Abstract
African trypanosomosis is a disease of high economic importance in animals and humans caused by an apicomplexan haemoparasite of the genus Trypanosoma. Canine trypanosomosis is relatively common in Nigeria because of the high prevalence of the insect vector. The haematology, erythrocyte, and serum sialic acid levels of dogs experimentally infected with Federe strain of Trypanosoma brucei brucei was studied in a total of five (5) 4-month old dogs. Three of the dogs were randomly selected and inoculated subcutaneously with 1 × 106 of T. brucei, while the remaining two dogs served as the uninfected control. The parasitaemia, packed cell volume (PCV), total and differential white blood cell count, erythrocyte surface sialic acid (ESSA) and free serum sialic acid (FSSA) were determined twice a week. The lymphocyte/monocyte ratio (LMR) and neutrophil/lymphocyte ratio (NLR) were calculated. The infected dogs had a significantly lower PCV, total white blood cell count (TWBC), LMR and ESSA compared to the uninfected control group. There was a strong positive correlation between NLR and PCV decrease, moderate negative correlation between LMR and PCV decrease, strong negative correlation between LMR and ESSA changes, and a moderate positive correlation between ESSA changes and parasitaemia. The higher NLR in trypanosome infection associated with decreasing levels of PCV indicates a poor prognosis, and NLR may be a useful tool in estimating trypanosomosis effect on PCV in dogs. Similarly, the moderate negative and strong negative correlations between LMR with decreases in PCV and ESSA respectively indicates poor prognosis in the response to trypanosomosis in dogs. Thus, NLR, LMR and ESSA levels can be used as markers for predicting disease outcome in trypanosome infection in dogs. This is, however, not the case with parasitaemia as the clinico-pathologic effect of trypanosomosis cannot be estimated from the level of parasitaemia alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trypanosomosis is a protozoan disease of vertebrates caused by an apicomplexan haemoparasite of the genus Trypanosoma. The disease has been reported in all domestic animals and humans, and has economic importance due to losses in production, weight loss, low milk yield, reduced capacity to work and abortion (Murray et al. 1990). Trypanosomosis is recognized as a major barrier to the development of subSaharan Africa (Ohaeri and Eluwa 2011). It is endemic in Africa compared to any other disease in a particular location (Murray et al. 1990) and continues to spread even to areas initially free of trypanosomes and the insect vectors (Majekodunmi et al. 2013).
All breeds of dogs are susceptible to trypanosomosis (Annette et al. 2006; Akpa et al. 2008). Canine trypanosomosis is relatively common in Nigeria because of high prevalence of Glossina species in most parts of the country (Ahmed 2007). Dogs get infected through tsetse fly (Glossina species) bite as well as other biting flies such as tabanids, stomoxys, and triatomid bugs, and ingestion of infected fresh animal carcass (Raina et al. 1985; Uilenberg 1998). The disease in dogs was formally known to be caused by T. cruzi and T. evansi alone (Jones et al. 2000). Currently, several trypanosome species including T. congolense and T. brucei have been reported to cause canine trypanosomosis (Samdi et al. 2006). Canine trypanosomosis caused by T. brucei presents an acute and fatal disease (Ikede and Losos 1972; Taylor and Authie 2004) leading to fever, corneal opacity, severe oedema, myocarditis and central nervous system derangements, aside anaemia which is the most prominent feature of African animal trypanosomosis (Franciscato et al. 2007; Nwoha and Anene 2011). This may be due to extensive tissue damage caused by the extravasation of the parasite into the body tissues, vasculitis, increased vascular permeability, thrombosis and release of sialidase which cleaves erythrocyte sialic acid leading to erythrophagocytosis and anaemia (Esievo et al. 1990).
Some animal breeds such as the Muturu and N’dama cattle have been reported to be trypanotolerant as they can survive, remain productive and gain weight despite trypanosome infection (Naessens 2006). Trypanotolerance in dogs and the role of erythrocyte surface sialic acid in the pathogenesis and pathology of canine trypanosomosis is yet to be substantially studied. And there is limited information on the correlation between the level of trypanosome infection and the clinic-pathologic findings in trypanosomosis.
