Abstract
Biochar amendment of soil may ameliorate inherently infertile soils, such as in the typical coconut (Cocos nucifera L.) growth areas along tropical coasts, where, moreover, temporary moisture stress commonly occurs. We conducted a pot experiment to evaluate the effects of biochar soil amendment (1% w/w) produced from Gliricidia sepium stems (BC-Gly) and rice husks (BC-RiH) on the growth of coconut seedlings and on N and P uptake mediated by mycorrhizae under wet or dry conditions in a Sandy Regosol. The pots were divided into root and hyphal zones by a nylon mesh, where 15N labelled N and P nutrients were only provided in the hyphal zone. Under wet conditions, biochar application did not affect plant growth, while under dry conditions, the BC-Gly increased root and plant growth similar to that under wet conditions. BC-Gly increased the acidic pH of the soil to a neutral level, and the microbial community shifted towards a higher fungal abundance. The P accumulated (Pacc) in roots was higher with BC-Gly and BC-RiH under dry and wet conditions, respectively. Pacc weakly correlated with the abundance of arbuscular mycorrhizal fungi (AMF) in the hyphal zone. With BC-Gly roots showed lower N derived from fertilizer. We conclude that biochar application has no impact on crop growth under wet conditions, while under dry conditions, BC-Gly stimulates crop growth and P uptake, probably through liming induced P availability but also possibly by some enhancement of AMF growth. The shift in the fungal-oriented microbial community and reduced plant fertilizer N uptake suggested that BC-Gly acted as an additional N source.
Graphical Abstract

Highlights
-
Application of biochar under wet moisture condition did not impact coconut seedling growth.
-
Under dry moisture BC-Gly enhanced crop growth similar to wet treatments and plant P uptake.
-
AMF growth was not impacted by biochar amendment aside from a stimulation in dry soil by BC-Gly.
-
The N rich BC-Gly likely provides N to the plants via fungi stimulated biochar decomposition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Poor soil management and continuous cropping of inherently low fertile, limited arable lands in humid tropical areas constitute alarming challenges for agricultural activities in this region (Hartemink 2002). Restoration of these soils is crucial to sustaining the productivity of the cropping systems. Sanchez et al. (2003) identified low nutrient capital, moisture stress, very low organic matter (OM) levels, and poor biological cycling as the major constraints in alleviating tropical soil fertility issues. In this context, coconut (Cocos nucifera L.) is a popular perennial plantation oil crop grown widely in the coastal areas of tropical countries, especially in Sri Lanka. The prevailing strongly weathered sandy soils in these coastal belts display inherently low fertile conditions (Loganathan et al. 1982). Most often, in coconut plantations in Sri Lanka, the topsoil contains less than 10 g kg−1 organic carbon (OC) and 1.3 g kg−1 total N, with plant-available P below 9 mg kg−1 and exchangeable K levels less than 0.12 mg kg−1 (Herath et al. 2007; Loganathan et al. 1982; Mapa 2020). Moreover, due to cation exchange capacity usually as low as 0.5–7.0 cmol(+) kg−1, positively charged nutrients are readily leached out. Not only accelerated soil fertility depletion due to the continuous harvest of nuts throughout the long (average of 60 years) productive lifespan of coconut plantations (Somasiri et al. 2010), but also prolonged droughts causing soil moisture stress with recent climate change effects (Kumarathunge 2014; Naveendrakumar et al. 2018; Siswanto et al. 2016) pose major challenges to the industry. Particularly during the drier months of the year (July–September and January–March), an average amount of 3.5 mm day−1 evapotranspiration surplus in combination with the limited water-holding capacity of the low OM containing, coarse-textured coconut plantation soils endangers productivity. Thus, for several reasons soil fertility restoration is much needed to sustain the productivity and continuity of coconut plantations.
Among the many approaches used (chemical fertilizers with compost or manure, mulching, incorporation of plant residues, and growing cover crops) to ameliorate low-fertile soils, the application of carbon-rich pyrolyzed exogenous organic matter (termed ‘biochar’) has caught the attention of scientists in recent decades due to its recalcitrant nature and long residence time in soil (Spokas 2010). Sufficient scientific evidence is available on the influence of biochar on improving crop performance (Akhtar et al. 2014), increasing soil nutrient availability (Lehmann et al. 2003), reducing nutrient loss through higher cation retention (Downie et al. 2012; Laird et al. 2010), maintaining suitable soil pH in acidic soils (Jeffery et al. 2011), enhancing water retention under moisture stress conditions (Akhtar et al. 2014; Downie et al. 2012) and stimulating biological processes through changes in the soil microbial community and abundance (Ameloot et al. 2013, 2015; Domene et al. 2014; Grossman et al. 2010; Thies and Rillig 2012). Even though the above improvements are directly attributed to the unique characteristics of the applied biochar, the understanding of the mechanisms by which biochar influences soil biological processes mediated by microbes is still very limited (Lehmann et al. 2011). Biological soil fertility is nevertheless of crucial importance for perennial crops, which, in contrast to short annual crops, rely more strongly on symbiotic associations for plant nutrient provision. Moreover, with inherent fast leaching losses of mobile nutrients such as N and K from sandy soils in a tropical environment, the gradual release of nutrients from OM-breakdown is probably also more critical for the growth of a year-round growing crop such as coconut.
A number of mechanisms occur in the rhizosphere environment to make nutrients available to plants from the bulk soil, and microorganisms associated with plant roots play a dominant role (Rengel and Marschner 2005). Arbuscular mycorrhizal fungi (AMF) associations are common with most terrestrial plant species (80%) and are profoundly known to be present under nutrient-limited environments, capable of translocating nutrients (i.e., P and N) to the plant system (Churchland and Grayston 2014; Willis et al. 2013), and under abiotic stress conditions such as drought and salinity (Begum et al. 2019; Gianinazzi et al. 2010). It has been shown that biochar application favors AMF abundance and colonization of roots (Shen et al. 2016; Warnock et al. 2007). In addition, biochar and AMF together can improve plant P availability and help plants overcome moisture stress conditions (Li and Cai 2021; Shen et al. 2016). However, despite these promising first studies on the potential of biochar to improve soil biological fertility and plant growth, at this point care should be taken not to extrapolate the results to other soil/crop/biochar combinations. First, too few studies exist on the effects of biochar on the productivity of perennial crops (to our knowledge, there are no studies on Cocos nucifera L.) for which stimulatory or possible adverse biochar effects on biological fertility likely differ compared to annual crops. For instance, depending on climate perennial crops might face periods with drought as well as sufficient moisture availability. Second, biochar quality depends strongly on the feedstock and on the production process, as does its effect on soil processes. We (Jegajeevagan et al. 2016) previously showed that a substantial contrast exists in the biochemical composition and degradability of biochar produced in a well-controlled pyrolysis reactor and with local artisanal techniques. The net effect of biochar produced from local feedstocks via artisanal pyrolysis will need to be ascertained experimentally. Third, many studies involve external inoculation of AMF/beneficial microorganisms together with biochar (Warnock et al. 2007), which may have shaped an overoptimistic image of the beneficial effects of biochar on soil fertility versus when biochar alone would have been applied in a field situation. Last, it has remained largely unclear to what extent the observed stimulatory effects on crop growth are derived from direct nutrient supply from mineralization of labile biochar constituents or from indirect stimulation of biological soil fertility. While the former mechanism will be only temporary until available nutrient biochar pools are depleted, stimulation of biological fertility may exert a prolonged beneficial effect on perennial crop growth.
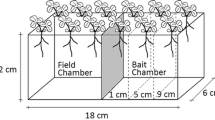
In this study, the following questions were addressed: (i) Does the application of biochar affect coconut crop growth? (ii) Does the application of biochar affect plant nutrient (N and P) uptake? (iii) Is there a beneficial effect on microbial/AMF-assisted nutrition acquisition? These research questions were tested under stressful and normal field moisture conditions to understand the effects of biochar on helping coconut plantations overcome the issues related to drought with current climate change. To elucidate the plant nutrient acquisition mediated by soil microbiota, we used an experimental setup described by Shen et al. (2016) with root-exclusion study containers and modified it to suit coconut seedlings (Fig. 1). The root-exclusion technique was combined with the addition of 15N-labelled KNO3 and Ca3(PO4)2 along with a P diffusion barrier to further differentiate the plant uptake mediated by microbes/mycorrhizae from direct root uptake.
