Abstract
Introduced, non-native organisms are of global concern, because biological invasions can negatively affect local communities. Arbuscular mycorrhizal (AM) fungal communities have not been well studied in this context. AM fungi are abundant in most soils, forming symbiotic root-associations with many plant species. Commercial AM fungal inocula are increasingly spread worldwide, because of potentially beneficial effects on plant growth. In contrast, some invasive plant species, such as the non-mycorrhizal Alliaria petiolata, can negatively influence AM fungi. In a greenhouse study we examined changes in the structure of a local Canadian AM fungal community in response to inoculation by foreign AM fungi and the manipulated presence/absence of A. petiolata. We expected A. petiolata to have a stronger effect on the local AM fungal community than the addition of foreign AM fungal isolates. Molecular analyses indicated that inoculated foreign AM fungi successfully established and decreased molecular diversity of the local AM fungal community in host roots. A. petiolata did not affect molecular diversity, but reduced AM fungal growth in the greenhouse study and in a in vitro assay. Our findings suggest that both introduced plants and exotic AM fungi can have negative impacts on local AM fungi.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Invasive species are an increasing phenomenon at a global scale and represent range expansions into non-native habitats. Invasions can lead to changes in community structure, decrease diversity of native species and alter ecosystem functioning (Hooper et al. 2005). Several mechanisms have been proposed to explain why certain species are successful invaders, including release from natural enemies (Agrawal et al. 2005), novel weapons (Callaway and Ridenour 2004), propagule pressure (Lockwood et al. 2005) or specific life-history traits such as high growth rates or resource-use efficiency (Funk and Vitousek 2007). Invaders may also profit from multiple beneficial traits, but often the most important mechanisms of invasiveness remain elusive. Invasive macroorganisms have been relatively well studied, but only recently have we begun making progress in our understanding of microbial invasions (Desprez-Loustau et al. 2007; van der Putten et al. 2007). This is especially true for soil microorganisms, which either directly or through differential feedbacks alter growth and success of native and invasive plants (Callaway et al. 2004; Klironomos 2002). Here we focus on arbuscular mycorrhizal (AM) fungi (phylum Glomeromycota), widespread root symbionts that form associations with the majority of plant species (Smith and Read 2008).
The promise of potentially beneficial effects of AM fungi on plant growth and nutrition has lead to an increase in the use of commercial AM fungal inocula applied as bio-fertilizers (Schwartz et al. 2006). Such inocula often consist of a single AM fungal genotype and are deliberately introduced to locations where they may either not be native or be present at an abundance that would allow co-existence with other native AM fungi. In most instances it remains unclear whether these inoculants successfully establish, and if so, whether they impact the local AM fungal communities. On the other hand, there is also increasing evidence that invasive plant species alter AM fungal communities (Hawkes et al. 2006; Mummey and Rillig 2006), possibly through allelopathic chemicals produced by roots or other tissues (Zhang et al. 2007). Native to Europe, Alliaria petiolata Bieb. Cavara & Grande (Brassicaceae) is an aggressive biennial invader of forest understories and edge habitats throughout North America. Studies using A. petiolata plant tissues, extracts or soils conditioned by A. petiolata have shown that A. petiolata may be allelopathic to some North American plants and AM fungi (Callaway et al. 2008; Meekins and McCarthy 1999; Prati and Bossdorf 2004; Roberts and Anderson 2001; Vaughn and Berhow 1999). Being non-mycorrhizal, the inhibitory effects of A. petiolata on ectomycorrhizal and AM fungi may negatively affect mycorrhizal-dependent native plants thereby facilitating the propagation and success of A. petiolata (Stinson et al. 2006; Wolfe et al. 2008). Negative effects of introduced organisms on AM fungal diversity may be ecologically important, because changes in AM fungal composition may alter plant diversity and ecosystem productivity (Grime et al. 1987; van der Heijden et al. 1998). AM fungi are ancient plant symbionts (Remy et al. 1994) and several AM fungal species, including Glomus intraradices Schenk & Smith (a species commonly used as a commercial inoculant), have global distributions (Opik et al. 2006).
The aim of the present work was to study the interplay of two introduced G. intraradices isolates (and their mixture), the invasive plant A. petiolata, and a native Canadian AM fungal field community naïve to A. petiolata. We chose to study this topic in a controlled greenhouse setting and not in the field, because of the potential risks associated with the release of non-native microbes (Schwartz et al. 2006). Two closely related, yet distinct G. intraradices genotypes from Switzerland and Québec were used (Croll et al. 2008; Koch et al. 2004), the latter being widely used for commercial inoculation purposes. Both strains originate from within the native and invaded range of A. petiolata, respectively. Based on the findings of Callaway et al. (2008), differentiation in resistance to A. petiolata may not only be present among areas that vary in A. petiolata invasion history, but also among closely related fungal genotypes from within the current range of A. petiolata. We also tested whether synergistic effects between these two related strains could be detected, by comparing their mixture to the effects of the two strains as monocultures. We expected that A. petiolata would have more detrimental effects on the diversity and abundance of AM fungi from a native Alliaria-naïve Canadian field community than addition of potentially non-native G. intraradices genotypes, because G. intraradices has previously been documented to frequently occur at this field site (Hart and Klironomos 2002). We also hypothesized that in the presence of Alliaria or its extracts, the growth of the Canadian G. intraradices isolate is more negatively affected than that of its Swiss counterpart.
