Abstract
Biochar may alleviate plant water stress in association with arbuscular mycorrhizal (AM) fungi but research has not been conclusive. Therefore, a glasshouse experiment was conducted to understand how interactions between AM fungi and plants respond to biochar application under water-stressed conditions. A twin chamber pot system was used to determine whether a woody biochar increased root colonisation by a natural AM fungal population in a pasture soil (‘field’ chamber) and whether this was associated with increased growth of extraradical AM fungal hyphae detected by plants growing in an adjacent (‘bait’) chamber containing irradiated soil. The two chambers were separated by a mesh that excluded roots. Subterranean clover was grown with and without water stress and harvested after 35, 49 and 63 days from each chamber. When biochar was applied to the field chamber under water-stressed conditions, shoot mass increased in parallel with mycorrhizal colonisation, extraradical hyphal length and shoot phosphorus concentration. AM fungal colonisation of roots in the bait chamber indicated an increase in extraradical mycorrhizal hyphae in the field chamber. Biochar had little effect on AM fungi or plant growth under well-watered conditions. The biochar-induced increase in mycorrhizal colonisation was associated with increased growth of extraradical AM fungal hyphae in the pasture soil under water-stressed conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For Mediterranean or subtropical rain-fed cropping systems, drought frequency and duration are likely to outweigh beneficial projections of cereal crop growth enhancement by increases in CO2 concentration and temperature (Turner et al. 2011). For these systems in southwestern Australia, mean annual soil moisture is expected to decrease by about 25 % over the next 20 years, which is likely to cause changes in soil biological processes (Borken and Matzer 2009; Talmon et al. 2011). By 2030, it is predicted that the area affected by drought in this region will at least double, and may experience twice the frequency of drought (Kirono et al. 2011). Thus, potential benefits of soil amendments to improve the effectiveness of access to water in soil, as may occur through enhanced mycorrhizal colonisation, requires further investigation.
Arbuscular mycorrhizal (AM) fungi are widely claimed to be able to aid in alleviation of plant water stress under soil water deficit conditions (Ruiz-Lozano et al. 2012). This is likely to occur via a combination of mechanisms, either directly associated with enhancing plant water uptake or indirectly through modification of the rhizosphere soil environment (Augé 2001; Ruiz-Lozano 2003; Mickan 2014). One hypothesised direct mechanism is that extraradical mycelium facilitates access to microsites of water/nutrients within soil pores or beyond the root depletion zone, which are inaccessible to roots or root hairs (Khalvati et al. 2005; Ruth et al. 2011). Indirectly, AM fungi may improve the water holding capacity of soil by contributing to stabilisation of soil aggregates and by protecting soil organic matter in association with their extensive network of extraradical hyphae (Augé 2001; Rillig 2004; Gianinazzi et al. 2010), which entangles or otherwise interconnects soil particles depending on the proportion of coarse soil particles (Degens et al. 1996; Tisdall and Oades 1982). Mechanisms by which hyphal strands bind soil particles include the secretion of glomalin, a ‘sticky’ proteinaceous hydrophobic substance (Gianinazzi et al. 2010; Simard et al. 2012). Based on the understanding of these interactions, it has been claimed that AM fungi can be more important to plant growth under dry conditions than when soil moisture and nutrients are adequate (Al-Karaki et al. 2004).
Biochars prepared from a variety of organic sources are receiving attention because of their potential to improve soil fertility and water holding capacity (Lehmann and Joseph 2009; Atkinson et al. 2010; Joseph et al. 2010). Some biochars may directly increase the water holding capacity of soil through an increase in porosity and surface area (Verheijen et al. 2010), but biochars do not all have the same porosity (Jaafar et al. 2015) and can differ in their ability to improve soil water holding capacity depending on their origin and pyrolysis temperature (Jeong et al. 2016). Thus, incorporating biochar with appropriate chemical and physical properties into soil may enable plants to withstand water stress in drought-prone areas (Kammann et al. 2011; Baronti et al. 2014; Das and Sarmah 2015; Ojeda et al. 2015). Indirect benefits of biochar may also occur due to increased colonisation of roots by AM fungi (Warnock et al. 2007), which influences nutrient availability to plants (Taffouo et al. 2013) and soil water retention through enhancement of soil structure (Ogawa and Okimori 2004; Atkinson et al. 2010). These potential benefits have been interpreted as improved drought resistance associated with greater efficiency of water and nutrient uptake (Ruiz-Lozano et al. 1995; Augé 2004). Most studies have used controlled conditions, but it has been claimed that biochar may alleviate plant water stress under soil water deficit conditions in association with AM fungi under field conditions (Solaiman et al. 2010). However, biochar may not change or may even decrease mycorrhizal colonisation (e.g. Warnock et al. 2010; Nzanza et al. 2012), but this has not been investigated under water-limiting conditions.
If application of biochar can increase soil water holding capacity (Ogawa and Okimori 2004; Karhu et al. 2011) and promote growth of AM fungi under soil water deficit conditions (Warnock et al. 2007; Lehmann et al. 2011; Liang et al. 2014), this could be important for agriculture where drier conditions are predicted, such as in southwestern Australia (Hall et al. 2010). Biochar may enable extraradical hyphae to access microsites of phosphorus that may be inaccessible to plant roots due to their larger diameter (Hammer et al. 2014). However, the potential benefits of interactions between biochar and AM fungi have not been demonstrated in water-stressed field soils with a naturally occurring community of AM fungi.