2 Materials and methods
2.1 Soil sampling and characterization
Soil for this study was collected in June, 2018 from a mono-cropped matured coconut plantation situated in the heart of the “Coconut Triangle” in Puttalam District of North Western Province, Sri Lanka (7° 20′ 06.0″ N 79° 52′ 57.8″ E), owned by the Coconut Research Institute of Sri Lanka. The soil belongs to the Great Soil Group of Sandy Regosol (Ustic Quartzipsamments) and is classified under the Madampe soil series, which commonly occurs on depositional surfaces on coastal plains and flood plains in the low country intermediate zone (ca. 30 m.a.s.l), where coconut plantations are concentrated on the island (Mapa 2020). The soil is a moderately well-drained, very deep, sand-textured soil and is also considered highly suitable (land suitability classification for coconut is S1) for coconut cultivation (Somasiri et al. 1994) without any physical limitations for plant growth. Despite this S1 classification, the soil has a very low soil organic carbon content, low nutrient levels and displays poor microbial activity (Nirukshan et al. 2016) (Table 1). This typical low chemical and biological fertility is widespread in the area and thus it could be considered suitable for studying the impacts of biochar on coconut growth and nutrient uptake.
Soil was collected at a depth of 0–30 cm from three random locations in the center squares of monocropped coconut land. The field moist soil was mixed thoroughly, and larger plant debris was removed by sieving through a 6 mm mesh on-site. The soil was then shipped to Ghent University, Belgium. The physicochemical properties and general details of the soil are given in Table 1.
2.2 Biochar production and characterization
The biochar for this experiment was produced in Lunuwila, Sri Lanka, in January–April 2018. Two different biochars from two types of farm-based waste biomass were produced in a farmer-scale biochar production facility of the Coconut Research Institute of Sri Lanka. The so-called ‘Kiln cum Retort’ method was used because it is adoptable by farmers with limited financial means. Feedstock selection for biochar production was performed based on the availability and alternative uses of the particular farm-based waste biomass locally available in the surroundings of the coconut-growing areas. The mature stem part of Gliricidia sepium, a leguminous tree grown intercropped with coconuts, and rice husks were identified as potential suited materials to produce biochar. The moisture content of the biomass was brought to 15–20% through sundrying before pyrolysis. A detailed description of the feedstock selection and production of biochar is given in the Additional file 1. In brief, the biomass was filled inside metal drums (used oil barrels) with securely closed lids, having small holes to facilitate the removal of volatile matter and syn gas while limiting air flow into the drum during pyrolysis. The barrels were then placed into the burning kiln for approximately 3–4 h until the pyrolysis was complete. Completion was determined based on the collection of syngas that escaped from the small holes in the barrels. The temperature inside the barrels during the pyrolysis process was measured frequently using thermocouples, and the maximum temperature reached was between 400 °C and 600 °C. At the end of the pyrolysis, the barrels were opened and the biochar was quenched with water to prevent further combustion and was left to dry in the air for several days until a moisture content of less than 15% was reached. Both biochar types were separately stored in polythene bags until further use. Gliricidia Stick Biochar (BC-Gly) was crushed manually to reduce the particle size before storage. The particle size of the rice husk biochar (BC-RiH) was already quite uniform and small, and therefore, no further crushing was needed. Biochar from both types was then shipped to Ghent, Belgium, for the coconut pot growth experiment.
Three random subsamples from separate polythene bags for each biochar type were taken for biochar characterization (Table 2). Both biochar samples were analyzed for their proximate composition (moisture content, volatile matter, ash and fixed carbon content) according to the method described in Singh et al. (2017). The pH and EC of the biochar samples were measured at a 1:10 (w/v) ratio with deionized water after shaking for 90 min using an electrical conductivity electrode and a pH meter. The specific surface area was determined with a Tristar 3000 gas sorption analyzer (Micromeritics, Inc., Norcross, GA, USA) at 77 K to obtain the internal surface area, pore volume and pore size using BET and BJH theory, respectively. However, the pore volume could not be accurately quantified due to the negative values for the isotherm. Bulk density was assessed following ASTM D 2854-96 standards. The particle size distribution of each biochar sample was analyzed using 50 g of dry sample using a stack of sieves to separate different particle size fractions by shaking for 10 min. The sieve sizes ranged from 0.01 mm to 10 mm. Biochar C and N contents were measured with a CN analyzer (Leco, USA), and the H and O contents were measured using an ONH analyzer (Eltra ONH 2000, Germany). The elemental P, Ca, Mg, and K were detected after subjecting the biochar samples to closed-vessel microwave HNO3 digestion and measured using ICP‒OES (iCAP Pro, Thermo Fisher). The S content was measured following the ASTM C 816-85 standards.
2.3 Coconut-planting materials
Open pollinated coconut seednuts of variety CRIC 60 (Sri Lankan Tall × Sri Lankan Tall) from the Coconut Genetic Resources Centre, Ambakelle, Sri Lanka, were used to propagate seedlings for this experiment. Forty mature seednuts of uniform sizes were selected from the healthy stock of seed coconuts for planting and were air transported under a protected environment (to preserve their viability) to Ghent University, Belgium. Soon after arrival in Belgium on the 15th of September, 2018, the seeds were laid for germination in sand beds in the tropical greenhouse of the Faculty of Bioscience Engineering (Ghent University) in Melle, Belgium. Seeds started to germinate in mid-November, 2018, and were allowed to grow for 2 months in sand beds at which point all plants had reached at least the two-leaf stage. Seedlings were randomized between the various experimental treatments (see Sect. 2.4) based on their height, girth of the nut at the collar region and the number of leaves.
2.4 Greenhouse experiment with the root-exclusion study container technique
A six-and-a-half-month pot experiment with coconut plants was run in the tropical greenhouse of the Faculty of Bioscience Engineering (Ghent University) in Melle, Belgium, from the 15th of January to the 1st of August 2019. The experimental system described by Shen et al. (2016) with soil containers with a root-exclusion zone was used in this study with slight modifications to accommodate coconut seedlings for up to 6–7 months (Fig. 1). The soil containers were split into two compartments, where the upper chamber and lower chamber was separated by a 29 µm root-exclusion nylon mesh. By planting the coconut seedling on the upper part, the root growth was restricted into the upper chamber (the ‘Root zone’). Root access to the bottom part was impeded, but fungal hyphae could pass this barrier freely (the ‘Root-excluded hyphal zone’). The experimental pots were composed of two PVC cylinders (Upper cylinder 25 cm, Bottom cylinder 10 cm, and diameter 25 cm), which were connected by a PVC collar, and the pots were closed at the bottom end with a PVC cap. Plant nutrients were only added to the bottom part where roots could not access and plant nutrient uptake from this compartment occurred through the transfer of nutrients mediated by AMF/microbial processes.
The experiment included biochar-amended treatments prepared from two different feedstocks, Glyricidia sticks (Gly) and rice husks (RiH) (described above in Sect. 2.2) and a nonamended soil as a control. All three biochar treatments were combined with ‘Wet’ and ‘Dry’ soil moisture treatments. For the ‘Wet’ treatment, the soil was maintained at 40% water-filled pore space (WFPS), i.e. near field capacity, a condition that prevails throughout the rainy months of the year between April–June and October–December. For the ‘Dry’ treatment, the soil was maintained at 18% WFPS, representative of the soil moisture levels during the drier months of January–March and July–August. For the biochar-included treatments, sieved biochar (< 10 mm) was mixed homogeneously with soil at 1% w/w dry weight base prior to being filled in both chamber compartments. In addition to biochar, for all treatments, including the control, the soil in the bottom chamber received plant nutrients, viz. 0.69 g of Ca3(PO4)2 and 1.5 g of 15 N-labelled KNO3 with 10% 15N atom excess, equivalent to a field application rate of 28 kg P ha−1 and 42 kg N ha−1, respectively. The soil was filled into the cylindrical chambers to match a bulk density of 1.40 g cm−3 up to a height of 30 cm, of which 10 cm was in the bottom chamber and 20 cm was in the upper root zone. To prevent the nutrients (especially P) from entering the root zone by passive diffusion, a thin layer of 2–3 mm OM-free Iron (Fe)-coated sand was placed below the root-exclusion mesh as a “P-Diffusion barrier”.
