytized by MCs of BM in the pathogenesis of anemia in T. vivax-infected Sokoto Red goats while early onset of MC hyperplasia and erythrophagocytosis are indicators of susceptibility to trypanosomosis in this breed of goats and perhaps other susceptible ruminants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Goat keeping has been identified as one of the most profitable enterprises in Nigeria and contributing significantly to agricultural gross domestic product (GDP), providing income, employment, food, and manure (Nuru 1984., Otuma 2005., Ikegwuonu 2013). However, animal African trypanosomosis has been known for many years as a major constraint to livestock value chains and food security in several parts of sub-Saharan Africa including Nigeria (Onyiah 1997; Swallow 2000; Adamu et al. 2011; Abenga 2015) and presently constitutes an emerging threat to ruminants in parts of Asia and Southern America (Jones and Davila 2001; Cadioli et al. 2012). However, factors responsible for the pathogenesis of the disease are not yet fully known. The Sokoto Red breed of goats, though one of the most prolific in production, shows high susceptibility to African trypanosomosis and is believed to be one of the factors that limit its distribution to the Northern Savannah. Anemia is a dominant pathological feature and important cause of death in trypanosome-infected animals while the severity of hematological changes is dependent on species of infecting trypanosome and host factors which determine the extent of bone marrow responses (Anosa 1988a, b). Logan-Henfrey et al. (1999) identified bone marrow responses as a key determinant factor of trypanotolerance in cattle as it determines the ability for hemopoietic cell regeneration and control of anemia. This was supported by light and electron microscopic studies of sequential biopsies of bone marrow which showed key differences between Trypanosome congolense-infected trypanotolerant N’dama and trypanosusceptible Boran cattle. However, not much is known on the role of such responses in different breeds of goats. Trypanosoma vivax is a dominant and highly pathogenic trypanosome affecting goats and other ruminants in Nigeria (Omotainse et al. 2000; Anyaegbunam and Okafor 2013; Gimba 2015) and other parts of sub-Saharan Africa (Anosa 1999) while the non-tsetse-transmitted T. vivax constitutes an important economic risk to ruminants beyond tsetse belts of Africa such as South and Central America and Asia (Silva et al. 1999; Jones and Davila 2001). In this study, an attempt was made to assess the nature of bone marrow responses in experimental acute trypanosomosis of Nigerian Sokoto Red breed of goats infected with T. vivax. This was with the view to identifying the roles of bone marrow in the susceptibility or otherwise of this breed of goats to trypanosomosis which could be harnessed in the research towards control of this disease.
Materials and methods
Research animals
Six adult Sokoto Red breed of goats of mixed sexes weighing 12.3 to 20.7 kg body weight were used for the study. All the animals were purchased from local markets around Kaduna metropolis, North Central Nigeria, and screened for hemoparasites before use. All the animals were parasitologically negative for trypanosomes by either hematocrit centrifugation technique or by buffy coat method. After the acclimatization period which lasted for 3 weeks, four of the goats made up of two males and two females were randomly selected to serve as the infected group while the remaining two made up of one male and one female served as the uninfected control group.
Trypanosome species
Trypanosoma vivax isolated from cattle in Federe area in Kaduna State, North Central Nigeria, was used for the study. The parasites were cryopreserved in liquid nitrogen in our laboratory in Vom, Plateau state, Nigeria, and sub-passaged later into a donor female Sokoto Red breed of goat from where they were harvested from the jugular blood for inoculation. An estimated 2 × 106 parasites were inoculated into the goats intra-peritoneally (Lumsden et al. 1973).
Sample collection and examination methods
Whereas daily parasitemia was determined from blood obtained from the ear vein of the infected goats for the determination of onset of parasitemia, blood samples obtained by weekly venipuncture of the jugular vein of all the goats into ethylene diamine tetracetic acid (EDTA) bottles were used for determination of the packed cell volume (PCV) of the animals (Kelly 1979). Bone marrow smears were however obtained from the sternum of terminally recumbent infected and control goats. The bone marrow thin smears were air-dried, fixed in absolute alcohol for 20 min (Dacie and Lewis 1984), and stained with Wright’s stain. Differential marrow cell counts based on 500 nucleated cells were made by light microscopy as described by Anosa et al. (1992).
The studies were done in accordance with the ethical guidelines of the Nigerian Institute for Trypanosomiasis Research, Kaduna, Nigeria, which to a greater extent is in conformity with the guiding principles for biomedical research involving animals as issued by the Council of International Organizations of Medical Sciences (CIOMS). The animals were therefore kept in good, clean, and hygienic housing and given clean water and humane handling during sample collection in the course of the experiment as specified in the guidelines.
Statistical analysis
The data collected was statistically analyzed to test the significant differences in the groups using, t test, Microsoft Excel 2010. In all cases, values of P < 0.05 were considered significant.