Materials and Methods
Two isolates of the AM fungal species G. intraradices were used. Both cultures (DAOM 197198 [named DAOM 181602 in Koch et al. (2004)] and B3) were started from single spores. The Swiss isolate (B3) originates from an agricultural field near Tänikon (Switzerland) and was obtained from the laboratory of Prof. Ian R. Sanders (University of Lausanne). The Canadian isolate (DAOM 197198) originates from an Ash forest near an agricultural field in Pont-Rouge (Quebec) and its genome is currently being sequenced (Martin et al. 2008). We obtained in vitro cultures of this isolate from Premier Tech Biotechnologies (Rivière-du-Loup, Quebec, Canada), who uses this strain as the active ingredient in their commercial inocula Myke®-Pro. Both isolates had previously been cultured in axenic root organ cultures (ROCs) on M-medium for over 6 years (Becard and Fortin 1988) and were clonally re-cultured under standardized conditions (on Petri dishes containing M-Medium and transformed carrot host roots) at least two times at the University of Guelph prior to any experiments. The axenic culture procedures and the isolation history of the Swiss isolate are described in detail in Koch et al. (2004). A. petiolata has not been observed in the native field of the Swiss isolate (T. Anken, personal communication), but this site lies within the native range of A. petiolata in Europe. We do not know whether A. petiolata has occurred at the field site where the Canadian isolate originates from, but the invaded A. petiolata range in eastern Canada includes that area.
Experiment 1: Greenhouse study
We collected field soil from one location at an old-field meadow, the long-term mycorrhizal research site (LTMRS), on the campus of the University of Guelph in October 2006. Sieved field soil (5 mm mesh width) was stored in an open 50 L plastic container at room temperature for drying until it was used in the experiment. While G. intraradices has been documented at the LTMRS, to date A. petiolata has not been observed there (Hart and Klironomos 2002; Klironomos 2002). A. petiolata frequently occurs in and around Guelph, locally forming dense stands of almost pure monocultures (A.M. Koch, personal observations).
Fifty 1-L pots with saucers were used in full factorial design with the following three factors, (1) soil type (sterilized or unsterilized field soil), (2) A. petiolata (exposed or unexposed), and (3) G. intraradices inoculation type (the Swiss isolate B3, the Canadian isolate DAOM 197198, their mixture, or no inoculation). We prepared inocula for each of the two isolates by pooling and thoroughly homogenizing (with a spoon) eight 15-week-old ROC plates (9 cm diameter, containing M-medium, transformed carrot roots, hyphae and spores). The mixed inoculum consisted of a 1:1 (v:v) mixture of the two G. intraradices inocula-types. We added 15 ml of inoculum to each pot. The non-mycorrhizal treatments received 15 ml of inoculum also obtained from ROC plates but containing uncolonized carrot roots and no AM fungi. The bottom of each pot consisted of a 200 ml layer of Turface (a montmorillonite clay, Turface Athletics MVP, Profile Products LLC, Buffalo Grove, IL, USA), which was covered with 200 ml of sterilized (steam autoclaved twice at 121°C for 60 min 2 weeks before the start of the experiment) or unsterilized field soil. In pots containing A. petiolata, we transplanted single, flowering second-year A. petiolata plants of approximately equal size into this soil layer, with the A. petiolata main root centered in each pot. All A. petiolata plants used were collected in a 5 × 5 m patch next to the Greenhouse of the Axelrod Building of the University of Guelph, approximately 1.5 km away from the LTMRS.
On the first day of the experiment (13 May 2007) second year A. petiolata plants of similar size were carefully dug up. Their roots were washed with water to remove all visible soil particles and then placed into pots. G. intraradices inoculum was equally distributed on top of the soil layer and all soils were then covered with 200 ml Turface. One hundred eighty Sorghum vulgare var. sudanense seeds (variety CADAN 99B, Browning Seed Inc., Plainview, TX, USA) were added to each pot and covered by a 2 cm layer of Turface. We chose S. vulgare as host species due to its capacity to establish symbioses with a wide range of AM isolates across the entire Glomeromycota (i.e. the INVAM culture collection, see http://invam.caf.wvu.edu/index.html, representing all major AM taxa). As such mycorrhizal responses to inoculations and/or A. petiolata introduction with this host may underestimate responses in other, more specialized, mycotrophic hosts. Three pots were established for each soil/A. petiolata/AMF treatment combination with the following exceptions. We set up four pots for both A. petiolata treatments with unsterilized field soil to which no G. intraradices was added. A. petiolata blend (see below) was accidentally added to one pot containing no A. petiolata plant. This pot was subsequently considered as +A. petiolata resulting in four pots of unsterilized field soil with isolate B3 and A. petiolata, and only two pots containing sterilized field soil, the isolate B3 and no A. petiolata. Each pot also received 2 ml of a microbial filtrate (made from a 200 ml vol : 200 ml vol mixture of field soil and soil recovered from the patch where A. petiolata plants and roots were sampled, stirred in 800 ml of sterile H2O, and passed through a 20 μm mesh), to minimize differences in non-AM microorganisms. The pots were then randomly arranged on a greenhouse bench and watered three times per week as needed. Temperature in the greenhouse ranged from 23 to 35°C. Day length was a minimum of 16 h, supplemented with artificial lights from 6 am to 10 pm when necessary. Because A. petiolata plants started wilting within 2 weeks after transplantation, probably due to the transplant shock or natural senescence, we added additional A. petiolata extract to each pot that contained an A. petiolata plant on 7 June 2007. For this, we sampled and washed the roots of 30 s-year A. petiolata individuals from the same A. petiolata population, blended 109 g fresh weight (fw) A. petiolata roots in 1.7 L water and added 60 ml of the resulting filtrate to each pot. This equates to approximately 3.2 mg A. petiolata tissue equivalents per g soil, which has been estimated to represent expected exposure levels in the field (Callaway et al. 2008).