Following the assemblage of the pots and filling of soil (with biochar and plant nutrients), two-month-old, two-leaf-sized coconut seedlings were planted in the upper chamber. Due to the presence of the seed nut, the soil in the upper chamber was displaced by the volume of the nut buried into the soil surface. Nevertheless, by adjusting the volume of the buried part, the soil height in the upper chamber was uniformly maintained in all pots at 23 cm. During the first two weeks (14 days) of the experiment all pots were maintained at 50% WFPS to provide the opportunity for the seedlings to recover from the transplanting shock. After the 14th day, the moisture levels described above were continuously maintained. The soil moisture content was monitored by regularly weighing the pots on a field balance and adjusted if needed. The pots were placed in a tropical greenhouse compartment along a randomised complete block design on a single plant growth table. The average air temperature and humidity were 28 °C and 82.2%, respectively.
The experiment had a factorial design, consisting of two factors (biochar and water). Three levels in the Biochar: two types of biochar (BC-Gly and BC-RiH) along with a control (BC-CON), each with two levels of moisture (Dry or Wet conditions) were added in triplicate, bringing the total number of pots to 18.
2.5 Plant growth, harvesting and sampling
At the end of the 6.5-month experimental period, plant-growth parameters such as plant height, number of leaves, and girth at the collar region were measured. Following this, the coconut plants were cut at the collar region, and the aboveground biomass (Shoot) was separated. Following this, the upper compartment was dismantled, and the soil along with the belowground biomass (Roots) was exposed. The root-exclusion mesh and the bottom chamber were carefully observed for signs of breaching of roots into the bottom chamber. The soil attached to the root system from the upper chamber was removed until all the root system was exposed. From the bottom chamber, the P diffusion barrier with Fe-coated sand was carefully removed with a very thin layer of soil, and the remaining soil was further mixed. Soil clods were crushed and sieved through a 2-mm sieve to remove root fragments (for the upper compartment only) and larger biochar particles. From each chamber, representative subsamples were separately taken in appropriate amounts and stored immediately in an ice box until the samples were transported to the laboratory. Thereafter subsamples for phospholipid fatty acids (PLFAs) were stored in a freezer (− 20 °C) and the rest of the sampled soil was stored at 4 °C for nutrient analysis on the following day. The roots growing outside the seed nut and the part of the main/crown roots inside the coconut husk were considered ‘Roots’. The Shoot and Roots samples were transported to the lab for further processing and analysis. Roots were washed with running water to remove soil particles attached to the roots. After washing, a few root fragments from the lateral roots from each experimental unit were sampled and stored in 50% ethanol for the observation of AMF root colonisation without affecting the weight of the root biomass estimation. The shoot and roots were then dried separately at 75 °C to a constant weight and weighed to determine the dry mass.
2.6 Soil pH, soil mineral N and plant-available P
The day after soil sampling, soil mineral N (NH4+-N and NO3–-N) contents were determined from the samples stored at 4 °C. Exchangeable NH4+ and NO3– were determined in 1:5 soil: 1 M KCl extracts by means of a continuous flow auto analyser (Skalar, The Netherlands). A subsample of the soils was air-dried. Soil pH-KCl was measured using a glass electrode in a 1:2.5 soil: KCl suspension, and available phosphorus was assessed by extraction of the soil with ammonium lactate–acetic acid (extraction ratio 1:20) and measured by ICP‒OES.
2.7 Microbial community analysis—PLFA fingerprinting
Soil subsamples stored at − 20 °C were freeze-dried for further phospholipid fatty acid (PLFA) extraction and analysis of the abundance of biomarkers of various soil microbial groups. PLFA extraction was completed following a procedure described in detail by Moeskops et al. (2010). Briefly, lipids were extracted from soil and fractionated into neutral lipids, glycolipids, and phospholipids by means of solid phase extraction and fractionation cartridges. Subsequently, the separated PLFAs were transformed to fatty acid methyl esters by transesterification, which were then redissolved in hexane with its respective 19:0 internal standard. The concentrations of various fatty acid methyl esters in the extracts were finally measured by GC/MS analysis using a Thermo Focus GC coupled to a Thermo DSQ quadrupole MS (Thermo Fisher Scientific Inc. Waltham, USA). PLFA biomarkers were grouped as derived from Gram-positive (G +) bacteria (i14:0, i15:0, a15:0, i16:0, a16:0, i17:0, a17:0 and i19:0), Gram-negative (G-) bacteria (cy19:0, cy19:0, cy17:0, cy17:0 and 18:1ω7c), fungi (18:1ω9, 18:2ω6 and 18:3ω3) and AMF (16:1ω5) (Kozdroj and van Elsas 2001; Sleutel et al. 2012; Yengwe et al. 2018). The 20:5 PLFA was also used as a supportive AMF biomarker to confirm changes in AMF populations (Olsson et al. 1995; Olsson 1999).
2.8 Plant nutrient analysis and 15N analysis
The dried plant samples (shoots and roots) were homogenised in a laboratory scale blender and a subsample of the homogenised material was ground-milled to pass through a 0.4-mm sieve with an ultracentrifugal mill (ZM 200, Retsch Germany) as described by Okito et al. (2004). Cations (K, Ca, Mg and Na) together with P were extracted with 1 N HNO3 after ashing the plant samples, and concentrations were determined using ICP‒OES with an iCap-6300 spectrometer (Thermo Scientific). Plant subsamples (Shoots 3 mg ± 0.5 mg; and Roots 6 mg ± 0.5 mg) were analysed for total N and δ15N using an elemental analyser (ANCA-SL, PDZ Europa, UK) coupled to an isotope ratio mass spectrometer (20–20, SerCon, UK). The % 15N atom excess (% 15 N a.e.) was calculated by subtracting 0.3663 (15N natural abundance) from the measured atom % 15N. The utilization % of 15N-labelled fertilizer was calculated according to Zapata (1990) as the ratio of the N taken up by the plant originating from the fertilizer and the amount of N applied as fertilizer:
where, QNdff is the quantity of the N in the plant sample that is derived from the labelled fertilizer (g Plant−1):
and the total N accumulated in the plant, Nacc (in g N plant−1):
and Ndff, the 15 N enrichment of the plant sample relative to the fertilizer
2.9 Arbuscular mycorrhizal colonisation
The collected fine root fragments were then stained according to a modified protocol of the ink–acid technique of Vierheilig et al. (1998) for microscopic detection of AMF-colonization. For each treatment, 10 bleached and stained root fragments were mounted onto microscope slides and inspected through a compound microscope (Ceti Topic-T, Belgium) at a magnification of × 100–200 for visual observations of AMF structures such as vesicles, arbuscules and hyphae. Based on the observation of either one of these structures, the presence or absence of root infection was noted. This procedure did allow quantification of the extent of root length colonisation per root but was instead used only to confirm the presence of AMF infection in each root sample. The number of AMF-colonized roots out of 10 samples per plant was then compared between treatments.
2.10 Statistics
All presented values are means ± standard errors based on three replicate measurements. Two-way ANOVA was used to determine the effects of biochar (BC-Gly, BC-RiH, and BC-CON) and moisture level (Dry and Wet) and their interactions on plant biomass (shoots and roots), PLFAs of different microbial groups, plant N and P concentrations, % Ndff, QNdff, fertilizer N utilization, soil 15N-NO3− abundance, and soil NO3− concentration. In addition, one-way ANOVA was used to examine the biochar effects within the Dry and Wet treatments. If there were significant treatment effects, individual means were compared using Fisher’s least significance differences (LSD) test. The nonparametric Kruskal–Wallis test was used if the conditions of normality were not fulfilled. Likewise, independent samples t-tests were used to detect differences between the Dry and Wet treatments for individual biochar treatments. All statistical tests were conducted in IBM SPSS Statistics 27 (SPSS, Inc., Chicago, USA).