This study was designed to investigate the correlation between parasitaemia and some haematological parameters, erythrocyte and serum sialic acid changes, and leukocyte ratios in dogs following experimental infection with T. brucei brucei Federe strain.
Materials and methods
Five (5) 4-month old dogs (three females and two males) weighing averagely 4 kg were used for the study. The dogs were purchased from the breeder at 1 month old, acclimatised for three months, during which they were dewormed using Caniverm®, a combination fenbendazole (15 mg/kg), pyrantel emboate (14 mg/kg), and praziquantel (5 mg/kg). The dogs were also vaccinated against canine distemper, hepatitis, leptospirosis, parvoviral enteritis and parainfluenza using a pentavalent DHLPP vaccine before the commencement of the experiment. They were fed and given water ad libitum. The Trypanosoma brucei brucei (Federe strain) was provided by the Nigerian Veterinary Research Institute (NVRI) Vom Jos, Plateau State. The strain of T. brucei was obtained from cattle and subpassaged in Wistar rat before used for the infection of the dogs.
The five dogs were randomly selected and tagged numbers 1, 2, 3, 4, and 5. The numbers 1, 2, and 3 were the infected group and were inoculated subcutaneously with 1 × 106 Federe strain of T. brucei brucei (Herbert and Lumsden 1976), while the remaining two dogs tagged 4 and 5 served as the uninfected control group.
Blood samples were collected twice a week from the dogs for a total of 3 weeks. Five (5) mL of blood was obtained from each animal from the cephalic vein. Two (2) mL of the sample was dispensed into sample bottles containing potassium diamine tetra acetate (K2EDTA) anticoagulant for complete blood count, while 2 mL of the blood sample was dispensed into a sample bottle containing 0.3 mL of freshly prepared acid citrate dextrose as anticoagulant for the preparation of erythrocyte membranes, and 1 mL was dispensed into a plain vacutainer and used for the separation of serum. The obtained sera from centrifugation at 5000g for 5 min was kept frozen (− 20 °C) until when needed for analysis. Haemoglobin-free erythrocyte membranes (erythrocyte ghosts) were prepared as described by Dodge et al. (1963). Complete blood count was determined from the EDTA samples as described by Coles (1986). Parasitaemia (log equivalence values and corresponding number of parasites per mL of blood) was determined as described by Herbert and Lumsden (1976).
The thiobarbituric acid (TBA) assay (Aminoff 1961; Engstler et al. 1995) was used to measure the free serum sialic acid (FSSA). The erythrocyte ghosts were incubated with 0.125 N H2SO4 at 80 °C for 1 h to liberate the bound sialic acid (Warren 1963). The erythrocyte surface sialic acid (ESSA) was thereafter measured as described for FSSA.
Haematological and sialic acid values were reported as mean ± standard error of mean (SEM). The leukocyte ratios (neutrophil:lymphocyte ratio, NLR; lymphocyte:monocyte ratio, LMR) were calculated from their absolute values respectively. Regression analysis was used to compare the parasitaemia with the haematological parameters and sialic acid values obtained for each sampling days as difference of the observed value from the preinfection value. Student t test was used to compare the haematology and sialic acid for the infected and control groups using Graph Pad Prism 5. Values of p ≤ 0.05 were considered significant.
Results
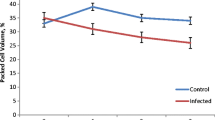
The haematological and sialic acid values in Nigerian dogs infected with T. brucei are shown in Table 1. The scatter plots showing the correlation of PCV decrease and parasitaemia, total white blood cell count TWBC and parasitaemia, ESSA decrease and parasitaemia, and FSSA changes and parasitaemia in dogs are all shown in Figs. 1, 2, 3, and 4 respectively. The mean PCV value for the uninfected group (control) was 31.20 ± 1.24% while that of the infected group was 21.39 ± 1.39%. There was a statistical significant difference between the PCV of the infected and that of the control group (p = 0.0006), and a weak positive correlation between PCV decrease and parasitaemia (r = 0.13). The mean TWBC for the control group was 12.66 ± 2.01 (× 103/μL) while the mean TWBC for the infected group was 6.760 ± 1.05 (× 103/μL). There was a statistical significant difference between the TWBC of the infected and that of the control group (p = 0.0228), and a weak negative correlation between TWBC and parasitemia (r = − 0.25).