Results
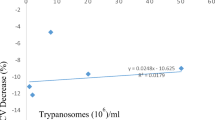
The mean PCV of the infected goats dropped only slightly (P > 0.05, Fig. 1) at 2 to 3 weeks post infection (PI) when the goats died of fulminating parasitemia. The mean terminal PCV of the infected goats at terminal recumbency was 26.01 ± 1.4% while that of control goats at 3 weeks was 31.01 ± 2.83%. Accompanying bone marrow cytological changes in the infected group were characterized by marginal erythroid hyperplasia (Fig. 2) above that of the control group (Table 1). The mean myeloid:erythroid (M:E) ratio was 0.66 ± 0.31:1 for infected goats and 0.89 ± 0.01:1 for control goats. The granulocytic precursors were generally depressed below those of control goats. Those affected included myeloblasts, premyelocytes, myelocytes, metamyelocytes, and segmenters which constitute the marrow granulocytic storage pool or reserve. As a result, the granulocytic maturation rate (ratio of non-mitotic to mitotic granulocytes) of infected goats was 3.15 ± 0.6 while that of control goats was 2.42 ± 0.0. Bone marrow macrophage numbers were significantly higher (P < 0.05) in the infected goats than in control goats. Macrophages constituted 7.0 ± 2.4% of the total numbers of marrow cells enumerated in the infected goats as against 2.5 ± 0.1% in the control goats. Phagocytic activities of the macrophages as demonstrated by light microscopy are summarized on Table 2. 29.02 ± 3.5% of the macrophages phagocytized mature red blood cells (RBC), 2.44 ± 2.07% phagocytized mature or band white blood cells (WBC) (Figs. 3), 1.52 ± 1.30% phagocytized both mature RBCs and WBCs while none phagocytized mitotic immature blood cells. The macrophages in the bone marrow of control goats phagocytized no blood cells. Mitotic figures accounted for 0.9 ± 0.5% of marrow cells in infected goats as against 0.4 ± 0.0% in control goats.
Bone marrow smear of a control Sokoto Red goats showing normal distribution of cells with fewer number of normoblasts (arrowed) and numerous mature granulocytes and myelocytes and that of b the T. vivax-infected group with marginal increase in normoblast numbers (arrowed) and fewer but immature granulocytes
Discussion
The mild nature of anemia in the T. vivax-infected goats characterized by slight drop in PCV and early death of the goats 2 to 3 weeks PI suggest that although the Sokoto Red breed of goats are highly susceptible to T. vivax, death may result from immunologic and other pathogenic factors besides anemia. The corresponding cytological changes in the bone marrow of T. vivax-infected goats though moderate were observable by light microscopy. These changes were similar to those observed in the first phase of T. congolense-infected Boran cattle reported by Anosa et al. (1997a, b) and were characterized by little changes in the erythroid and granulocytic cells. In many cases, erythroid hyperplasia and myeloid hypoplasia are consistent features of bone marrow cytological change in African trypanosomosis, thereby leading to a drop in the myeloid:erythroid ratio and gross expansion of the red marrow in long bones during the acute phase of trypanosome-induced anemia (Anosa 1988a). These were demonstrated in T. vivax-infected calves (Anosa et al. 1992), T. congolense-infected Boran cattle (Anosa et al. 1997a), and T. gambiense-infected vervet monkeys(Abenga 1997) as well as in trypanotolerant N’dama cattle infected with T. vivax and T. congolense (Anosa 1999).
Although there were only slight differences in the erythroid and granulocytic cell numbers in the infected and control goats, there was a marked increase in the number of macrophages in the bone marrow of the infected animals suggesting they played roles in the early susceptibility of this breed of goats to T. vivax (Anosa et al.,1997b). Anosa et al. (1997b) had observed similar macrophage proliferation and activation as early as 15 days post infection before the development of patent parasitemia between 21 and 24 days post infection in T. congolense-infected Boran cattle. This early activation and proliferation of macrophages are believed to arise from indirect mechanisms such as dispersal of trypanosome antigens in the bone marrow and local production through the circulation of cytokines generated by host cells such as T-lymphocytes that are primed by trypanosome or trypanosome products (Anosa et al. 1997b). The macrophages in the bone marrow of infected goats phagocytized mainly mature RBCs and few numbers of neutrophils and eosinophils. This suggests that the phagocytosis of blood cells follows a pattern, and mature cells form the first cell types to be phagocytized while phagocytosis of immature (nucleated RBCs and young WBCs) follows in the chronic phase of trypanosomosis. Cytophagia by MC in the BM of trypanosome-infected animals was first described by Anosa et al. (1992) in T. vivax-infected cattle and has not been described in Sokoto Red goat and may play major roles in precipitating pancytopenia and ineffective hemopoiesis in trypanosome infection. MC phagocytic activities in the BM of trypanosome-infected animals were earlier observed in T. congolense-infected Boran (Anosa et al. 1997b) and N’dama cattle and in vervet monkeys infected with T. gambiense (Abenga 1997). Macrophage hyperplasia in the BM of trypanosome-infected rats also showed correlation with severity of anemia (Abenga et al. 2017) while MCs were reported to play a central role in trypanosomiasis-associated anemia (Stijlemans et al. 2010).