3 Results
3.1 Biochar characteristics
The chemical properties of the biochar used in this experiment are presented in Table 2. Both biochar types exhibited alkaline pH, with BC-Gly having a higher pH (10.1) than BC-RiH (8.3). BC-Gly also had a 30% higher fixed carbon content and nearly 40% lower ash content than BC-RiH. The nitrogen levels in both biochar types were less than 1%, with nearly similar C/N ratios. Both biochar types contained important plant nutrients, including potassium (K), phosphorus (P), magnesium (Mg), calcium (Ca) and sulfur (S).
3.2 Plant-growth measurements
The effect of biochar amendment on plant growth at the end of the experiment is presented in Fig. 2 for both soil moisture regimes. According to the two-way ANOVA, only the moisture regime significantly (p < 0.05) affected any of the growth parameters tested, and no interaction existed with the biochar factor. Nevertheless, we found that under dry moisture conditions, the belowground root biomass and total plant biomass differed between the biochar treatments (p < 0.05), with higher biomass in the BC-Gly treatment than in the nonamended BC-CON and BC-RiH treatments. Likewise, there were no growth differences among the three biochar treatments under wet moisture condition.
Effect of biochar on coconut seedling growth under dry and wet moisture conditions. a Shoot biomass. b Root biomass. c Total plant biomass given in grams of dry matter per pot. Different lowercase and capital letters indicate significantly different biomasses (One-way ANOVA and LSD; p ≤ 0.05) between biochar treatments for the dry and wet moisture conditions, respectively. Asterisks indicate statistically significant differences (independent samples t-test, p ≤ 0.05) between dry and wet moisture conditions within each biochar treatment; ns- indicates ‘not significant’. Vertical bars represent standard errors of the means (n = 3)
Independent-samples t-tests between dry and wet conditions of a particular biochar treatment showed that plant growth was lower under dry conditions in the case of the BC-Con and BC-RiH (p < 0.05) treatments. Interestingly, no similar effect of moisture condition on the growth of plants existed for the BC-Gly treatment. Although not significant, with BC-Gly under dry conditions, the root/shoot ratio was larger (0.56) than that of the plants grown under wet conditions (0.43). Similar, yet insignificant, trends could also be observed for the other growth parameters tested, such as the number of new leaves developed, girth and height of the plants (data not shown).
3.3 Plant nutrient status
The measured plant N and P contents are presented in the Table 3. Irrespective of the differences observed in plant biomass, only minor statistically insignificant variation existed in the nitrogen accumulated (Nacc) in the plants between biochar treatments. An exception was a significant biochar effect (p < 0.05; Table 3) on the root N-content, which was approximately 2 mg g−1 lower in BC-Gly amended pots than in unamended and BC-RiH treated pots. Plant tissue N concentrations were all affected by soil moisture condition (Shoot, p < 0.05; Root, p < 0.001; and Entire Plant, p < 0.05; Table 3), with nearly 3.18 mg g−1 higher concentrations under dry conditions (Table 4).
In contrast to nitrogen, accumulated phosphorus (Pacc) in plants was significantly affected by soil moisture condition, with approximately a third to nearly doubled Pacc under wet conditions in roots and shoots. Specifically, Pacc in the total plant was higher under wet conditions in the BC-Con and BC-RiH treatments (independent samples t-tests). Biochar treatment did not impact Pacc overall, but one-way ANOVAs of Pacc in the roots in the dry and wet treatments separately showed that with BC-Gly under dry conditions and BC-RiH under wet conditions, Pacc was significantly higher.
The final soil mineral N and plant available P contents are presented in Additional file 1: Table S1. Overall, very low amounts of mineral N were left in the soil at the end of the pot experiment. The soil mineral N content was on average larger in the Dry than in the Wet soils (p < 0.001), in both the root and hyphal zones. In the dry soils, slightly less N was left in the root zone of the BC-Gly pots than in the BC and BC-RH pots (p < 0.05), while less mineral N was left in the hyphal zone of the BC-Zero treatment (p < 0.01). Final plant-available P was generally invariable between moisture and biochar treatments, except for 20–40% lower levels in the root zone of the BC-Zero treatment (p < 0.01).
3.4 Nutrient transfer from the bottom chamber—15N transfer
As shown in Fig. 3 and Additional file 1: Fig. S1, under dry moisture conditions there was a significantly higher plant bottom-chamber N-uptake, irrespective of biochar treatment. Moisture conditions strongly impacted fertilizer N-uptake, with nearly 31% to 37% of added fertilizer N utilized in plant biomass under dry conditions and only 2% to 8% under wet conditions (Fig. 4), resulting in a 6- to 18-fold higher 15N enrichment in both roots and shoots under dry conditions than under wet conditions (Additional file 1: Fig. S1). In addition, the uptake N utilization between above- and belowground biomass also differed, with average enrichment ratios of roots to shoots (R/S) > 1, and a higher ratio (p < 0.05) in case of the dry (1.53) than the wet moisture condition (1.34).
15N derived from 15N labelled fertilizer (Ndff) expressed as the percentage of N in shoots (bars above the x-axis) and roots (bars below the x-axis). Different lowercase and capital letters indicate significantly different means (One-way ANOVA and LSD; p ≤ 0.05) between biochar treatments under dry and wet moisture conditions, respectively. Asterisks * indicate significantly higher values in dry than in the wet moisture condition within a biochar treatment; Vertical bars represent standard errors of the means (n = 3)
The total 15N transferred to the plants as a percentage of added fertilizer N. Different lowercase and capital letters indicate significantly different means (One-way ANOVA and LSD; p ≤ 0.05) between biochar treatments under dry and wet moisture conditions, respectively. Asterisks * indicate significantly higher values for the dry than for the wet moisture condition within a biochar treatment; Vertical bars represent standard errors of the means (n = 3)
The Ndff (expressed as the percentage of the total N accumulated in tissues; Fig. 3) of the roots (6.15–10.62%) was larger than that of the shoots (4.35–6.45%) under dry conditions. Even though moisture appeared to be the dominant factor for the uptake of N from the bottom chamber, there was also a marginal influence of biochar on the percentage of Ndff in roots (Fig. 3) in the dry soils. Namely, there was a lower percentage of Ndff in case of BC-Gly than in the unamended control and BC-RiH grouped together. However, this lower percentage of N derived from fertilizer in roots did not translate into a lower percentage of 15N taken up from added fertilizer (Fig. 4).
3.5 Soil microbial community structure and abundance
The effect of biochar under two different moisture conditions on the abundance of several major microbial groups in the root zone (upper compartment) was assessed by PLFA-analysis (Table 3). In the bottom soil layer, roots were largely excluded via a < 29 µm nylon mesh, allowing only microbial structures in symbiosis with roots such as the extraradical hyphae of AMF, to pass through. To study the overall effect of the treatments on the soil microbial community, the root zone was considered representative of the actual rooting zone of coconut plants. The hyphal zone (bottom compartment) differs from the rhizosphere environment of coconuts and PLFA measurements were used to observe whether biochar × moisture treatments impacted AMF growth in the hyphal zone (Fig. 5).