Plants were harvested on 11 July 2007. For each pot, we counted and excised all Sorghum shoots. Shoots where dried for 3 days at 70°C and weighed. Roots where washed and representative subsamples of about 3 g fw were collected from each pot and stored in 50% ethanol and subsequently used for staining and quantification of AM fungal colonization (McGonigle et al. 1990). Root subsamples of about 0.5 g (fw) were collected into 1.5 ml Eppendorf tubes and immediately placed on ice. These subsamples were then stored at −80°C before being freeze-dried for use in terminal restriction fragment length polymorphism (T-RFLP) analysis. This method enables AM fungal communities to be fingerprinted by analyzing gene polymorphism in a ~380 bp long section of the large subunit (LSU) rDNA (Mummey and Rillig 2007). Each subsample was individually shredded and a sub-sample of 20 mg weighed into each well of a 96 well block for DNA extraction (NucleoSpin® 96 Plant, Macherey–Nagel Inc., Bethlehem, PA, USA). A nested PCR protocol was used to amplify DNA from the AM fungi. The fungal community was amplified with LR1/FLR2 primers (Trouvelot et al. 1999; Van Tuinen et al. 1998). The PCR product was then used as template for a second PCR using the 5′-labeled primer pair FLR3-FAM/FLR4-VIC (Applied Biosystems, Foster City, CA, USA) to amplify AM fungi (Gollotte et al. 2004). Both PCRs were comprised of a 30 μl reaction mix containing final concentration of 1× Green GoTaq® Reaction Buffer (Promega, Madison, WI, USA), 1.7 mM MgCl2, 0.13 mM of each dNTP, 0.33 mM of each primer and 1.25 u GoTaq® DNA Polymerase and 1.5 μl of template DNA. Products of the first PCR were diluted 1/100 for the second PCR. Both PCRs consisted of an initial denaturation step at 93°C for 3 min followed by 35 cycles (93°C for 1 min, 58°C for 1 min, 72°C for 1 min) and a final extension step of 10 min at 72°C in a Mastercycler®ep thermocycler (Eppendorf, Hamburg, Germany). PCR product sizes were verified by gel electrophoresis with a 1 kb GeneRulerTM DNA ladder (Fermentas, Burlington, ON, Canada) as a standard. After the second PCR, products were purified using a QIAquick96® cleanup kit (Qiagen Inc.), before being separately digested with the restriction enzymes AluI and MboI (Invitrogen Inc., Burlington, ON, Canada). The restriction digestion, comprised of a 20 μl reaction mix containing 8 μl of purified PCR product (total of 60 ng DNA), 1× REact®1 and 2 buffer (AluI and MboI, respectively) and 2 U of enzyme, was incubated for 4 h at 37°C. T-RF sizes in each sample were determined using an ABI 3730 DNA Analyzer with LIZ-500 (Applied Biosystems, Foster City, California, U.S.A.) as the size standard.
Experiment 2: In vitro study
We also used ROCs for an in vitro assay (Becard and Fortin 1988; Koch et al. 2004) and manipulated the concentrations of A. petiolata extract in the M-Medium to test responses of the two G. intraradices isolates. In the control treatment, no A. petiolata extract was added. The A. petiolata extract consisted of a 1:1 (v:v) mixture of extracts enriched in flavonoids and glucosinolates isolated from first year A. petiolata plants as in Callaway et al. (2008). We used M-medium with five different A. petiolata extract concentrations (AECs), 0, 1.8, 3.6, 5.4 and 7.2 μl A. petiolata extract per ml M-Medium, respectively, to test whether different AECs affect growth of AM fungi. The second highest concentration used in our experiment (5.4 μl A. petiolata extract per ml M-Medium) corresponded approximately to the concentrations of the combined flavonoid and glucosinolate fractions used by Callaway et al. (2008) that was shown to negatively affect spore germination of AM fungi. For each isolate we inoculated five 25 ml Petri dish plates containing M-Medium with each of the five AECs, for a total of 50 plates. Thus, we had a factorial experimental design with two AM isolates and five AECs. We measured AM hyphal densities of each plate 21, 34, 46, 55, 69, 84 and 99 days after inoculation; lengths of carrot roots were measured 21, 34, 46, 69 and 105 days after inoculation as described previously (Koch et al. 2004, 2006).