A twin chamber microcosm experiment was used in the present study to simulate effects of biochar on root colonisation of subterranean clover and on extraradical AM fungal hyphae production in a pasture soil under water stress. It was hypothesised that the biochar would increase mycorrhizal colonisation of roots and plant growth under water stressed conditions and more AM fungal hyphae would be formed in the soil. Increased mycorrhizal colonisation could arise from increased hyphal growth in the soil (see Fig. 1 in Abbott and Gazey 1994) initiated by interactions between AM fungi and biochar surfaces as for biochar stimulation of growth of Rhizoctonia solani (Copley et al. 2015). This hypothesis accords with evidence of increased growth of AM fungi inside roots associated with biochar (Jaafar 2014; Vanek and Lehmann 2014; Elzobair et al. 2016) and hyphal proliferation as more roots become colonised (Wilson 1984). For situations where biochar does increase mycorrhizal colonisation, this could also occur via direct stimulation of colonisation of roots by AM fungi, leading to more AM fungal hyphae in soil, although there is little evidence of this (Jaafar et al. 2014). Indirect influences of biochar on AM fungal proliferation in roots or in soil could result from biochar stimulating root growth (Bruun et al. 2014; Abiven et al. 2015; Hammer et al. 2015).
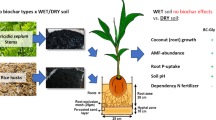
Schematic representation of the twin chamber microcosm separated by a central 38-μm mesh to allow hyphal penetration from the field chamber (containing pasture soil) to the bait soil chamber (containing irradiated pasture soil) and showing the location of the plants (Trifolium subterraneum). The field chamber was amended with either 0 or 2 % w/w biochar. Both chambers shared the same water treatments
Materials and methods
Soil and biochar sources
A field soil was selected based on P availability between locations with different land use histories at The University of Western Australia Farm Ridgefield near Pingelly, Western Australia (S 32° 30′ 23″–E 116° 59′ 31″). The site chosen had been cropped the previous year with wheat and was currently in annual pasture. The agricultural soil is classified as a sandy loam Grey Orthic Tenosol (Isbell 2002). Dry field soil (0–10 cm) was sampled on 9 July 2012, and chemical analyses (Table 1) showed that it had a relatively low P availability for an agricultural soil in the region. The soil was sieved (<4 mm), thoroughly mixed, and half was treated with 25 kGy gamma irradiation to eliminate AM fungi (Stotzky and Mortensen 1959), and the chemical composition was reanalysed. Biochar was produced by Simcoa Pty Ltd. at 600 °C for 24 h from Jarrah (Eucalyptus marginata Sm.) wood, with a high carbon and low nutrient content (Table 1). The biochar source had previously been shown to increase colonisation of roots by AM fungi in a rainfed cropping system (Solaiman et al. 2010).
Experimental system design
A twin chamber microcosm system was used. Pots, constructed from non-transparent PVC-plates, consisted of two uniform non-draining soil chambers of 120 mm × 180 mm × 60 mm (H × L × W) and volume of 800 mm3. A common wall consisting of two layers separated the two chambers: one nylon net with a pore size of 38 μm and another 8-mm nylon grid (Fig. 1). The pore size of the 38-μm nylon net allowed fungal hyphae to pass this barrier whilst excluding plant roots. The 8-mm nylon grid gave support to the nylon mesh, preventing the weight of soil and root penetration breaching the separating barrier. Field soil was placed in one chamber (field chamber) and the same irradiated field soil in the other (bait chamber). The adjacent bait chamber was used to detect AM fungal hyphae passing from the field soil through the mesh barrier (which excluded roots) into the irradiated soil using bait plants and to quantify extraradical hyphal growth in response to biochar addition to the field chamber.
Biochar was incorporated homogeneously into the field soil at a rate of 0 and 2 % w/w prior to placing in the field chamber. All potted soil was maintained at 55 % field capacity (FC) until day 20, when water treatments were initiated. Both chambers, and each soil treatment (0 and 2 % biochar), received either a water-stressed or well-watered treatment equivalent to 30 and 60 % FC, respectively, and were thereafter maintained for the rest of the experiment. A soil water retention curve was used to calculate the field capacity of the soil, where 100 % field capacity was −10 kPa and permanent wilting point was −1500 kPa. Control soil and soil amended with either 0 or 2 % biochar was firmly packed to a bulk density of 1.4 g/cm3 into a core of 54 mm in diameter and 10 mm in height and saturated with water at atmospheric pressure, and its gravimetric water content was determined. Triplicate batches of identical, saturated samples were transferred to porous ceramic plates, placed in pressure chambers, and subsequently equilibrated at matric potentials of −10, −100, and −1500 kPa. Gravimetric water contents were measured after equilibration and then adjusted to volumetric values using bulk density.