Effect of biochar on AMF PLFAs 16:1ω5 (Bars) and 20:5 (Marker point) under dry and wet moisture conditions in the hyphal zone (bottom compartment). Different lowercase letters and capital letters indicate significantly different concentrations (nmol g−1 dry soil) of PLFA 16:1ω5 (One-way ANOVA and LSD; p ≤ 0.05) between the biochar treatments under dry and wet moisture conditions, respectively, Vertical bars represent standard errors of the means (n = 3)
In contrast to the plant-growth parameters, the microbial community structure appeared to be affected by the biochar factor only (p < 0.05) in both compartments. Even though the total microbial biomass estimated by the total PLFAs was not affected by biochar application, a significant effect existed on the abundance (in nmol g−1 dry soil) of fungi, Gram-positive, Gram-negative bacteria, and total bacterial PLFAs. Specifically, BC-Gly amendment increased the concentration of fungal PLFAs (p < 0.05) while reducing bacterial PLFAs, including Gram-positive and Gram-negative groups (p < 0.05). Most prominently, the ratio of fungal to bacterial markers was two- to fivefold larger for the Gy-BC treatment than for the control or BC-PH treatment (p < 0.05). This shift toward a more fungal-composed microbial community with the addition of BC-Gly was more apparent under the dry than the wet soil condition although two-way ANOVA did not show a significant biochar x moisture interaction.
In the hyphal zone, biochar treatment had a significant effect on the concentration of AMF-related PLFAs 16:1ω5 and 20:5 (Fig. 5), whereas the moisture factor and the interaction with the biochar factor did not have any impact. Under wet conditions there were significantly higher AMF marker PLFA abundances with BC-RiH, while under dry soil conditions, BC-Gly had the highest AMF marker abundances (Fig. 5). The PLFA 16:1ω5 was used as an AMF biomarker, but it is known not to be entirely specific, as it also occurs in the membrane of Gram-positive bacteria. However, another (Olsson et al. 1995; Olsson 1999) AMF marker, viz. 20:5-PLFA, which does not occur in Gram-positive bacteria, followed a similar pattern, and its concentration correlated well with PLFA 16:1ω5 (R2 of 0.77, Fig. 5). In the attempt to assess root infection in the coconut plants, it was observed that all plants in the experimental unit had AMF infections; however, the sampling procedure did not provide the opportunity to robustly quantify the AMF colonisation rate (see Sect. 2.8).
4 Discussion
4.1 Overall crop-growth response to biochar–soil moisture treatment combinations
The reported biochar impact on seed germination and seedling growth in the literature ranges from inhibition to stimulation. Undesirable substances present in freshly produced biochar such as crystalline silica, dioxin, polyaromatic hydrocarbons (PAHs) and phenolic compounds, are known to adversely impact early plant growth (Dutta et al. 2017; Paymaneh et al. 2018), especially at higher application rates (Ali et al. 2021). In our study with biochar applied at 1% (w/w), such growth retardations were not observed, and the effect of biochar on the coconut seedlings during their early plant growth was rather neutral to positive (Fig. 2). Plant growth at the end of the pot experiment more prominently depended on the established soil moisture condition. The average plant growth under wet conditions was higher than under dry conditions, indicating that moisture was the limiting factor on seedling growth. Generally, coconuts are considered extravagant in regard to water consumption and are indeed very vulnerable to moisture stress during their early growth stages (Liyanage and Mathes 2010). In addition, the growth of the plants under wet moisture conditions was invariable across the control and biochar treatments, suggesting a favorable growth environment with an unimpeded provision of nutrients and water.
Under dry moisture conditions, instead, plant growth depended on biochar treatment. Specifically, the BC-Gly treatment stimulated root growth, which increased the overall plant growth similar to the growth observed in the wet treatment. A number of factors could have co-contributed to this improved root and overall plant growth. First, the increase in the initial very acidic soil pH toward a neutral level (Additional file 1: Table S1) by BC-Gly favored coconut plant growth, as the optimum soil pH for coconuts is 6.4–7.0 (Child 1974). The stronger liming effect of BC-Gly compared to BC-RiH was expected, as biochar produced from legumes (such as Gliricidia sepium) inherits alkalinity from their feedstock, and legumes accumulate more alkali in their plant biomass during growth as a result of the unbalanced uptake of cations and anions compared with non-legumes (Yan et al. 1996). At the same time, in line with our study biochar produced from rice husk has also been reported to have little impact on soil pH (Palansooriya et al. 2019; Yuan and Xu 2011). Second, the unique characteristics of biochar, such as its porous internal structure, high specific surface area, and low bulk density, are some of the factors that improve soil hydraulic properties, helping plants withstand drought conditions (Edeh et al. 2020; Pratiwi and Shinogi 2016; Singh Karam et al. 2022; Wakamiya et al. 2022). BC-Gly might accordingly have reduced plant moisture stress in the typical low SOC sandy soil used here. However, then a likewise plant stimulatory effect should have occurred with BC-RiH, which was not the case. In fact, with its finer particle size and higher specific surface area, compared to BC-Gly (Table 1), BC-RiH should have more effectively improved soil water properties (Edeh et al. 2020), refuting the idea that the BC-Gly stimulatory effect on coconut was related to an alleviation of moisture stress. Pratiwi and Shinogi (2016) showed that biochar produced from rice husk applied below a 2% (w/w) rate did not improve the available water content of the soil. Here biochars were added at a rate of only 1%; therefore, it seems logical that soil water provision would have been insufficiently improved by either the BC-Gly or BC-RiH treatment. Third, biochar amendment may have directly or indirectly improved plant nutrient provisioning, as will be further discussed in Sects. 4.2 and 4.3.
4.2 Plant P-uptake and AMF development
The stimulated root and overall growth with BC-Gly added under dry conditions coincided with nearly doubled Pacc (Table 3) in the roots of the BC-Gly treatment, indicating that plant P-provisioning was improved. This may logically have been the result of direct P-delivery from biochar or, alternatively, the stimulation of AMF association and P-uptake from the root-exclusion compartment. The lower final soil plant-available P level of the root zone in the pots with no biochar added (Additional file 1: Table S1) supports this.
From a meta-analysis, Xiang et al. (2017) and Zou et al. (2021) concluded that biochar often induces root growth and thereby also root P accumulation. Indeed, in the dry BC-Gly treatment, root biomass nearly doubled compared to the BC-RiH and control pots. However, we may probably exclude that this expanded rooting system on its own per se stimulated P uptake, given that the soil that was used for this experiment was very low in plant available P (Table 1). More importantly, the coconut roots coiled together to form a thick root mat that laid on top of the root-exclusion mesh. If biochar acted as the main source of P, such a concentration of roots should not occur given that biochar was uniformly mixed in the upper chamber. Moreover, the total P content of the BC-Gly was considerably lower than that of the BC-RiH, for which instead no stimulatory effect on root and plant growth was observed. Fertilizer P was only added to the bottom chamber, separated by the 29 µm mesh to prevent root penetration (Fig. 1) and thus largely also P-uptake. Zoysa et al. (1997) observed root mat formation as well in a root study container with Camellia japonica L. and found that this created a rhizosphere environment just below the mesh, favoring direct nutrient uptake from this region. However, in our experiment, such direct uptake of P from the bottom chamber by passive movement of P across a concentration gradient was prevented by the establishment of an Fe-coated sand layer that acted as a diffusion barrier. Therefore, it is unlikely that the roots would have directly accessed the P in the bottom soil chamber in our experiment and that promotion of the rooting system by BC-Gly directly would have improved soil P uptake from the root-exclusion compartment.