Statistical analyses
In Experiment 1 AM community analysis consisted of determining the profile (sizes, peak heights and areas) of terminal restriction fragments (T-RFs) in each sample using GeneMapper® software v. 3.5 (Applied Biosystems, Foster City, California, U.S.A.). The Microsoft-Excel macro Treeflap (Rees et al. 2004), available at http://www.wsc.monash.edu.au/~cwalsh/treeflap.xls, was used to convert fragment sizes to the nearest integer, aligning them with their respective peak heights side by side in two columns. We then developed an R script (R Development Core Team 2007; available upon request from the authors) that standardizes the amount of total fluorescence among sample profiles (Dunbar et al. 2001) and binary codes T-RFLP fingerprints. Presence/absence matrices for each enzyme/primer combination were combined into a single matrix. We used the pooled total number of detected T-RFs per sample as a surrogate of molecular diversity (i.e., T-RF richness, indicative of AM fungal ribotype richness) and compared treatments by analysis of variance (ANOVA, see below). Only T-RF richness was considered in this study and not the height or area of peaks, because the latter parameters may not be indicative of relative abundance of ribotypes. The influence of inoculation and A. petiolata treatments and their interactions on community composition was tested by distance-based redundancy analysis (db-RDA) (Legendre and Anderson 1999). Bray Curtis’ coefficients of similarity were first calculated between samples and used to compute principal coordinates in PrCoord 1.0 (part of Canoco version 4.51, Biometris, Wageningen, The Netherlands). All the principal coordinate axes (PCAs) were then exported to Canoco and treated as “species” data. Factors (A. petiolata, AMF addition and soil sterilization) were entered as dummy binary variables (one level per column) and their effects tested by using the combined levels of a factor as the explanatory variable in the model while removing the variance explained by the other factors by entering their levels as covariates. The significance of such models was tested with a Monte-Carlo test based on 999 permutations. Where appropriate we used forward selection of environmental variables and conducted Monte-Carlo permutation tests based on 999 permutations. The results of the ordination of the AM fungal community composition, as assessed by PCR-T-RFLP, were displayed as a PCA ordination diagram.
Growth traits of plants (total shoot dry weight of hosts, number of host shoots) and AM root colonization traits (percent root lengths colonized by arbuscules, vesicles and hyphae) were analyzed separately by multivariate analysis of variance (MANOVA), followed by univariate ANOVA using fully factorial three-way models and planned contrast analyses (CoA). Additionally, since Callaway et al. (2008) found that A. petiolata affects North American AM fungi more negatively than their European counterparts, we compared these two G. intraradices isolates in a separate analysis to investigate whether a similar pattern could be detected. MANOVA and ANOVA results gave overall qualitatively similar results. For simplicity, we present only significant F-statistics of (M)ANOVAs and CoAs in the result section. To meet the requirements of the statistical tests, variables were transformed if necessary (Zar 1984). In Experiment 2, we used the repeated measures of extraradical hyphal density (H) for each plate to estimate hyphal growth rates (gr) for consecutive measures at days y and z after the start of the experiment, as
From these growth rates, the maximum hyphal gr and time when maximal growth occurred (estimated by the average of days y and z) were calculated for each of the 50 replicate plates. Response variables were analyzed by ANOVA and repeated measures ANOVA. Repeated measures ANOVA was performed on the last four dates only, because of non-normality of data (many zero-values) for the previous dates. In both experiments correlations among variables were assessed by Pearson’s correlation coefficient R and (M)ANOVAs were performed with the statistical software JMP 5.0 (SAS Institute Inc. Cary, NC, USA).
Results
Experiment 1: AM fungal community responses assessed by T-RFLP
The detected T-RF richness in roots differed among AM inocula types (F 3,42 = 3.67, P < 0.05, Fig. 1a). The highest T-RF richness was found in field soil, when no G. intraradices inoculum was added. Sterilizing the field soil reduced T-RF detection (F 1,42 = 21.23, P < 0.0001), especially in the absence of G. intraradices addition. Removal of T-RFs belonging to G. intraradices profiles (corresponding to the samples from sterile soil) from T-RFLP profiles of G. intraradices inoculated field soil samples eliminated all detected T-RFs in three samples. Overall no direct effect of A. petiolata on T-RF richness was observed, except in sterilized field soil without G. intraradices addition where T-RF-richness was lower in presence of A. petiolata. However, the T-RF sizes present in these non-inoculated sterile controls were highly variable in quantity and sizes and inconsistent among replicates (data not shown). When analyzing field soil samples only, inoculation by G. intraradices significantly reduced overall T-RF richness (CoA, F 1,27 = 8.30, P < 0.01), irrespective of presence or absence of A. petiolata or inoculum type. Db-RDA of AM fungal community assemblage in field soil showed that the inoculation treatments accounted for a significant proportion of the variance (Trace = 0.44, F = 5.84, P < 0.001, Fig. 1b), whereas the presence of A. petiolata did not have a significant effect (Trace = 0.08, F = 0.79, P < 0.88). The analysis of G. intraradices treatments indicates that there was an overlap between soil sterilization treatments (Trace = 0.095, F = 1.25, P < 0.15), giving further support to the hypothesis that G. intraradices became dominant upon inoculation of field soil (Fig. 1b). In field soil, a significant proportion of the variance was explained by the different G. intraradices inocula (Trace = 0.17, F-ratio = 2.26, P < 0.05, Fig. 1b). As in sterilized soils, the two isolates of G. intraradices also formed separate clusters in field soil, whereas the samples of the mixture of the two isolates were clustered between the individual isolates (Fig. 1b).