Subterranean clover (Trifolium subterraneum L.) was used as a donor and bait plant because it was an existing pasture legume where the soil was collected. Seeds of subterranean clover (var Seaton Park) were soaked in 50 % ethanol/50 % H2O overnight, and rinsed with deionised water. Three pairs of seeds were planted at a 1-cm depth, at increasing distances (1, 5 and 9 cm) from the separating mesh barrier on 10 July 2012 (Fig. 1). No rhizobial inoculum was used because the subterranean clover plants growing in the pasture from where the soil was collected were known to be well nodulated (personal observation). Pots were placed under trace element glasshouse conditions, under well-watered (60 % FC) and water-stressed (30 % FC) conditions, according to the experimental design.
The experiment used a factorial design, consisting of two factors (biochar and water), each with two levels (+/− biochar for field chamber, water stressed/well watered for all chambers). Because there is a complex relationship between root colonisation by AM fungal communities and extraradical hyphal growth over time, three destructive harvests for each treatment (field and bait chamber) were performed at 35, 49, and 63 days after seedling emergence (DAE). A randomised complete block design was selected to accommodate three replications of each treatment at each destructive harvest (two water treatments, either +/− biochar (field chamber)) to give a total of 36 microcosms. Verification that irradiation of the bait soil eliminated AM fungi was tested using one microcosm at each harvest with irradiated soil in both chambers.
Plant growth and AM fungal root colonisation assessment
At each harvest, shoots were cut from each pot and roots were washed free of soil and organic matter for both the field and bait chambers. Shoot and root biomass were recorded after oven drying at 70 °C for 72 h for plants from each side of the barrier. Both fresh and dry weights of roots were recorded. Prior to drying root subsamples, 0.2-g fresh weight of bait plants at distances of 1, 5 and 9 cm from the mesh, and two 0.2-g samples from the field chamber, were taken to assess mycorrhizal colonisation. Root subsamples were cut into ∼1-cm pieces and stained for assessment of AM fungal colonisation (Abbott and Robson 1981; Giovannetti and Mosse 1980) by scoring more than 100 intercepts at ×100 magnification at 35, 49, and 63 DAE. The occurrence of morphotypes of AM fungi was noted at the time of mycorrhizal assessment (Abbott 1982; Shi et al. 2012).
Extraradical hyphal length assessment
Soil cores (1-cm diameter) were taken from within the bait chamber at distances of A: 1, B: 5 and C: 9 cm (Fig. 1) from the separation barrier and to a depth of 7 cm. A single soil core was taken from the centre of the field chamber. On removal, all soil cores were stored in a dark cool room at 5 °C until aqueous hyphal extraction within 14 days of each harvest. Hyphal length in the soil was measured as described by Jakobsen et al. (1992).
Plant P analysis
Oven-dried shoot and root material from field and bait chambers was ground and digested in 3:1 HNO3-HClO4 (Johnson and Ulrich 1959) and the P concentration in the digest measured by the molybdenum-blue method (Murphy and Riley 1962). Shoot P uptake per chamber was calculated by multiplying shoot P concentration by total shoot weights.
Statistical analysis
The experimental design used presents a typical factorial experiment with three main factors: soil, water and harvest. To aid better understanding and interpretation of the data, the factors soil and water were combined in a one factor treatment, consisting of four levels: (i) well-watered/biochar (WWBC), (ii) water-stressed/biochar (WSBC), (iii) well-watered/no biochar (WWNB) and (vi) water-stressed/no biochar (WSNB). The main effects of treatment, harvest and their interaction were of primary interest.
Two types of statistical models were used for data analyses: an unbalanced ANOVA and a linear mixed model (repeated measures analysis). The latter was used to model the correlation structure of the measurements performed on the same pot at different distances (1, 5 and 9 cm) from the central mesh barrier extending into the bait chamber. The response variables analysed were total foliar P uptake in pots, foliar P uptake concentration, root biomass, shoot biomass, AM fungal colonisation (%) and hyphal length and mycorrhizal root weight. Where responses exhibited homogeneous error in the variance of residuals, a logarithmic or square root transformation were applied in order to stabilise the variance. All analyses were performed using GenStat 15th Edition (2013, VSN International, UK) and R 3.01 (R Core Development Team, Austria, 2013).
Results
Field chamber (with naturally occurring AM fungi)
Plant growth
Application of biochar to the pasture soil decreased total shoot mass by 27 %, 35 days after emergence (DAE) in the water-stressed treatment in the field chamber (Fig. 2a). By 49 DAE, there was no effect of biochar application on shoot mass. However, by 63 DAE, biochar application increased mean shoot mass by 25 %, within the water-stressed treatment (Fig. 2a). Root mass of well-watered plants in the field chamber was increased by biochar application at 49 DAE with no effect at the other harvests (Fig. 2b). For the well-watered treatment, biochar application had no effect on either dry shoot or root biomass within the field chamber, at any harvest (Table 2).