The neutrophil lymphocyte ratio (NLR) of the infected group was 0.66 ± 0.13 while that of the control group was 0.51 ± 0.13. There was no statistical significant difference between the NLR of the dogs infected with Trypanosoma brucei and those not infected (p = 0.43). The scatter plot showing the correlation between parasitaemia and NLR, PCV decrease and NLR, and ESSA decrease and NLR are presented in Figs. 5, 6, and 7 respectively. There was a very strong positive correlation (r = 0.85) between PCV decrease and NLR, a weak positive correlation (r = 0.27) between the rate of loss of ESSA and NLR, and a weak negative correlation between parasitaemia and NLR (r = − 0.25). The lymphocyte monocyte ratio (LMR) of T. brucei infected dogs was 32.43 ± 2.57 while that of the control was 48.33 ± 6.35. There was a statistical significant difference (p = 0.04) between the LMR of the infected and those of the control group. The scatter plot of the correlation between parasitaemia and LMR, PCV decrease and LMR, and ESSA decrease and LMR are shown in Figs. 8, 9, and 10 respectively. There was a moderate negative correlation (r = − 0.47) and a very strong positive correlation (r = 0.92) between PCV decrease and LMR, and ESSA loss and LMR respectively. There was, however, a very weak negative correlation (r = − 0.10) between parasitaemia and LMR.
The mean ESSA for the uninfected control group was 3.37 ± 0.40 mg/mL compared to 2.33 ± 0.26 (mg/ml) for the infected group. There was a slight statistical significant difference between the ESSA of the infected and that of the control group (p = 0.0493). There was a moderate positive correlation between ESSA decrease and parasitaemia (r = 0.41), and a moderate positive correlation between ESSA decrease and PCV decrease (r = 0.43). The mean FSSA of the uninfected control group (1.02 ± 0.13 mg/mL) was not statistically different from that of the infected group (1.26 ± 0.21 mg/ml). A weak negative correlation between the changes in FSSA values and parasitaemia (r = − 0.36) was also observed.
Discussion
Dogs were experimentally infected with the Federe strain of Trypanosoma brucei brucei to study their haematological response as well as the changes in erythrocyte surface sialic acid (ESSA) and free serum sialic acid (FSSA). Following the infection of the dogs with 1 × 106 Trypanosoma brucei, there was a significant decrease in PCV from the preinfection values. The PCV of the infected dogs were significantly reduced compared to the uninfected control group. This agrees with the response of Nigerian local dogs infected with the same strain of trypanosomes as reported by Abenga et al. (2016). Similar PCV response was reported in dogs infected with Trypanosoma congolense (Abenga et al. 2005) and in other livestock species (Franciscato et al. 2007; Nwoha and Anene 2011). Anaemia is a main feature of trypanosomosis in this study, the mean decrease in PCV from the value of the control was 9.76 corresponding to 31.28%. This is less than the 36% postinfection PCV reduction reported by Abenga et al. (2016). This study shows a percentage PCV decrease per parasite (×106) of 1.93% contrary to the 3.82% per Log equivalent value (LEV) reported by Abenga et al. (2016). However, the minimal decrease in PCV of the infected group from the control values may be attributed to the ability of the dogs to control anaemia in canine trypanosomosis. The weak positive correlation (r = 0.13) between PCV decrease and parasitaemia may be due to the migration of the parasites to tissues from the vasculature thereby presenting a false lowered parasite load on the sampling days (Losos 1986; Abubakar et al. 2005).
The total white blood cell (TWBC) count of the infected dogs decreased by 46.6% from the control value, and 2.92% per parasite (× 106). This is slightly higher than the 43.6% drop in TWBC reported by Abenga et al. (2016) and 0.22 TWBC drop per LEV of parasitaemia. The TWBC decrease in this study agrees with reports of T. brucei infection by Anosa (1988) and Abenga et al. (2016) but disagrees with Igbokwe and Anosa (1989) in sheep T. vivax infection where leucocytosis was reported. The leukopenia observed may be due to granulopoiesis depression, increased tissue demand for leucocytes, or phagocytosis in liver, bone marrow and spleen (Taylor and Authie 2004).