Experiment 1. a Effect of A. petiolata and AM fungal inoculation on the average number of detected T-RFLP fragments (T-RFs) in field and sterilized field soil. Bars represent mean values (±S.E.M.). G. intraradices inoculation treatments are abbreviated as NA (no G. intraradices inoculum added), +CA (addition of the Canadian G. intraradices isolate), +CH (addition of the Swiss G. intraradices isolate), and +Mix (addition of a 1:1 mixture of both isolates). b Ordination diagram of principal components 1 and 2 (Axis 1 and Axis 2) obtained from distance-based redundancy analysis. The distance between symbols (each representing an individual sample) in the diagram approximates the dissimilarity of their T-RF composition measured by their Euclidean distance. Different symbols and shadings represent inoculation treatments and soil sterilization treatments, respectively (see sub-panel in Figure). NA samples from sterilized soils were highly irregular due to unspecific primer binding and, are not shown.± A. petiolata samples are not marked differently because A. petiolata did not have a significant effect on T-RF composition. Numbers in parentheses indicate the amount of variance accounted for by each principal component axis
Experiment 1: Fungal and plant growth responses
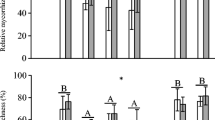
Our AM fungal inoculation treatments significantly differed (F 9,102 = 6.51, P < 0.0001, Fig. 2). In sterilized soils, no AM-specific structures (i.e. vesicles and arbuscules) were observed in the roots of control plants that received no addition of G. intraradices inoculum (Fig. 2a, b). Overall fungal root colonization was lower in pots where the Canadian isolate was added compared with pots where the Swiss isolate was present either as a pure culture or a mixture (CoA, F 3,32 = 3.82, P < 0.05). However, inoculation by G. intraradices in field soil did not significantly alter root colonization by AM fungi compared to non-inoculated samples (CoA, F 3,16 = 0.31, P = 0.81). A. petiolata negatively affected levels of root colonization by AM fungi (F 3,32 = 3.16, P < 0.05, Fig. 2). When analyzing only the effect of adding the Swiss and the Canadian isolate as pure cultures, A. petiolata significantly reduced fungal growth (F 3,14 = 5.01, P < 0.05). Addition of the Swiss isolate resulted in higher root colonization than inoculation by the Canadian isolate (F 3,14 = 4.88, P < 0.05), and we detected a significant three-way interaction between the two AM fungal isolates, A. petiolata and the sterilization of soil (F 3,14 = 3.85, P < 0.05). In field soil A. petiolata reduced root colonization of both isolates similarly, whereas in sterilized soils, A. petiolata had a negative effect on growth of only the Canadian isolate.
Experiment 1. Effect of A. petiolata and AM fungal inoculation on the percentage of S. vulgare root length colonized by a arbuscules, b vesicles and c hyphae in field and sterilized field soil. Bars represent mean values (±S.E.M.). Figure captions are as in Fig. 1a
Plant growth was significantly altered by sterilization and G. intraradices treatments (F 2,33 = 607.96 and F 6,68 = 4.51, P < 0.001 in both cases, respectively, Fig. 3). Sterilization of soils almost doubled plant shoot dry weight and tended to increase the number of shoots (F 2,33 = 607.96, P < 0.0001, Fig. 3). There was a significant interaction between soil sterilization and AM fungal inoculation (approximate F 6,68 = 5.01, P < 0.001, Fig. 3). Adding G. intraradices to sterilized soils significantly reduced plant shoot weight overall relative to controls (no G. intraradices inoculum added; CoA, F 1,34 = 10.33, P < 0.01). In field soil, addition of the isolate mixture resulted in the highest shoot dry weights and number of shoots. This interaction remained significant when the controls (no G. intraradices addition) were removed from the analysis (F 4,48 = 2.83, P < 0.05). Neither did we detect a significant effect of A. petiolata on host growth (F 2,33 = 0.39, P = 0.68) nor a significant A. petiolata interaction with any of the other main factors (P > 0.58 for all interactions, Fig. 3).
Experiment 1. Effect of A. petiolata and AM fungal inoculation on total shoot dry weight (a), and number of shoots (b) of S. vulgare at harvest in field and sterilized field soil. Figure captions are as in Fig. 1a
Experiment 2: In vitro study
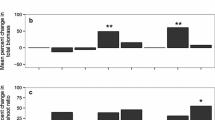
Final hyphal lengths did not significantly differ between the two isolates (F 1,40 < 0.01, P = 0.96), but significantly decreased with increasing concentration of A. petiolata extract in the growth medium (F 4,40 = 3.81, P < 0.01; Fig. 4a). Maximal hyphal growth rates were positively correlated with the final hyphal density (R = 0.74, P < 0.0001) and the two isolates differed significantly when maximal hyphal growth occurred (F 1,40 = 12.82, P < 0.01; Fig. 4b). The Canadian isolate had a delayed maximal growth relative to the Swiss isolate, especially at intermediate AECs. However, when no A. petiolata extract was added and at high AECs, responses of the two isolates were similar. These trends explained the significant 3-way interaction among measurement date, A. petiolata treatment and isolate in a repeated measures ANOVA on hyphal lengths (approximate F 12,120 = 1.97, P < 0.05). The Swiss isolate had transiently higher hyphal densities than the Canadian isolate after 55 and 69 days of growth, especially at low AECs (data not shown). Final root lengths were not significantly altered by any of the treatments (data not shown) and were negatively correlated with hyphal densities at the last measurement (R = −0.3020, P < 0.05).