Total dry shoot (a) and dry root mass (b) of Trifolium subterraneum harvested at 35, 49 and 63 days after emergence (DAE) for the water-stressed (30 % field capacity) treatment in the field chamber. BC = 2 % w/w biochar, NB = 0 % w/w biochar applied to the field chamber. Error bars show standard errors of the mean (n = 3); *p < 0.05
AM fungal colonisation
For the field chamber exposed to the water stress treatment, biochar application increased the percentage root length colonised by AM fungi at 35 DAE (by 47 %) and at 63 DAE (by 26 %) (Fig. 3a). Under water-stressed conditions, percentage root length colonisation decreased from 35 and 49 to 63 DAE, both with and without biochar application (Fig. 3a). Biochar amendment in the well-watered treatment had no effect on AM fungal colonisation (Table 2).
AM fungal colonisation of roots of Trifolium subterraneum (% root length colonised) (a) and hyphal length in soil (b) for the field chamber at 35, 49 and 63 days after emergence (DAE) for the water-stressed (30 % field capacity) treatment. BC = 2 % w/w biochar, NB = 0 % w/w biochar applied to the field chamber. Error bars show standard errors of the mean (n = 3); *p < 0.05
Extraradical hyphal length
At 35 DAE under water stress, hyphal length in the field chamber did not respond to biochar application (Fig. 3b). However, biochar application increased hyphal length at 49 DAE (by about 30 %) and at 63 DAE (by about 46 %) in the water-stressed soil (Fig. 3b). For well-watered soil, biochar application had no effect on hyphal length until 63 DAE, where it was increased by 64 % (Table 2).
Plant P uptake
Foliar P uptake by subterranean clover growing in the field chamber under water stress was unaltered by addition of biochar at 35 and 49 DAE, but it was increased by 24 % at DAE 63 (Fig. 4a). Under this condition, foliar P concentration increased with biochar application and was 20 % higher at the first harvest (35 DAE). There was no difference in foliar P concentration at consecutive harvests (49 and 63 DAE) for the water-stressed plants (Fig. 4c). However, foliar P uptake and concentrations in well-watered plants growing in the field chamber decreased with biochar application at 63 DAE with biochar application by about 36 % at 63 DAE (Table 2).
Phosphorus uptake into foliage of Trifolium subterraneum in the water-stressed (30 % field capacity) treatment for a field chamber ± biochar, b adjacent bait chamber (no biochar) and c foliar P (%) for the field chamber. BC = 2 % w/w biochar, NB = 0 % w/w biochar applied to the field chamber. Error bars show standard errors of the mean (n = 3); *p < 0.05; ***p < 0.001
Bait chamber
Plant growth and P uptake
Shoot mass in the bait chamber increased when biochar was added to the adjacent field chamber (Supplementary Fig 1). In this case, under water stress, shoot biomass increased by 33 % at DAE 35 and by 21 % at DAE 49, but at DAE 63, there was no effect of biochar application. There was no effect of biochar application to the field chamber on plant growth in the bait chamber for well-watered soil (Supplementary Fig 1). There was no effect of biochar application to the field chamber on root mass of plants in the bait chamber for either water-stressed or well-watered conditions (Supplementary Fig. 2). Foliar P uptake by subterranean clover growing in the bait chamber increased when biochar was applied in the field chamber at DAE 49 by 43 % and DAE 63 by 80 %, under water-stressed conditions (Fig. 4b).
AM fungal colonisation
For both well-watered and water-stressed treatments, percent root length colonised by AM fungi within the bait chamber decreased with increasing distance from the field chamber (Fig. 5). There was no effect of biochar applied to the well-watered field chamber on colonisation in the bait chamber, but there was an effect when the soil was water stressed (Fig. 5). In this case, biochar application to the field chamber altered mycorrhiza formation within the bait chamber in a consistent manner at each harvest. Percent root length colonised in the bait chamber under water stress increased at 35 DAE (128 %), 49 DAE (86 %) and 63 DAE (153 %) in plants at a distance of 1 cm from the mesh barrier when biochar was applied to the field chamber (Fig. 5), but there were no differences in mycorrhizal colonisation at distances of 5 or 9 cm from the mesh barrier for any harvest.
AM fungal colonisation of roots of Trifolium subterraneum (% root length colonised) within the bait chamber with distance (1, 5 and 9 cm) from the mesh barrier at 35, 49 and 63 days after emergence (DAE) for well-watered and water-stressed treatments. WSBC = water stress/biochar, WWBC = well watered/biochar, WSNB = water stress/no biochar, WWNB = well watered/no biochar. Biochar applied to the field chamber only. Error bars show standard errors of the mean (n = 3)
Extraradical hyphal length
Hyphal length in the bait chamber was unaffected by biochar application to the field chamber at 35 DAE for water-stressed soil (Supplementary Fig. 3). By day 49, biochar amendment increased (42 %) hyphal length in the bait chamber at a distance of 1 cm from the mesh barrier, but not at distances of 5 or 9 cm. Biochar application did not alter hyphal length within the well-watered bait chamber (Supplementary Fig 3). For all water and biochar treatments, hyphal length decreased in the bait chamber with increasing distance from the barrier between the field and bait chambers.