A stimulatory effect of BC-Gly on AMF development could alternatively have led to higher Pacc in the roots among the dry condition treatments. The somewhat higher AMF abundance in the hyphal zone with BC-Gly under dry conditions (p < 0.05) (Fig. 5) indeed suggests that the possibility of plants accessing the bottom chamber via AMF association was improved with BC-Gly addition. However, the presence of AMF PLFA biomarkers does not necessarily indicate the presence of active root infection of AMF. Therefore, we attempted to quantify and compare the presence of AMF infection in the coconut roots via microscopic observations as well. Such infections were confirmed, but their extent could not be reliably determined in this experiment. This was due to the unsuccessful depigmentation of heavily pigmented 2nd order lateral roots, leaving only the possibility to visualize the very fine 3rd order roots, which had low colonization in terms of length of root fragments observed. Such fine lateral roots are known to lack the development of cortical cells and are thus less actively involved in indirect nutrient absorption (Lee et al. 2004; Gutjahr and Paszkowski 2013; Valverde-Barrantes et al. 2016). Moreover, although root Pacc and AMF abundance showed a similar trend across all treatments, the positive correlation between these two variables was not strong (R = 0.42; p = 0.059). Regardless, we may conclude that the application of BC-Gly did not adversely affect AMF development. The significant increase in the soil pH by BC-Gly could have improved conditions for AMF development, but it is not possible to deconvolute this effect from other biochar effects, as increasing acidic soil pH by application of strongly alkaline biochar is also known to increase soil P availability (Cui et al. 2011). BC-Gly and BC-RiH increased the acidic pH of the soil to neutral (6.4–6.9) and slightly acidic (4.3–5.03) levels (Additional file 1: Table S1), respectively. Particularly with BC-Gly amendment, optimal conditions for P-availability (from added fertilizer or already present in soil) were created, while fixation by soluble Al and perhaps Fe as well and their hydrous oxides would still have limited P-availability with BC-RiH addition. Although differences in final plant available P content of the hyphal zone were statistically insignificant, its ordination across treatments viz. BC-Gly > BC-RiH > BC-Zero supports this interpretation (Additional file 1: Table S1).
4.3 Plant N uptake and AMF development
There was a significantly higher N utilization of the 15N isotope labelled fertilizer from the root inaccessible bottom chamber in the dry treatment compared with the wet treatment (Figs. 3 and 4). Similar to P, this may have been the result of the promoted uptake of water and nutrients by AMF. Such mycorrhiza supported uptake of NO3− from dry soil was also proven by Tobar et al. (1994) for lettuce with a somewhat similar experimental setup. However, as we observed no relationship between AMF abundance and the quantity of plant-N derived from fertilizer (QNdff), nor with final soil mineral N in the hyphal zone (Additional file 1: Table S1), this seems rather unlikely. In fact, most studies, including the one by Tobar et al. (1994), proved a contribution of AMF to N acquisition with external soil AMF-inoculation only, which was not the case in our experiment. It should be considered that, unlike much less mobile phosphate, upward convective transport or diffusion of NO3− from the lower to the upper soil compartment instead contributed to plant fertilizer-N uptake. However, such diffusive transport of NO3− from the bottom to upper chamber should have been larger under wet moisture conditions, while in fact, fertilizer N uptake was in contrast a factor of eight times lower, strongly suggesting that its contribution to plant-uptake was minimal. At the end of the experiment, there was also no contrast in soil mineral N between both compartments in the wet treatment (Additional file 1: Table S1), although such a gradient would have been required to enable upward diffusive transport. More likely, a transpiration pull of water and dissolved nitrate from the underlying root-excluded compartment might have caused the overall extra fertilizer N uptake when the soil was maintained under dry conditions (Gomes and Prado 2007). The very low final soil mineral N levels in the root compartment, equivalent to just 2–5 kg N ha−1, clearly demonstrated that plants would have required this extra N for their growth.
Although adding biochar did not affect total fertilizer plant N uptake, it did impact the percentage of fertilizer N used by roots vs. native soil N under dry conditions (Figs. 3 and 4). Strikingly, under dry conditions, although BC-Gly amendment increased root growth and Pacc in the roots, it lowered the percentage of Ndff compared to the control and BC-RiH treatments. This suggests that the application of BC-Gly under dry conditions reduced plant nitrogen stress to some extent. BC-Gly could have acted as a microbial substrate and N source, thus in part relieving the need for fertilizer N uptake, which was lower in the BC-GL dry treatment, while still improving coconut crop growth and overall N uptake (Table 3). As a leguminous tree crop, Gliricidia sepium feedstock yielded higher N levels in the BC-Gly biochar than in the BC-RiH biochar. Moreover, the volatile matter content of the BC-Gly biochar was double that of the BC-RiH biochar, and as explained before, it was more alkaline. All three parameters (N content, volatile matter content and pH) have been linked to increased soil N supply following biochar amendment (Ameloot et al., 2013), indicating that BC-Gly in particular would have improved coconut N availability by its partial mineralization. In this context, it is noteworthy that within the six-and-a-half-month span of this experiment, BC-Gly application strongly shifted the microbial community toward a fungal dominant structure, while this was not the case for BC-RiH. It would seem unlikely that this shift toward more fungi was simply driven by the increase in soil pH caused by biochar amendment, as it is generally accepted that the biomass of fungi relative to bacteria is instead higher in more acidic soils because of the greater tolerance of fungi to acidity (Bååth and Anderson 2003). In general, most studies have found that biochar favors bacteria over fungi due to such an increase in pH (Ameloot et al. 2013; Dai et al. 2021; Pathy et al. 2020). Aside from altering the soil pH, biochar could have altered the microbial community by constituting a microbial substrate. Recalcitrant, high C/N ratio substrates are indeed colonized by fungi, as they are able to use them more efficiently than bacteria (Hunt et al. 1987; Newman 1985). This has been attributed to greater rates of production of extracellular cellulolytic enzymes, lower nitrogen requirements (Alexander 1978), and an overall ability to colonize nonlabile substrates more rapidly than bacteria (Gray and Baxby 1968; Tribe 1960). The larger share of volatile matter and thus likely bioaccessible part of BC-Gly compared to BC-RiH may explain why similar shifts in the PLFA fungi to bacteria ratio did not occur with BC-RiH. Owing to their lower N-requirement and higher C/N compared with bacteria, this fungal-dominated degradation of BC-Gly would logically have stimulated net N mineralization. From the generally very low soil mineral N levels in all treatment combinations and compartments that were moreover observed only once (at the end of the experiment), it is not possible to prove or disprove this interpretation. A soil incubation study employing 13C- and 15N-labelled biochar (produced from 13C-pulse-labelled and 15N-fertilized plants) could be used instead to verify that BC-Gly acted as N-source.
5 Conclusion
The application of biochar to coconut growing Sandy Regosols appears to have no impact on crop production when the water supply is guaranteed, but, depending on the biochar type, it can stimulate root and crop growth when plants are continuously faced with water stress. It seems unlikely that such an effect is caused by improved water supply with biochar added. It rather seems tied to a liming effect and improvement of nutrient availability. The exclusive stimulatory effect of BC-Gly on coconut crop root biomass and alongside higher root Pacc could be tied to a liming-caused increased P-availability. Here we found indications that such could at least partly have been due to stimulated AMF-growth, but this conclusion remains tentative, pending further experimental proof. The observed lower utilization of fertilizer N with BC-GLy added might be due to a direct N-fertilizer effect of the BC-Gly itself and/or alternatively stimulated native SOM N mineralization. The observed shift toward a more fungal-oriented microbial community suggests the former, but unequivocal proof would require the use of 15N-labelled biochar and closer follow-up of plant N uptake alongside soil mineral N. These positive plant-growth promotional effects of BC-Gly under dry moisture conditions could be considered promising for coconuts grown on low SOC sandy soils. Last, the N-fixing Glyricidia sepium legume tree would appear to form an ideal low-cost biochar feedstock, as it is N-rich and very common to large parts of the (Sub)Tropics.
Availability of data and materials
Data has been included as additional files and will be made available on reasonable request.