Discussion
AM fungal community and host plant responses to inoculation by G. intraradices
We used T-RFLP to characterize AM fungal communities in host plant roots. In our greenhouse study, addition of G. intraradices inocula to a native Canadian field soil resulted in a drastic decrease of detected T-RFs obtained from roots (Fig. 1). Since we also characterized T-RFLP profiles of G. intraradices in sterilized substrates, we were able to estimate to which extent the shift in community assemblage was toward the introduced profiles. We found that addition of G. intraradices to field soil clearly had a negative effect on native AM diversity. However, due to limitations of the technique (Avis et al. 2006), we cannot conclude that G. intraradices outcompeted all native AM fungi. Fungi occurring at low frequency may have not been detected, and some G. intraradices specific T-RFs were also present in the field soil. However, our findings suggest that, irrespective of the identity of the isolate, introduced G. intraradices successfully established and became dominant. This finding is relevant since the Canadian isolate used in our experiments is currently spread worldwide as commercial inoculum. Our inoculum was obviously very infective, demonstrating that some commercial inocula have the potential to establish and (at least temporarily) disrupt the diversity of local AM fungal communities. To assess the impact of introduction of exotic AM fungi to native AMF communities we call for long-term studies and AM community surveys of field sites where fungal inoculants have been applied in the past. Potential benefits in plant growth should be weighed with the possibility of irreversible long-term alterations of soil microbial communities when applying non-native inocula. Consistent with our findings, pre-inoculation of plants with two Glomus strains greatly decreased the detected ribotype richness of another native AM fungal community in roots of hosts that were transplanted into field soil, relative to plants that were not pre-inoculated (Mummey et al. 2009). In contrast, inoculation of an agricultural soil with a commercial inoculant containing the Canadian isolate of G. intraradices at the rate of application recommended by the supplier did not affect the resident AM fungal community in maize roots (Antunes et al. 2009). These findings suggest that successful establishment of an inoculant may depend on the amount and type of inoculum and/or the diversity and genotypic identities found in the target community. Limited inoculum addition may reduce both the inoculants initial rate of establishment and its subsequent impact on native AM fungi. In a more natural setting (i.e., unmanaged soils) where native AM fungi may already have formed intact hyphal networks and colonized most of the plant roots, additional non-native AM fungi may be less likely to become problematic invaders. Success of AM fungal isolates also depends on the identity of host plants (Ehinger et al. 2009; Klironomos 2003) and other environmental factors such as the local climate, soil properties, or the local AM community composition (Maherali and Klironomos 2007). An AM fungal inoculant could also directly interact with resident genotypes of the same species, since closely related, but genetically distinct AM fungi can anastomose and exchange genetic information (Croll et al. 2009; Croll and Sanders 2009). Whether or not the addition of an inoculant is beneficial or harmful to its native conspecifics remains to be explored. Our findings highlight that, at least at high inoculum application rates, introduced non-native AM fungi may negatively impact resident AM fungi. Future experiments should also investigate whether repeated inoculant-applications at lower rates would have similar effects.
Plant growth was strongly enhanced in sterilized field soil, likely a result of combined effects of nutrient release, or a reduced presence of soil biota or pathogens. Relative to non-inoculated controls, G. intraradices addition decreased host growth in sterilized field soil, possibly by competition for nutrients, similar to the negative correlation between plant and fungal growth observed in Experiment 2. On the other hand, in field soil where a native community was already present, inoculation by G. intraradices increased the number of shoots, possibly by enhancing seed germination, seedling survival (van der Heijden 2004) or protection from root pathogens (Sikes et al. 2009; Wehner et al., 2010). Interestingly, we also found that the combined mixture of both G. intraradices isolates had different effects on plant growth relative to separate effects of the two isolates, revealing an unexpected intraspecific synergism. This suggests that functional complementarity may not only occur among (Jansa et al. 2008; Maherali and Klironomos 2007), but also within AM fungal species. Thus, environmental factors, genotype identity and diversity within AM species alter host plant performance from antagonistic to beneficial (Koch et al. 2006), possibly due to altered resource uptake or pathogen protection (Johnson et al. 1997; Newsham et al. 1995).
Host plant and AM fungal community responses to A. petiolata
Allelochemicals produced by certain invasive plants, including A. petiolata, have been reported to limit the growth of native plants (Bais et al. 2003; Dorning and Cipollini 2006; Ridenour and Callaway 2001; Stinson et al. 2006). However, A. petiolata did not significantly alter the growth of host plants in our experiments. Thus AM-inhibitory effects caused by A. petiolata, as evidenced by an overall reduction in levels of AM root colonization and hyphal growth, corroborate previous reports of negative effects of A. petiolata allelochemicals on mycorrhizal fungi (Callaway et al. 2008; Roberts and Anderson 2001; Stinson et al. 2006; Wolfe et al. 2008). Contrary to our hypothesis, A. petiolata did not significantly affect community composition and richness of local AM fungi found in field soil in our greenhouse study. This suggests that different native AM fungal taxa were affected similarly by A. petiolata. Exposure to actively growing first-year rosettes, which are known to vary in chemistry from second year plants, may have different effects. In sterilized substrates A. petiolata altered TRF-composition only of non-mycorrhizal controls, but these effects are likely biologically meaningless resulting from unspecific primer binding, since no AM fungi were visible in these roots. On the other hand A. petiolata had a more negative effect on the growth of the Canadian isolate than on the Swiss isolate in both experiments. Even though we have not tested replicate G. intraradices isolates from Europe and North America, these findings support the existence of transcontinental divergence in A. petiolata-tolerance as suggested by the findings of Callaway et al. (2008); random effects are unlikely to produce such a predicted pattern. However, in field soil without G. intraradices inoculation A. petiolata did not entirely suppress AM root colonization by local AM fungi, which could reflect sub-lethal concentrations of allelochemicals, possibly due to microbial degradation of A. petiolata metabolites in field soil (Barto and Cipollini 2009; Gimsing et al. 2006, 2007; Tsao et al. 2000) or a certain inherent tolerance of local AM to A. petiolata. The latter interpretation is consistent with findings of two recent studies that analyzed AM communities in host plants from patches that were invaded or uninvaded by A. petiolata. First-year A. petiolata plants did not affect AM fungal T-RF richness within roots of sugar maple seedlings from eight sites in Ohio and Massachusetts, but altered the AM fungal community composition in half of the sites monitored (Barto E. K., unpublished data). Similarly, A. petiolata did not alter levels of AM root colonization of three plant species at one site in Pennsylvania and only affected AM communities in the roots of one plant species (Burke 2008).