Discussion
The twin chamber microcosm designed here to assess the capacity of biochar to influence mycorrhizal colonisation, and hyphal spread in soil has proved useful for investigating how AM fungi may facilitate interactions between biochar and plant growth. Despite the difficulty in identifying AM fungal hyphae in soil (Abbott and Robson 1985), use of this twin chamber microcosm confirmed that changes in hyphal length in the field soil did include changes in length of mycorrhizal hyphae. Previously, biochar-mycorrhizal interactions have been thought to increase plant growth either by direct effects associated with nutrient uptake and water holding capacity of the soil or indirectly through interactions with other beneficial soil biological processes (see reviews by Warnock et al. 2007; Lehmann et al. 2011). The mechanisms of biochar/mycorrhizal fungi interactions hypothesised by Warnock et al. (2007) included biochar serving as a refuge for soil fungi and bacteria, and indeed, it has been shown recently that AM fungi can penetrate and access microsites within biochar pores under artificial conditions (Hammer et al. 2014). However, when biochar was investigated in an agricultural field soil containing naturally occurring AM fungi, there was little evidence that AM fungi hyphae proliferated around biochar particles (Jaafar et al. 2014).
Inconsistencies have been reported in responses of plant growth following amendment of field soil with biochar in relation to root colonisation by AM fungi (Blackwell et al. 2009; Warnock et al. 2010; Nzanza et al. 2012), and mechanisms underlying eventual effects have not been demonstrated for naturally occurring communities of AM fungi in field soil. Indeed, previous experiments investigating mechanisms of effects of biochar on mycorrhiza have only been conducted by inoculating plants with mycorrhizal fungi in soils modified to eliminate the mycorrhizal infectivity (Vanek and Lehmann 2014), in root organ cultures (Hammer et al. 2014), or in sandy soil amended with organic potting media (LeCroy et al. 2013). Under these artificial conditions, biochar has been shown to increase AM fungal colonisation, although this may not translate in increased plant growth in the short term (LeCroy et al. 2013), or to increased P uptake through the AM fungal pathway (Vanek and Lehmann 2014; Hammer et al. 2014).
Biochar-induced increases in the proportion of roots colonised by AM fungi, together with an associated increase in mycorrhizal hyphae and therefore hyphal exploration of a greater volume of soil for water exploration (Mickan 2014), were both possible mechanisms for alleviation of water stress in the subterranean clover plants grown under soil water deficit in the experiment reported here. Consequently, the plants growing in soil amended with biochar would have experienced less water stress and been able to invest more carbon in shoot growth than were plants grown under water-stressed conditions (Quilliam et al. 2012). However, it is not possible to determine whether the increase in extraradical hyphae in soil was a direct response to interactions between AM fungal hyphae and biochar surfaces or to an indirect response of biochar-root interactions that stimulated AM fungal growth inside roots that led to proliferation of hyphae in the soil. There is no direct relationship between the extent of colonisation of roots by AM fungi and the length of AM hyphae in soil (Abbott et al. 1992). The length of hyphae in soil can differ with different species of AM fungi, different quantities of infective inocula and differences in root growth (Abbott and Robson 1985; Abbott et al. 1992).
The present study highlights the importance of including multiple harvests to assess responses that have the capacity to change over time, especially plant and fungal growth (Augé 2001). The initial decrease under water stress in shoot weight in the field chamber with biochar application is consistent with previous observations of subterranean clover showing a negative growth response in both germination and early vigour when exposed to a woody biochar with similar characteristics to that used here (Solaiman et al. 2012). Early negative plant growth associated with biochar has also been observed for sorghum which may be due to parasitism of AM fungi under high nitrogen conditions in the short term (LeCroy et al. 2013). In contrast to water-stressed conditions, there was no significant difference in shoot mass in response to biochar application when the soil was well watered at any of the harvest times. This concurs with other experimental evidence showing no net change in plant growth with biochar application (Prendergast-Miller et al. 2011). Biochar addition under water stress, as used in the present investigation, increased root mass at 49 DAE but had no effect at harvests taken at 35 or 93 DAE. Other studies have shown variable effects of biochar on root growth (Jones et al. 2012; Kammann et al. 2011; Major et al. 2010), and the time of sampling may be partly responsible for these differences.
The decreasing trend in the proportion of roots colonised by AM fungi both with and without biochar application from the first to third harvests within the water-stressed treatment at first seems uncharacteristic of a typical growth relationship. This decreasing trend can be explained by the rate of plant growth compared with the rate of AM fungal colonisation. When plant root weight increases at a rate faster than AM fungi can colonise or spread laterally within the root system, a dilution effect can occur (Abbott and Robson 1984); this may explain the decrease in the proportion of roots colonised with time.
Shoot mass increased within the bait chamber for the first two harvests with the application of biochar to soil under water stress. This is interesting because biochar was placed only in the field chamber and was not present in the bait chamber. Changes in shoot mass in the bait chamber with application of biochar to the field chamber could be attributed to two processes: (i) through increased nutrient transport by mass flow from the biochar through the 38-μm separating mesh and (ii) direct connection between chambers via a mycorrhizal hyphal network. As the biochar used had a low nutrient content and was applied in a relatively small amount (2 % w/w), nutrient diffusion from the field chamber mediated by biochar to the bait chamber was unlikely. It is also unlikely because the soil in the bait chamber was higher in nutrients following irradiation, as has been shown previously (McNamara et al. 2003). In contrast, it is likely that a mycorrhizal hyphal network connected the two chambers, because roots became mycorrhizal in the bait chamber and levels decreased with increasing distance from the adjacent field chamber. Mycorrhizal networks can connect two or more plants allowing resource allocation though a source-sink relationship (Eason et al. 1991).