Response of neutrophil and proinflammatory cytokines in acute inflammation such as observed in T. brucei infection can be exaggerated, and therefore needs the modulatory effect of antiinflammatory or immunomodulatory cytokines in order to avoid overt tissue damage. Lymphocytes (particularly B lymphocytes) produce antibodies against infectious agents including trypanosomes. They also have immunoregulatory function through the release of interleukin (IL)-10, IL-4, and IL-5 which are antiinflammatory cytokines that control inflammation in order to achieve immunity with minimal immunopathology (Powrie et al. 1993; O’Garra and Murphy 1994). Higher levels of neutrophil count with decreased lymphocyte count could lead to an unregulated inflammatory response capable of tissue damage. Infection of dogs with T. brucei led to increased NLR but not significantly. However, the strong positive correlation between the drop in PCV and NLR indicates that the more severe the anaemia, the higher the NLR, indicating a poor prognosis following T. brucei infection.
The Federe strain of T. brucei infection in dogs showed a significant decrease in LMR and a moderate negative correlation with PCV decrease. The decreased LMR may be due to an increase level of monocytes in circulation in response to the infection. Monocytes emigrate into the tissues to form macrophages in trypanosomosis to clear the damage caused by trypanosomes and their sialidases, including necrotized cells and erythrocytes damaged by parasitaemia (Cnops et al. 2015; Stijlemans et al. 2015). The relative monocytosis and lymphopenia (decreased LMR) may also be responsible for the strong negative correlation between LMR and ESSA loss in this study, as more monocytes may be released to phagocytise erythrocytes which have been damaged by the cleavage of their ESSA by the parasites. This indicates a poor prognosis in dogs Trypanosoma brucei infection.
The erythrocyte surface sialic acid (ESSA) significantly differs in the control group when compared to the infected dogs. This is in agreement with Useh et al. (2007) in dogs and Ode et al. (2017) in cattle. This study shows a moderate positive correlation (r = 0.4) between PCV decrease and ESSA loss whereas Useh et al. (2007) observed a strong correlation (r = 0.68) between the losses in PCV and ESSA in dogs administered Clostridium chauvoei and Trypanosoma vivax sialidase. In this study, we report a moderate positive (r = 0.41) correlation between parasitaemia and ESSA loss. The moderate correlation observed in this study may be due to tissue migration of the parasite. It may also suggest that migrated parasites in tissues are able to produce sialidase which continue to cleave off ESSA despite the parasite’s absence in blood circulation. Erythrocytes found to have lost 10–20% of their ESSA are phagocytized and cleared from blood circulation giving rise to anaemia (Jancik and Schauer 1974). Results from this study can moderately predict that parasite load of 5.6 × 106/mL T. brucei (Federe strain) can cause a 10% decrease in ESSA and consequently lead to anaemia in dogs. However, the extent of the clinic-pathological damages caused by trypanosomosis in dogs cannot be directly quantifiable by the estimation of parasitaemia, and vice versa.
There was no significant increase in free serum sialic acid (FSSA) due to infection with T. brucei in dogs. This is similar to the report of Ode et al. (2017). There was also a weak negative (r = − 0.36) correlation between FSSA and parasitaemia. Blood stream forms of trypanosomes utilize part of the cleaved ESSA to form their variable surface glycoprotein which enables them to evade host’s immune response to the parasite (Taiwo and Ogunsanmi 2000; Coustou et al. 2012). The utilization of some of the cleaved sialic acids by the parasite may be responsible for the weak correlation in FSSA build-up in response to ESSA cleavage by the increased parasitaemia.
Conclusion
This study provides information on the correlation between parasitaemia and some haematological and sialic acid values in dogs infected with Federe strain of T. brucei. NLR is a strong positive indicator of anaemia, while LMR can be a strong negative predictor of erythrocyte desialylation and both NLR and LMR could be good prognostic parameters in canine trypanosomosis. The pattern of deviation of erythrocyte and serum sialic acid in relation to parasitaemia may be useful in comparing susceptibility to trypanosome infection in dogs and other animal species, where trypanotolerance may be a function of the number of parasites required to cause 10–20% decrease in ESSA.