References
Akhtar SS, Li G, Andersen MN, Liu F (2014) Biochar enhances yield and quality of tomato under reduced irrigation. Agric Water Manag 138:37–44. https://doi.org/10.1016/J.AGWAT.2014.02.016
Alexander M (1978) Introduction to soil microbiology. John Wiley and Sons, New York
Ali L, Xiukang W, Naveed M et al (2021) Impact of biochar application on germination behavior and early growth of maize seedlings: insights from a growth room experiment. Appl Sci 11:1–13. https://doi.org/10.3390/app112411666
Ameloot N, De Neve S, Jegajeevagan K et al (2013) Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol Biochem 57:401–410. https://doi.org/10.1016/j.soilbio.2012.10.025
Ameloot N, Sleutel S, Das KC et al (2015) Biochar amendment to soils with contrasting organic matter level: effects on N mineralization and biological soil properties. GCB Bioenergy 7:135–144. https://doi.org/10.1111/gcbb.12119
Bååth E, Anderson TH (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol Biochem 35:955–963. https://doi.org/10.1016/S0038-0717(03)00154-8
Begum N, Qin C, Abass Ahanger M et al (2019) Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front Plant Sci. https://doi.org/10.3389/fpls.2019.01068
Child R (1974) Coconuts, 2nd edn. Longman Group Ltd, London
Churchland C, Grayston SJ (2014) Specificity of plant-microbe interactions in the tree mycorrhizosphere biome and consequences for soil C cycling. Front Microbiol 5:1–20. https://doi.org/10.3389/fmicb.2014.00261
Cui HJ, Wang MK, Fu ML, Ci E (2011) Enhancing phosphorus availability in phosphorus-fertilized zones by reducing phosphate adsorbed on ferrihydrite using rice straw-derived biochar. J Soils Sediments 11:1135–1141. https://doi.org/10.1007/s11368-011-0405-9
Dai Z, Xiong X, Zhu H et al (2021) Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes. Biochar 3:239–254. https://doi.org/10.1007/s42773-021-00099-x
Domene X, Mattana S, Hanley K et al (2014) Medium-term effects of corn biochar addition on soil biota activities and functions in a temperate soil cropped to corn. Soil Biol Biochem 72:152–162. https://doi.org/10.1016/J.SOILBIO.2014.01.035
Downie A, Crosky A, Munroe P (2012) Physical properties of biochar. In: Biochar for Environmental Management: Science and Technology. Taylor and Francis, pp 13–32
Dutta T, Kwon E, Bhattacharya SS et al (2017) Polycyclic aromatic hydrocarbons and volatile organic compounds in biochar and biochar-amended soil: a review. GCB Bioenergy 9:990–1004. https://doi.org/10.1111/gcbb.12363
Edeh IG, Mašek O, Buss W (2020) A meta-analysis on biochar’s effects on soil water properties—new insights and future research challenges. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.136857
Gianinazzi S, Gollotte A, Binet MN et al (2010) Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20:519–530. https://doi.org/10.1007/s00572-010-0333-3
Gomes FP, Prado CHBA (2007) Ecophysiology of coconut palm under water stress. Braz J Plant Physiol 19:377–391. https://doi.org/10.1590/S1677-04202007000400008
Gray TRG, Baxby P (1968) Chitin decomposition in soil: II. The ecology of chitinoclastic micro-organisms in forest soil. Trans Br Mycol Soc 51:293–309. https://doi.org/10.1016/S0007-1536(68)80064-6
Grossman JM, O’Neill BE, Tsai SM et al (2010) Amazonian anthrosols support similar microbial communities that differ distinctly from those extant in adjacent, unmodified soils of the same mineralogy. Microb Ecol 60:192–205. https://doi.org/10.1007/s00248-010-9689-3
Gutjahr C, Paszkowski U (2013) Multiple control levels of root system remodeling in arbuscular mycorrhizal symbiosis. Front Plant Sci 4:1–8. https://doi.org/10.3389/fpls.2013.00204
Hartemink AE (2002) Soil science in tropical and temperate regions—some differences and similarities. Adv Agron 77:269–292. https://doi.org/10.1016/S0065-2113(02)77016-8
Herath HMIK, Saumyakumara APA, Tennakoon NA (2007) Soil fertility status of common coconut growing soil series in Sri Lanka
Hunt HW, Coleman DC, Ingham ER et al (1987) The detrital food web in a shortgrass prairie. Biol Fertil Soils. https://doi.org/10.1007/BF00260580
Jeffery S, Verheijen FGA, van der Velde M, Bastos AC (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric Ecosyst Environ 144:175–187. https://doi.org/10.1016/J.AGEE.2011.08.015
Jegajeevagan K, Mabilde L, Gebremikael MT et al (2016) Artisanal and controlled pyrolysis-based biochars differ in biochemical composition, thermal recalcitrance, and biodegradability in soil. Biomass Bioenerg 84:1–11. https://doi.org/10.1016/j.biombioe.2015.10.025
Kozdroj J, van Elsas JD (2001) Structural diversity of microorganisms in chemically perturbed soil assessed by molecular and cytochemical approaches. J Microbiol Methods 43:197–212
Kumarathunge D (2014) Extreme temperature events in three major coconut growing agroecological regions of Sri Lanka. In: Proceedings of the Fifth Symposium on Plantation Crop Research. pp 287–298
Laird D, Fleming P, Wang B et al (2010) Biochar impact on nutrient leaching from a midwestern agricultural soil. Geoderma. https://doi.org/10.1016/j.geoderma.2010.05.012
Lee KJ, Park H, Lee IS (2004) Morphology of arbuscular mycorrhizal roots and effects of root age and soil texture on the mycorrhizal infection in Panax ginseng C.A. Meyer. J Ginseng Res 28:149–156
Lehmann J, Pereira da Silva Jr J, Steiner C, et al (2003) Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments
Lehmann J, Rillig MC, Thies J et al (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43:1812–1836. https://doi.org/10.1016/j.soilbio.2011.04.022
Li M, Cai L (2021) Biochar and arbuscular mycorrhizal fungi play different roles in enabling maize to uptake phosphorus. Sustain 13:1–15. https://doi.org/10.3390/su13063244
Liyanage LVK, Mathes DT (2010) Effect of irrigation on establishment and early growth of coconut (VAR.CRIC 60) in the dry zone of Sri Lanka. Cocos 7:1. https://doi.org/10.4038/cocos.v7i0.2057
Loganathan P, Dayaratne PMN, Shanmuganathan RT (1982) Evaluation of the phosphorus status of some coconut-growing. J Agric Sci 99:25–33. https://doi.org/10.1017/S002185960005499X
Mapa RB (2020) Soil research and soil mapping history. In: Mapa RB (ed) The soils of Sri Lanka. Springer International Publishing, Cham, pp 1–11
Moeskops B, Sukristiyonubowo BD et al (2010) Soil microbial communities and activities under intensive organic and conventional vegetable farming in West Java, Indonesia. Appl Soil Ecol 45:112–120. https://doi.org/10.1016/j.apsoil.2010.03.005
Naveendrakumar G, Vithanage M, Kwon HH et al (2018) Five decadal trends in averages and extremes of rainfall and temperature in Sri Lanka. Adv Meteorol. https://doi.org/10.1155/2018/4217917
Newman EI (1985) The rhizosphere: carbon sources and microbial populations. Ecol Interact Soil Plants, Microbes Anim 107–121
Nirukshan GS, Herath I, Wijebandara I, Dissanayake PD (2016) Soil microbial population and activity affected by fertilizer and manure addition in a coconut growing Sandy Regosol. In: Vidhanaarachchi VR., Herath HMI., Meedahakumbura M., et al. (eds) 6th Symposium on Plantation Crop Research– “Plantation Agriculture towards National Prosperity.” Coconut Research Institute, Lunuwila, Sri Lanka, pp 163–172
Okito A, Alves BRJ, Urquiaga S, Boddey RM (2004) Isotopic fractionation during N2 fixation by four tropical legumes. Soil Biol Biochem 36:1179–1190. https://doi.org/10.1016/j.soilbio.2004.03.004
Olsson PA (1999) Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol Ecol 29:303–310. https://doi.org/10.1016/S0168-6496(99)00021-5
Olsson PA, Bååth E, Jakobsen I, Söderström B (1995) The use of phospholipid and neutral lipid fatty acids to estimate biomass of arbuscular mycorrhizal fungi in soil. Mycol Res 99:623–629. https://doi.org/10.1016/S0953-7562(09)80723-5