In conclusion, biochar applied to the agricultural soil with a naturally occurring community of AM fungi used in this study stimulated growth of extraradical hyphae in soil and increased mycorrhizal colonisation of roots. The observed biochar-induced increases in shoot mass when water was a limiting resource could therefore be related to the increase in AM fungal colonisation giving plants greater access to available soil P, as well as a water scavenging activity associated with the increased growth of extraradical hyphae in soil. As the water potential of the soil was the same with and without biochar amendment, it is unlikely that the observed effects on plant growth were related to possible benefits from the water holding capacity of the biochar. It is difficult to differentiate among complex interactions between hyphal growth from AM fungal propagules, growth of roots, their interception by hyphae and changes over time in resource allocation between roots and AM fungal hyphae. Time-course investigations are needed in order to separate the effects of biochar on different stages of the life cycle of AM fungi in agricultural soils differing in water and P availability.
References
Abbott LK (1982) Comparative anatomy of vesicular-arbuscular mycorrhizas formed on subterranean clover. Aust J Bot 30:485–499
Abbott LK, Gazey C (1994) An ecological view of the formation of VA mycorrhizas. Plant Soil 159:69–78
Abbott LK, Robson AD (1981) Infectivity and effectiveness of vesicular arbuscular mycorrhizal fungi: effect of inoculum type. Aust J Agric Res 32:631–639
Abbott LK, Robson AD (1984) The effect of root density, inoculum placement and infectivity of inoculum on the development of vesicular-arbuscular mycorrhizas. New Phytol 97:285–289
Abbott LK, Robson AD (1985) Formation of external hyphae in soil by four species of vesicular‐arbuscular mycorrhizal fungi. New Phytol 99:245–255
Abbott LK, Robson AD, Jasper DA, Gazey C (1992) What is the role of VA mycorrhizal hyphae in soil? In: Read DJ et al (eds) Mycorrhizas in ecosystems. CAB International, Wallingford, pp 37–41
Abiven S, Hund A, Martinsen V, Cornelissen G (2015). Biochar amendment increases maize root surface areas and branching: a shovelomics study in Zambia. Plant and Soil 1–11
Al-Karaki G, McMichael B, Zak J (2004) Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza 14:263–269
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Augé RM (2004) Arbuscular mycorrhizae and soil/plant water relations. Can J Soil Sci 84:373–381
Baronti S, Vaccari FP, Miglietta F, Calzolari C, Lugato E, Orlandini S, Pini R, Zulian L, Genesio L (2014) Impact of biochar application on plant water relations in Vitis vinifera (L.). Eur J Agron 53:38–44
Blackwell P, Riethmuller G, Collins M (2009) Biochar application to soil. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science and technology. Earthscan, London, pp 207–226
Borken W, Matzer E (2009) Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Glob Chang Biol 15:808–824
Bruun EW, Petersen CT, Hansen E, Holm JK, Hauggaard‐Nielsen H (2014) Biochar amendment to coarse sandy subsoil improves root growth and increases water retention. Soil Use Manag 30:109–118
Copley TR, Aliferis KA, Jabaji S (2015) Maple bark biochar affects Rhizoctonia solani metabolism and increases damping-off severity. Phytopathology 105:1334–1346
Das O, Sarmah AK (2015) The love-hate relationship of pyrolysis biochar and water: a perspective. Sci Total Environ 512:682–685
Degens BP, Sparling GP, Abbott LK (1996) Increasing the length of hyphae in a sandy soil increases the amount of water-stable aggregates. Appl Soil Ecol 3:149–159
Eason WR, Newman EI, Chuba PN (1991) Specificity of interplant cycling of phosphorus: the role of mycorrhizas. Plant Soil 137:267–274
Elzobair KA, Stromberger ME, Ippolito JA, Lentz RD (2016) Contrasting effects of biochar versus manure on soil microbial communities and enzyme activities in an Aridisol. Chemosphere 142:145–152
Gianinazzi S, Gollotte A, Binet MN, van Tuinen D, Redecker D, Wipf D (2010) Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20:519–530
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Hall DJM, Jones HR, Crabtree WL, Daniels TL (2010) Claying and deep ripping can increase crop yields and profits on water repellent sands with marginal fertility in southern Western Australia. Soil Res 48:178–187
Hammer EC, Balogh-Brunstad Z, Jakobsen I, Olsson PA, Stipp SL, Rillig MC (2014) A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol Biochem 77:252–260
Hammer EC, Forstreuter M, Rillig MC, Kohler J (2015) Biochar increases arbuscular mycorrhizal plant growth enhancement and ameliorates salinity stress. Appl Soil Ecol 96:114–121