The potential role of soil microbes in co-evolutionary outcomes for invasive species
In our short-term study, introducing non-native AM isolates to a native AM community had much more drastic effects than introducing A. petiolata. The introduced G. intraradices isolates became dominant, implying that drastic frequency shifts had occurred within the native AM fungal community inside host plant roots. Undoubtedly, long-term field studies are required to test whether a successful short-term establishment is also indicative of persistence. In light of our results, however, current practices of commercial AM fungal inocula applications to field sites means accepting the risks of potentially irreversible microbial invasions of unknown consequences (Schwartz et al. 2006).
Reported negative effects of A. petiolata on AM fungi and AM-dependent plants may enhance the success of A. petiolata in North America at some invaded sites (Callaway et al. 2008; Stinson et al. 2006). Thus, invasive species act as a new selective force altering above- and belowground native populations and communities in invaded sites. Selection for more A. petiolata-tolerant soil microbes, decomposers of A. petiolata phytochemicals, native natural enemies, or less toxic A. petiolata may counteract the success of and spread of A. petiolata over time (Burke 2008; Enright and Cipollini 2007; Lankau et al. 2009). The numerous on-going biological invasions around the globe offer possibilities to study ongoing co-evolutionary dynamics of native communities. An analysis of invasions by various organisms showed that many invasions become regulated at some point in time (Arim et al. 2006). This does not lessen the negative impacts that invaders cause locally, but rather suggests that there are mechanisms that regulate species in their new habitats. Invasive species find themselves in a novel environment, facing new selective pressures, which can result in evolutionary changes or niche shifts (Broennimann et al. 2007; Lee 2002; Schlaepfer et al. 2005). However, for many soil microbes, and AM fungi in particular, such investigations have rarely been undertaken. Even though these organisms escape our direct sight, they do have important effects on ecosystem function (Rillig 2004) and should not be ignored.
References
Agrawal AA, Kotanen PM, Mitchell CE, Power AG, Godsoe W, Klironomos J (2005) Enemy release? An experiment with congeneric plant pairs and diverse above- and belowground enemies. Ecol 86:2979–2989
Antunes PM, Koch AM, Dunfield KE, Hart MM, Downing A, Rillig MC, Klironomos JN (2009) Influence of commercial inoculation with Glomus intraradices on the structure and functioning of an AM fungal community from an agricultural site. Plant Soil 317:257–266
Arim M, Abades SR, Neill PE, Lima M, Marquet PA (2006) Spread dynamics of invasive species. Proc Natl Acad Sci USA 103:374–378
Avis PG, Dickie IA, Mueller GM (2006) A ‘dirty’ business: testing the limitations of terminal restriction fragment length polymorphism (TRFLP) analysis of soil fungi. Mol Ecol 15:873–882
Bais HP, Vepachedu R, Gilroy S, Callaway RM, Vivanco JM (2003) Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science 301:1377–1380
Barto EK, Cipollini D (2009) Half-lives and field soil concentrations of Alliaria petiolata secondary metabolites. Chemosphere 76:71–75
Becard G, Fortin JA (1988) Early events of vesicular arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol 108:211–218
Broennimann O, Treier UA, Muller-Scharer H, Thuiller W, Peterson AT, Guisan A (2007) Evidence of climatic niche shift during biological invasion. Ecol Lett 10:701–709
Burke DJ (2008) Effects of Alliaria petiolata (garlic mustard; Brassicaceae) on mycorrhizal colonization and community structure in three herbaceous plants in a mixed deciduous forest. Am J Bot 95:1416–1425
Callaway RM, Ridenour WM (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2:436–443
Callaway RM, Thelen GC, Rodriguez A, Holben WE (2004) Soil biota and exotic plant invasion. Nature 427:731–733
Callaway RM, Cipollini D, Barto K, Thelen GC, Hallett SG, Prati D, Stinson K, Klironomos J (2008) Novel weapons: Invasive plant suppresses fungal mutualists in America but not in its native Europe. Ecol 89:1043–1055
Croll D, Sanders IR (2009) Recombination in Glomus intraradices, a supposed ancient asexual arbuscular mycorrhizal fungus. BMC Evolutionary Biology 9:13
Croll D, Wille L, Gamper HA, Mathimaran N, Lammers PJ, Corradi N, Sanders IR (2008) Genetic diversity and host plant preferences revealed by simple sequence repeat and mitochondrial markers in a population of the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol 178:672–687
Croll D, Giovannetti M, Koch AM, Sbrana C, Ehinger M, Lammers PJ, Sanders IR (2009) Nonself vegetative fusion and genetic exchange in the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol 181:924–937
Desprez-Loustau M-L, Robin C, Buée M, Courtecuisse R, Garbaye J, Suffert F, Sache I, Rizzo DM (2007) The fungal dimension of biological invasions. Trends Ecol Evol 22:472–480