Palansooriya KN, Ok YS, Awad YM et al (2019) Impacts of biochar application on upland agriculture: a review. J Environ Manage 234:52–64. https://doi.org/10.1016/j.jenvman.2018.12.085
Pathy A, Ray J, Paramasivan B (2020) Biochar amendments and its impact on soil biota for sustainable agriculture. Biochar 2:287–305. https://doi.org/10.1007/s42773-020-00063-1
Paymaneh Z, Gryndler M, Konvalinková T et al (2018) Soil matrix determines the outcome of interaction between mycorrhizal symbiosis and biochar for Andropogon gerardii growth and nutrition. Front Microbiol 9:1–16. https://doi.org/10.3389/fmicb.2018.02862
Pratiwi EPA, Shinogi Y (2016) Rice husk biochar application to paddy soil and its effects on soil physical properties, plant growth, and methane emission. Paddy Water Environ 14:521–532. https://doi.org/10.1007/s10333-015-0521-z
Rengel Z, Marschner P (2005) Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytol 168:305–312. https://doi.org/10.1111/j.1469-8137.2005.01558.x
Sanchez PA, Palm CA, Buol SW (2003) Fertility capability soil classification: a tool to help assess soil quality in the tropics. Geoderma 114:157–185. https://doi.org/10.1016/S0016-7061(03)00040-5
Shen Q, Hedley M, Camps Arbestain M, Kirschbaum MUF (2016) Can biochar increase the bioavailability of phosphorus? J Soil Sci Plant Nutr 16:268–286. https://doi.org/10.4067/S0718-95162016005000022
Singh Karam D, Nagabovanalli P, Sundara Rajoo K et al (2022) An overview on the preparation of rice husk biochar, factors affecting its properties, and its agriculture application. J Saudi Soc Agric Sci 21:149–159. https://doi.org/10.1016/j.jssas.2021.07.005
Siswanto S, van Oldenborgh GJ, van der Schrier G et al (2016) Temperature, extreme precipitation, and diurnal rainfall changes in the urbanized Jakarta city during the past 130 years. Int J Climatol 36:3207–3225. https://doi.org/10.1002/joc.4548
Sleutel S, Bouckaert L, Buchan D et al (2012) Manipulation of the soil pore and microbial community structure in soil mesocosm incubation studies. Soil Biol Biochem 45:40–48. https://doi.org/10.1016/j.soilbio.2011.09.016
Somasiri LLW, Nadarajah N, Amarasinghe L, Gunathilake HA. (1994) Land suitability assessment of coconut growing areas in the coconut triangle. Coconut Research Institute of Sri Lanka
Somasiri LLW, Wijebandara DMDI, Panditharathne BDP et al (2010) Loss of nutrients in a high yielding coconut plantation through removal of plant materials from the field. Cocos 15:12. https://doi.org/10.4038/cocos.v15i0.2184
Spokas KA (2010) Review of the stability of biochar in soils: predictability of O:C molar ratios. Carbon Manag 1:289–303. https://doi.org/10.4155/cmt.10.32
Thies JE, Rillig MC (2012) Characteristics of biochar: biological properties. Biochar Environ Manag Sci Technol. https://doi.org/10.4324/9781849770552
Tobar R, Azcon R, Barea JM (1994) Improved nitrogen uptake and transport from 15N-labelled nitrate by external hyphae of arbuscular mycorrhiza under water-stressed conditions. New Phytol 126:119–122. https://doi.org/10.1111/j.1469-8137.1994.tb07536.x
Tribe HT (1960) Aspects of decomposition of cellulose in Canadian soils: I. Observations with the microscope. Can J Microbiol 6:309–316. https://doi.org/10.1139/m60-035
Valverde-Barrantes OJ, Horning AL, Smemo KA, Blackwood CB (2016) Phylogenetically structured traits in root systems influence arbuscular mycorrhizal colonization in woody angiosperms. Plant Soil 404:1–12. https://doi.org/10.1007/sl1104-016-282
Vierheilig H, Coughlan AP, Wyss U, Piché Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64:5004–5007. https://doi.org/10.1128/aem.64.12.5004-5007.1998
Wakamiya S, Hayakawa A, Takahashi T et al (2022) Physicochemical properties of biochar derived from wood of Gliricidia sepium based on the pyrolysis temperature and its applications. J Soil Water Conserv 77:322–330. https://doi.org/10.2489/jswc.2022.00083
Warnock DD, Lehmann J, Kuyper TW, Rillig MC (2007) Mycorrhizal responses to biochar in soil—concepts and mechanisms. Plant Soil 300:9–20. https://doi.org/10.1007/s11104-007-9391-5
Willis A, Rodrigues BF, Harris PJC (2013) The ecology of arbuscular mycorrhizal fungi. CRC Crit Rev Plant Sci 32:1–20. https://doi.org/10.1080/07352689.2012.683375
Xiang Y, Deng Q, Duan H, Guo Y (2017) Effects of biochar application on root traits: a meta-analysis. Glob Chang Biol Bioenergy 9:1563–1572. https://doi.org/10.1111/gcbb.12449
Yan F, Schubert S, Mengel K (1996) Soil pH changes during legume growth and application of plant material. Biol Fertil Soils 23:236–242. https://doi.org/10.1007/BF00335950
Yengwe J, Gebremikael MT, Buchan D et al (2018) Effects of Faidherbia albida canopy and leaf litter on soil microbial communities and nitrogen mineralization in selected Zambian soils. Agrofor Syst 92:349–363. https://doi.org/10.1007/s10457-016-0063-4
Yuan J-H, Xu R-K (2011) The amelioration effects of low temperature biochar generated from nine crop residues on an acidic Ultisol. Soil Use Manag 27:110–115. https://doi.org/10.1111/j.1475-2743.2010.00317.x
Zapata F (1990) Isotope techniques in soil fertility and plant nutrition studies. Use Nucl Tech Stud Soil Plant Relationships 61–128
Zou Z, Fan L, Li X et al (2021) Response of plant root growth to biochar amendment : a meta-analysis. Agronomy 11:1–14
Zoysa AKN, Loganathan P, Hedley MJ (1997) A technique for studying rhizosphere processes in tree crops: soil phosphorus depletion around camellia (Camellia japonica L.) roots. Kluwer Academic Publishers
Acknowledgements
Gertrude Scynthya Nirukshan is a doctoral candidate with a sandwich scholarship grant given for developing countries (Special Research Fund (BOF)-2017) by Ghent University Belgium, and this research is part of her doctoral research work. We sincerely thank Ghent University for granting this scholarship and funding this research. We sincerely acknowledge the great support given by Sujith Nayanakumara, Tharanga Madusantha, Don Benthara Anuruddha on producing the biochar required for this research project at the production facility of Coconut Research Institute of Sri Lanka. We also would like to thank Stefan Vidts from the tropical greenhouse, Mathieu Schatteman, Tina Coddens, Anne-Mie Terryn, Sophie Schepens from the lab of the Soil Fertility and Nutrient Management (SoFer) group and Katja Van Nieuland from the Isotope Bioscience Laboratory (ISOFYS) of Ghent University (Belgium) for their assistance during the pot trial and laboratory analysis.
Funding
This research has been financed by the Special research fund grant BOF.DCV.2017.0006.01 of Ghent University.
Author information
Authors and Affiliations
Contributions
All authors participated in conceiving the study. GSN: Conceptualization, methodology, investigation, formal analysis, data curation, data visualization, writing- original draft, review and editing. SR: Research administration-Sri Lanka, supervision, resources, writing-review. SS: Conceptualization, methodology, investigation, data validation, supervision, project administration, writing-review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationship that could have appeared to influence the work reported in this paper.
Supplementary Information
Additional file 1.
S1. Farmer scale production of biochar from agricultural wastes (Detailed description of biochar production procedure). Fig. S1. 15N enrichment in shoots and roots of coconut seedlings given as Atom % excess. Table S1. Soil pH, mineral N content and available P at the end of the experiment in the root and hyphal zone.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nirukshan, G.S., Ranasinghe, S. & Sleutel, S. The effect of biochar on mycorrhizal fungi mediated nutrient uptake by coconut (Cocos nucifera L.) seedlings grown on a Sandy Regosol. Biochar 4, 68 (2022). https://doi.org/10.1007/s42773-022-00192-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-022-00192-9