Isbell R (2002) The Australian soil classification (Vol. 4). CSIRO Publishing
Jaafar NM (2014) Biochar as a habitat for arbuscular mycorrhizal fungi. In: Solaiman ZM, Abbott LK, Varma A (Eds) Mycorrhizal Fungi: Use in Sustainable Agriculture and Land Restoration. Soil Biol 41: 297–313
Jaafar NM, Clode PL, Abbott LK (2014) Microscopy observations of habitable space in biochar for colonization by fungal hyphae from soil. J Integr Agric 13:483–490
Jaafar NM, Clode PL, Abbott LK (2015) Soil microbial responses to biochars varying in particle size, surface and pore properties. Pedosphere 25:770–780
Jakobsen I, Abbott LK, Robson AD (1992) External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum. New Phytol 120:371–380
Jeong CY, Dodla SK, Wang JJ (2016) Fundamental and molecular composition characteristics of biochars produced from sugarcane and rice crop residues and by-products. Chemosphere 142:4–13
Johnson CM, Ulrich A (1959) Analytical methods for use in plant analysis. Calif Agric Exp Sta Bull 767:25–78
Jones DL, Rousk J, Edwards-Jones G, DeLuca TH, Murphy DV (2012) Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol Biochem 45:113–124
Joseph SD, Camps-Arbestain M, Lin Y, Munroe P, Chia CH, Hook J, van Zwieten L, Kimber S, Cowie A, Singh BP, Lehmann J, Foidl N, Smernik RJ, Amonette JE (2010) An investigation into the reactions of biochar in soil. Soil Res 48:501–515
Kammann CI, Linsel S, Gößling JW, Koyro HW (2011) Influence of biochar on drought tolerance of Chenopodium quinoa Willd and on soil–plant relations. Plant Soil 345:195–210
Karhu K, Mattila T, Bergström I, Regina K (2011) Biochar addition to agricultural soil increased CH4 uptake and water holding capacity–results from a short-term pilot field study. Agric Ecosyst Environ 140:309–313
Khalvati MA, Hu Y, Mozafar A, Schmidhalter U (2005) Quantification of water uptake by arbuscular mycorrhizal hyphae and its significance for leaf growth, water relations, and gas exchange of barley subjected to drought stress. Plant Biol 7:706–713
Kirono DGC, Kent DM, Hennessy KJ, Mpelasoka F (2011) Characteristics of Australian droughts under enhanced greenhouse conditions: results from 14 global climate models. J Arid Environ 75:566–575
LeCroy C, Masiello CA, Rudgers JA, Hockaday WC, Silberg JJ (2013) Nitrogen, biochar, and mycorrhizae: alteration of the symbiosis and oxidation of the char surface. Soil Biol Biochem 58:248–254
Lehmann J, Joseph S (2009) Biochar for environmental management: science and technology. (Eds J. Lehmann & S. Joseph) Earthscan, Dunstan house 14a St Cross Street London, EC1N 8XA
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43:1812–1836
Liang C, Zhu X, Fu S, Méndez A, Gascó G, Paz-Ferreiro J (2014) Biochar alters the resistance and resilience to drought in a tropical soil. Environ Res Lett 9:064013
Major J, Lehmann J, Rondon M, Goodale C (2010) Fate of soil-applied black carbon: downward migration, leaching and soil respiration. Glob Chang Biol 16:1366–1379
McNamara NP, Black HIJ, Beresford NA, Parekh NR (2003) Effects of acute gamma irradiation on chemical, physical and biological properties of soils. Appl Soil Ecol 24:117–132
Mickan B (2014) Mechanisms for alleviating of water stress involving arbuscular mycorrhizal fungi. In: Solaiman ZM, Abbott LK, Varma A (Eds) Mycorrhizal Fungi: Use in Sustainable Agriculture and Land Restoration. Soil Biol 41:225–239
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Analyt Chim Acta 27:31–36
Nzanza B, Marais D, Soundy P (2012) Effect of arbuscular mycorrhizal fungal inoculation and biochar amendment on growth and yield of tomato. Inter J Agri Biol 14:965–969
Ogawa M, Okimori Y (2004) Pioneering works in biochar research, Japan. Aust J Soil Res 48:489–500
Ojeda G, Mattana S, Àvila A, Alcañiz JM, Volkmann M, Bachmann J (2015) Are soil-water functions affected by biochar application? Geoderma 249:1–11
Prendergast-Miller MT, Duvall M, Sohi SP (2011) Localisation of nitrate in the rhizosphere of biochar-amended soils. Soil Biol Biochem 43:2243–2246
Quilliam RS, Marsden KA, Gertler C, Rousk J, DeLuca TH, Jones DL (2012) Nutrient dynamics, microbial growth and weed emergence in biochar amended soil are influenced by time since application and reapplication rate. Agric Ecosyst Environ 158:192–199
Rillig MC (2004) Arbuscular mycorrhizae, glomalin and soil aggregation. Can J Soil Sci 84:355–363
Ruiz-Lozano JM (2003) Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 13:309–317
Ruiz-Lozano JM, Azcon R, Gomez M (1995) Effects of arbuscular-mycorrhizal Glomus species on drought tolerance: physiological and nutritional plant responses. Appl Environ Microbiol 61:456–460
Ruiz-Lozano JM, Porcel R, Bárzana G, Azcón R, Aroca R (2012) Contribution of arbuscular mycorrhizal symbiosis to plant drought tolerance: state of the art. In: Aroca P (ed) Plant Responses to Drought Stress. Springer, Berlin, pp 335–362