The detailed structural changes and process of phagocytosis by MC in the BM of trypanosome-infected animals have been demonstrated by transmission election microscopy (TEM) (Anosa et al. 1992, 1997b) involving four stages leading to the phagocytosis of cells in the hematopoietic compartment (HC). These include, firstly, target cell to MC attraction which was represented in the nucleated cells by the development of many microvilli extending towards the MCs at the zone of contract. Secondly and apparently occurring concomitantly with the first process was the development of enveloping pseudopodia by the MCs partially surrounding the cells being phagocytized at the zone of contact. Thirdly, there was subsequent adhesion of the cell to the MC with the disappearance of the microvilli previously developed by target cells, and lastly, the complete enveloping of the target cells by pseudopodia of the MCs results to endocytosis of the target cells. Phagocytosis of normoblasts, reticulocytes, and erythrocytes in the bone marrow had been identified as a major cause of dyserythropoiesis and ineffective reticulocyte response to anemia in Africa trypanosomosis (Anosa et al. 1992) besides suboptimal production of erythropoietin. Similarly, phagocytosis of neutrophils and eosinophils and their non-mitotic precursors is known to result in decrease in the marrow granulocyte reserve and contribute to dysgranulopoiesis. Significant increase in the number of mitotic cells in the marrow of T. vivax-infected goats however suggested that there was still optimal production of chemical mediators of hematopoiesis especially erythropoietin and eventual early response to the anemia in the infected goats which were probably still optimal in preventing anemia, arising from infecting trypanosomal activities as recently observed in Wister rats infected with T. brucei brucei (Yusuf et al. 2013). Hyperplasia of macrophages in other organs such as the spleen, liver, testes, lymph nodes, hemolymph nodes, and heart of trypanosome-infected animals (Anosa et al. 1997b) had also been reported.
Results of this investigation demonstrated that early macrophage hyperplasia coupled with early onset in phagocytic activities demonstrated in the Sokoto Red goats may be indication that they play roles in the susceptibility of this breed of goats to Trypanosome vivax. The observation of bone marrow cytological changes in the T. vivax-infected goats also raise the question of what mechanisms regulate the selective or preferential phagocytosis of mature blood cells and not immature cells by macrophages in acute trypanosomosis. Further studies in this area may provide clues to drug development and supportive treatments towards control of anemia in animal trypanosomosis.
References
Abenga JN (1997) Haematological and biochemical studies on green (vervet) monkeys infected with Trypanosoma brucei gambiense. M. V. Sc. Thesis, University of Ibadan, Ibadan, Nigeria
Abenga JN (2015) Effect of flooding on trypanosome infection rates in trade cattle at central abattoir, Makurdi metropolis, Benue State, North Central Nigeria. Annals of Research and Reviews in. Biology 8(5):1–6
Abenga JN, Samdi SM, Fajinmi FO, Kajgo AM (2017) Comparative bone marrow responses of albino rats experimentally infected with single and mixed species of Trypanosoma congolense and T. brucei and ability to control anemia. Afr J Clin Exp Microbiol 18(2):124–128. https://doi.org/10.4314/ajcem.v18i2.11
Adamu UO, Haruna MK, Ovbagbedia RP, Bizi R, Benjamin W, Malala UA, Enwezor FNC, Mohammed M (2011) Control of African trypanosomiasis in Nigeria: time to strengthen integrated approaches. Int J Anim Vet Adv 3(3):138–143
Anosa VO (1988a) Haematological and biochemical changes in human and animal trypanosomiasis part I. Revue Elev Med Vet Pays Trop 41:65–78
Anosa VO (1988b) Haematological and biochemical changes in human and animal trypanosomiasis part II. Revue Elev Med Vet Pays Trop 41:151–164
Anosa VO (1999) Bone marrow functions and pathology in trypanosomiasis in: Organization of African Unity/Scientific Technical and Research Commission Publication No. 119 Pp 298–300