References
Abenga JN, Ezebuiro CO, Kehinder D, Fajinmi AO, Samdi S (2005) Studies on anaemia in Nigerian local puppies infected with Trypanosoma congolense. Vet Arh 75(2):65–174
Abenga JN, Ode SA, Agishi G (2016) Haematological derangement patterns in Nigerian dogs infected with Trypanosoma brucei: a simple prototype for assessing tolerance to trypanosome infections in animals. Afr J Clin Exp Microbiol 17(1):25–34
Abubakar A, Iliyasu B, Yusuf AB, Igweh AC, Onyekwelu NA, Shamaki BA, Afolayan DO, Ogbadoyi EO (2005) Antitrypanosomal and haematological effects of selected Nigerian medicinal plants in Wistar rats. Biokemistri 17:95–99
Ahmed AB (2007) High trypanosome infections in Glossina palpalis palpalis in Southern Kaduna State, Nigeria. Sci World J 2(2):1–7
Akpa PO, Ezeokonkwo RC, Eze CA, Anene BM (2008) Comparative efficiency assessment of pentamidine isethionate and diminazene in dogs. Vet Parasitol 151:139–149
Aminoff D (1961) Methods of quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J 81:384–392
Annette KM, Erick A, Sheldon EL, Harry RH, Christiane ML (2006) Serological diagnosis of Trypanosoma cruzi: evaluation of three enzyme immunoassays and an indirect immunofluorescent assay. J Med Microbiol 55:171–178
Anosa VO (1988) Haematological and biochemical changes in human and animal trypanosomiasis part II. Rev Elev Med Vet Pays Trop 41:151–164
Cnops J, De Trez C, Stijlemans B, Keirsse J, Kauffmann F, Barkhuizen M, Keeton R, Boon L, Brombacher F, Magez S (2015) NK, NKT and CD8derived IFNγ drives myeloid cell activation and erythrophagocytosis, resulting in trypanosomosis associated acute anemia. PLoS Pathog 11(6):e1004964. https://doi.org/10.1371/journal.ppat.1004964
Coles EH (1986) Veterinary clinical pathology, 4th edn. W.B. Saunders Co, Philadelphia
Coustou V, Plazolles N, Guegan F, Baltz T (2012) Sialidases play a key role in infection and anaemia in Trypanosoma congolense animal trypanosomiasis. Cell Microbiol 14(3):431–445
Dodge JT, Mitchell C, Hanachan DJ (1963) The preparation and chemical characteristics of haemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys 100(1):110–130
Engstler M, Reuter G, Schauer R, Brun R (1995) Purification and characterization of a novel sialidase found in procyclic culture forms of Trypanosoma brucei. Mol Biochem Parasitol 54:21–30
Esievo KAN, Jaye A, Andrews JN, Ukoha AI, Eduviet LO, Saror DI, Njoku CO (1990) Electrophoresis of bovine erythrocyte sialic acids: existence of additional band in trypanotolerant N’Dama cattle. J Comp Pathobiol 102(4):357–361
Franciscato C, Lopes STA, Teixeira MMG, Monteiro SG, Wolkmer P, Garmatz BC (2007) Cao naturalmente infectado por trypanosome evansi em santa maria, RS, Brasil. Ciênc Rural 37(1):288–291
Herbert WJ, Lumsden WHR (1976) Trypanosoma brucei: a rapid “matching” method for estimating the host’s parasitaemia. Exp Parasitol 40:427–431
Igbokwe IO, Anosa VO (1989) Leucopenia in Trypanosoma vivax infection of sheep. Rev Elev Med Vet Pays Trop 42(2):219–221
Ikede BO, Losos GJ (1972) Spontaneous canine trypanosomiasis caused by T. brucei: meningo-encephalomyelitis with extravascular localization of trypanosomes in the brain. Bull Epizoot Dis Afr 20(3):221–8
Jancik J, Schauer R (1974) Sialic acid- a determinant of the life-time of rabbit erythrocytes. Hoppe Seylers Z Physiol Chem 355:395–400
Jones TC, Hunt RD, King NW (2000) Molestias causadas por protozoarios. In: Jone TC, Hunt D, King NW (eds) Patologia Veterinaria. 6thEd. Manole, Sao Paulo, pp 559–610