Ruth B, Khalvati M, Schmidhalter U (2011) Quantification of mycorrhizal water uptake via high-resolution on-line water sensors. Plant Soil 342:459–468
Shi P, Abbott LK, Banning NC, Zhao B (2012) Comparison of morphological and molecular genetic quantification of relative abundance of arbuscular mycorrhizal fungi within roots. Mycorrhiza 22:501–513
Simard SW, Beiler KJ, Bingham MA, Deslippe JR, Philip LJ, Teste F (2012) Mycorrhizal networks: mechanisms, ecology and modelling. Fungal Biol Rev 26:39–360
Solaiman ZM, Blackwell P, Abbott LK, Storer P (2010) Direct and residual effect of biochar application on mycorrhizal root colonization, growth and nutrition of wheat. Aust J Soil Res 48:546–554
Solaiman ZM, Murphy DV, Abbott LK (2012) Biochars influence seed germination and early growth of seedlings. Plant Soil 353:273–287
Stotzky G, Mortensen JL (1959) Effect of gamma radiation on growth and metabolism of microorganisms in an organic soil. Proc Soil Sci Soc Am 23:125–127
Taffouo VD, Ngwene B, Akoa A, Franken P (2013) Influence of phosphorus application and arbuscular mycorrhizal inoculation on growth, foliar nitrogen mobilization, and phosphorus partitioning in cowpea plants. Mycorrhiza 24:361–368
Talmon Y, Sternberg M, Grynzweig J (2011) Impact of rainfall manipulations and biotic controls on soil respiration in Mediterranean and desert ecosystems along an aridity gradient. Glob Chang Biol 17:1108–1118
Tisdall JM, Oades J (1982) Organic matter and water-stable aggregates in soils. J Soil Sci 33:141–163
Turner NC, Molyneux N, Yang S, Xiong Y, Siddique KHM (2011) Climate change in south-west Australia and northwest China: challenges and opportunities for crop production. Crop Pasture Sci 62:445–456
Vanek SJ, Lehmann J (2014) Phosphorus availability to beans via interactions between mycorrhizas and biochar. Plant Soil 1–19
Verheijen F, Jeffery S, Bastos AC, van der Velde M, Diafas I (2010) Biochar application to soils—a critical scientific review of effects on soil properties, processes and functions. EUR 24099 EN. Office for the Official Publications of the European Communities, Luxembourg, p 149
Warnock DD, Lehmann J, Kuyper TW, Rillig MC (2007) Mycorrhizal responses to biochar in soil—concepts and mechanisms. Plant Soil 300:9–20
Warnock DD, Mummey DL, McBride B, Major J, Lehmann J, Rillig MC (2010) Influences of non-herbaceous biochar on arbuscular mycorrhizal fungal abundances in roots and soils: results from growth-chamber and field experiments. Appl Soil Ecol 46:450–456
Wilson JM (1984) Comparative development of infection by three vesicular arbuscular mycorrhizal fungi. New Phytol 97:413–426
Acknowledgments
We appreciate the contributions of Ms Aimee Martin and Mr James Gee who volunteered their time in helping in the glasshouse, laboratory and potting rooms. Additionally, we thank the Soil Biology and Molecular Ecology Group at The University of Western Australia for providing the positive environment in which to conduct this experiment.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Dry shoot mass of Trifolium subterraneum in the bait chamber harvested at 35, 49, and 63 days after emergence (DAE) for the (a) water stressed (30 % field capacity) and (b) well watered treatments. BC = 2 % w/w biochar, NB = 0 % w/w biochar applied to the field chamber; WS = water stressed and WW = well watered treatments. Error bars show standard errors of the mean (n = 3). * significance p < 0.05. (PNG 27 kb)
Supplementary Fig. 2
Dry root mass of Trifolium subterraneum in the bait chamber harvested at 35, 49, and 63 days after emergence (DAE) for the (a) water stressed (30 % field capacity) and (b) well watered treatments. BC = 2 % w/w biochar, NB = 0 % w/w biochar applied to the field chamber; WS = water stressed and WW = well watered treatments. Error bars show standard errors of the mean (n = 3). * significance p < 0.05. (PNG 25 kb)
Supplementary Fig. 3
Hyphal length in soil in the bait chamber with distance (1, 5 and 9 cm) from the mesh barrier at 35 and 49 days after emergence (DAE) for well watered and water stressed treatments. WSBC = water stress/biochar, WWBC = well watered/biochar, WSNB = water stress/no biochar, WWNB = well watered/no biochar. Biochar applied to the field chamber only. Error bars show standard errors of the mean (n = 3). (PNG 36 kb)
Rights and permissions
About this article
Cite this article
Mickan, B.S., Abbott, L.K., Stefanova, K. et al. Interactions between biochar and mycorrhizal fungi in a water-stressed agricultural soil. Mycorrhiza 26, 565–574 (2016). https://doi.org/10.1007/s00572-016-0693-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-016-0693-4