Anosa VO, Logan-Henfrey LL, Shaw MK (1992) A light and electron microscopic study of changes in blood and bone marrow in acute hemorrhagic Trypanosoma vivax infected in calves. Vet Pathol 23:33–45
Anosa VO, Logan-Henfrey LL, Wells CW (1997a) The haematological of Trypanosoma congolene infection in cattle I. Sequential cytomorphological changes in the blood and bone marrow of Boran cattle. Comp Haematol Int 7:14–22
Anosa VO, Logan-Henfrey LL, Wells CW (1997b) The haematological of Trypanosoma congolene infection in cattle II. Macrophage structure and function in the bone marrow of Boran cattle. Comp Haematol Int 7:23–29
Anyaegbunam LC, Okafor OJ (2013) Trypanosomiasis in Red Sokoto and West African Dwarf goats at Ikpa abattoir, Nsukka, Enugu state, Nigeria. J Ent Zool St 1(5):29–39
Cadioli FA, Barnabe PA, Machado RZ, Teixeria MCA, Andre MR, Sampaio PH, Junior OLF, Tiexeria MMG, Marques LG (2012) First report of Trypanosoma vivax outbreak in dairy cattle in Sao Paulo state, Brazil. Revista Brasil Parasitol Vet 21(2):118–124. https://doi.org/10.1590/S1984-29612012000200009
Dacie JV, Lewis SM (1984) Practical haematology, 6th edn. Edinburgh, Churchillis Livingstone
Gimba UN (2015) Studies on the endemicity of pathogenic trypanosomes: an implication for human trypanosomiasis control in Gwagwalada town, FCT, Abuja. Biol Sci 2(12):29–39
Ikegwuonu NC (2013) Goat keeping as an enterprise: training pamphlet. Small Holder Foundation, Owerri
Jones TW, Davila AMR (2001) Trypanosoma vivax—out of Africa. (Review). Trends Parasitol 17(2):99–101. https://doi.org/10.1016/S1471-4922(00)01777-3
Kelly WR (1979) Veterinary clinical diagnosis, 2nd edn. Bailliere Tindall, London
Logan-Henfrey LL, Anosa VO, Wells CW (1999) The role of bone marrow in bovine trypanotolerance. I. Changes in blood and bone marrow in Trypanosoma congolense infected cattle. Com Haematol Int 9:198–207
Lumsden WHR, Herbet WJ, M’Neillage GJC (1973) Technique with trypanosomes. Churchill Livingstone, Edinburgh and London
Nuru S (1984) Paper 1: livestock research in Nigerian sub-humid zone. Proceedings of the second ILCA/NAPRI symposium held in Kaduna, Nigeria, 29th October – 2nd November, 1984. Eds. R. von Kaufmann, S. Chater and R. Blauch, International Livestock Center for Africa, Addis-Ababa, Ethiopia
Omotainse SO, Edeghere H, Omoogun GA, Elhassan EO, Thompson GA, Igweh AC, Ukah JCA, Ikenga MA, Halid I (2000) The prevalence of animal trypanosomosis in Konshisha Local Government Area of Benue State, Nigeria. Israel J Vet Med 55(4):142–144
Onyiah JA (1997) African animal trypanosomes: an overview of current status in Nigeria. Trop Vet 15:111–116
Otuma MO (2005) Evaluation of different crossbreeding programmes, season and sex on breed weight and linear traits of Nigerian goats. J Agric Food Environ Ext 4:35–37
Silva RAMS, Ramires L, Souza SS, Ortiz AG, Pereira SR, Davila AMR (1999) An outbreak of Trypanosoma vivax infection in a dairy herd in the Pantanal, Brazil. Revue Elev Med Vet Pays Trop 52:35–38
Stijlemans B, Vankrunkelsven A, Caljon G, Bockstal V, Guilliams M, Bosschaerts T, Beschin A, Raes G, Magez S, Baetselier PD. (2010) The central role of macrophages in trypanosomiasis-associated anemia: rationale for therapeautical approaches. Endocrine, Metab Imm Disorders-Drug Targets, 10: 000–000
Swallow BM (2000) Impacts of trypanosomosis on African Agriculture. PAAT Technical and Scientific series, vol. 2. FAO, Rome
Yusuf OS, Oseni BS, Olayanju AO, Hassan MA, Adewosun AA, Akele RY (2013) Acute and chronic effects of T. brucei brucei experimental infections on bone marrow and peripheral bleed cells in Wistar rats. Sch. J Appl Med Sci 1(6):1036–1040
Acknowledgements
Technical support was provided by staff of the Diagnostic Laboratory of the Department of Animal African Trypanosomiasis Research while Mallam Abubakar Yahaya took care of the animals.
Funding
This work was funded by the Nigerian Institute for Trypanosomiasis Research, Kaduna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Abenga, J., Idowu, T. Packed cell volume and bone marrow cytological responses in Trypanosoma vivax-induced acute trypanosomosis in Nigerian Sokoto Red goats. Comp Clin Pathol 27, 455–460 (2018). https://doi.org/10.1007/s00580-017-2613-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-017-2613-1