Losos GJ (1986) Infectious tropical diseases of domestic animals. Churchill Livingstone Inc., New York
Majekodunmi AO, Fajinmi A, Dongkum C, Picozzi K, MacLeod E, Thrusfield MV, Shaw APM, Welburn S (2013) Social factors affecting seasonal variation in bovine trypanosomiasis on the Jos Plateau, Nigeria. Parasit Vectors 6(293):1–9
Murray M, Trail JCM, D’Iteren GDM (1990) Trypanotolerance in cattle and prospects for the control of trypanosomiasis by selective breeding. Rev Sci Tech OIE 9(2):369–386
Naessens J (2006) Bovine trypanotolerance: a natural ability to prevent severe anaemia and haemophagocytic syndrome? Int J Parasitol 36(5):521–528
Nwoha RIO, Anene BN (2011) Clinical signs and pathological changes in dogs with single and conjunct experimental infections of Trypanosoma brucei brucei and Ancylostoma caninum. J Vet Parasitol 24(2):91–102
Ode S, Adamu M, Taioe M, Thekisoe O, Adamu S, Saror DI (2017) Molecular occurrence of trypanosomes, erythrocyte and serum sialic acid concentrations of Muturu and Bunaji cattle in Benue State, Nigeria. Veterinary Parasitology 242:10–13
O’Garra A, Murphy K (1994) Role of cytokines in determining T-lymphocyte function. Current Opinion in Immunology 6 (3):458–466
Ohaeri CC, Eluwa MC (2011) Abnormal biochemical and hematological inices in trypanosomiasis as a threat to heard production. Vet Parasitol 177:199–202
Powrie F, Menon S, Coffman RL (1993) Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunityin vivo. European Journal of Immunology 23(11):3043–3049
Raina AK, Kumar R, Rajora VS, Sridhar SRP (1985) Oral transmission of Trypanosoma evansi infection in dogs and mice. Vet Parasitol 18(1):67–69
Samdi SM, Abenga JN, Kalgo AM (2006) Trypanosomosis in hunting dogs in Kaduna, North Central Nigeria; Implications on the disease in humans. J Biomed Investig 4:15–18
Stijlemans B, Cnops J, Naniima P, Vaast A, Bockstael V et al (2015) Development of a pHrodo TMbased assay for the assessment of in vitro and in vivo erythrophagocytosis during experimental trypanosomosis. PLoS Neglect Trop D 9(3):e0003561. https://doi.org/10.1371/journal.pntd.0003561
Taiwo VO, Ogunsanmi AO (2000) Erythrocyte membrane Sialoglycoproteins. I. Qualitative and quantitative differences in zebu and taurine cattle and buffalo. J Appl Anim Res 18:199–208
Taylor KA, Authie ML (2004) Pathogenesis of animal trypanosomiasis. In: Maudlin I, Holmes PH, Miles MA (eds) The trypanosomiases. CABI Publishing, pp 331–353
Uilenberg G (1998) A field guide for the diagnosis, treatment and prevention of African animal trypanosomosis. FAO Rome, 158
Useh NM, Adenike IA, Adeiza AA, Nok AJ (2007) Erythrocyte surface sialic acid levels of clinically healthy mongrel and exotic (Alsatian and Terrier) breeds of dogs. Glycoconj J 24:491–495
Warren L (1963) Thiobarbituric acid assay of sialic acids. Meth Enzymol 6:463–465
Acknowledgments
The authors are grateful to the laboratory staff of the Department of Veterinary Parasitology and Entomology, College of Veterinary Medicine, University of Agriculture, Makurdi, for technical assistance in the laboratory work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ode, S.A., Acham, M.F., Anike, W.U. et al. Correlation between some haematological parameters, leukocyte ratios, parasitaemia and their prognostic value in canine trypanosomosis. Comp Clin Pathol 30, 129–135 (2021). https://doi.org/10.1007/s00580-021-03203-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-021-03203-6