Dorning M, Cipollini D (2006) Leaf and root extracts of the invasive shrub, Lonicera maackii, inhibit seed germination of three herbs with no autotoxic effects. Plant Ecol 184:287–296
Dunbar J, Ticknor LO, Kuske CR (2001) Phylogenetic specificity and reproducibility and new method for analysis of terminal restriction fragment profiles of 16S rRNA genes from bacterial communities. Appl Environ Microbiol 67:190–197
Ehinger M, Koch AM, Sanders IR (2009) Changes in arbuscular mycorrhizal fungal phenotypes and genotypes in response to plant species identity and phosphorus concentration. New Phytol 184:412–423
Enright SM, Cipollini D (2007) Infection by powdery mildew Erysiphe cruciferarum (Erysiphaceae) strongly affects growth and fitness of Alliaria petiolata (Brassicaceae). Am J Bot 94:1813–1820
Funk JL, Vitousek PM (2007) Resource-use efficiency and plant invasion in low-resource systems. Nature 446:1079–1081
Gimsing AL, Sorensen JC, Tovgaard L, Jorgensen AMF, Hansen HCB (2006) Degradation kinetics of glucosinolates in soil. Environ Tox Chem 25:2038–2044
Gimsing AL, Sorensen JC, Strobel BW, Hansen HCB (2007) Adsorption of glucosinolates to metal oxides, clay minerals and humic acid. Appl Clay Sci 35:212–217
Gollotte A, van Tuinen D, Atkinson D (2004) Diversity of arbuscular mycorrhizal fungi colonising roots of the grass species Agrostis capillaris and Lolium perenne in a field experiment. Mycorrhiza 14:111–117
Grime JP, Mackey JML, Hillier SH, Read DJ (1987) Floristic diversity in a model system using experimental microcosms. Nature 328:420–422
Hart MM, Klironomos JN (2002) Diversity of arbuscular mycorrhizal fungi and ecosystem functioning. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal Ecology. Springer, pp, pp 225–242
Hawkes CV, Belnap J, D’Antonio C, Firestone MK (2006) Arbuscular mycorrhizal assemblages in native plant roots change in the presence of invasive exotic grasses. Plant Soil 281:369–380
Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setala H, Symstad AJ, Vandermeer J, Wardle DA (2005) Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol Monogr 75:3–35
Jansa J, Smith FA, Smith SE (2008) Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol 177:779–789
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol 135:575–586
Klironomos JN (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417:67–70
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecol 84:2292–2301
Koch AM, Kuhn G, Fontanillas P, Fumagalli L, Goudet J, Sanders IR (2004) High genetic variability and low local diversity in a population of arbuscular mycorrhizal fungi. Proc Natl Acad Sci USA 101:2369–2374
Koch AM, Croll D, Sanders IR (2006) Genetic variability in a population of arbuscular mycorrhizal fungi causes variation in plant growth. Ecol Lett 9:103–110
Lankau RA, Nuzzo V, Spyreas G, Davis AS (2009) Evolutionary limits ameliorate the negative impact of an invasive plant. Proc Natl Acad Sci USA 109:15362–15367
Lee CE (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17:386–391
Legendre P, Anderson MJ (1999) Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol Monogr 69:1–24
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20:223–228
Maherali H, Klironomos JN (2007) Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316:1746–1748
Martin F, Gianinazzi-Pearson V, Hijri M, Lammers P, Requena N, Sanders IR, Shachar-Hill Y, Shapiro H, Tuskan GA, Young JPW (2008) The long hard road to a completed Glomus intraradices genome. New Phytol 180:747–750
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective-measure of colonization of roots by vesicular arbuscular mycorrhizal fungi. New Phytol 115:495–501
Meekins JF, McCarthy BC (1999) Competitive ability of Alliaria petiolata (garlic mustard, Brassicaceae), an invasive, nonindigenous forest herb. Int J Plant Sci 160:743–752
Mummey DL, Rillig MC (2006) The invasive plant species Centaurea maculosa alters arbuscular mycorrhizal fungal communities in the field. Plant Soil 288:81–90
Mummey DL, Rillig MC (2007) Evaluation of LSU rRNA-gene PCR primers for analysis of arbuscular mycorrhizal fungal communities via terminal restriction fragment length polymorphism analysis. J Microbiol Methods 70:200–204
Mummey DL, Antunes PM, Rillig MC (2009) Arbuscular mycorrhizal fungi pre-inoculant identity determines community composition in roots. Soil Biol Biochem 41:1173–1179
Newsham KK, Fitter AH, Watkinson AR (1995) Multi-functionality and biodiversity in arbuscular mycorrhizas. Trends Ecol Evol 10:407–411
Opik M, Moora M, Liira J, Zobel M (2006) Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J Ecol 94:778–790
Prati D, Bossdorf O (2004) Allelopathic inhibition of germination by Alliaria petiolata (Brassicaceae). Am J Bot 91:285–288
Rees GN, Baldwin DS, Watson GO, Perryman S, Nielsen DL (2004) Ordination and significance testing of microbial community composition derived from terminal restriction fragment length polymorphisms: application of multivariate statistics. Antonie Van Leewenhoek Int J Gen Mol Biol 86:339–347
Remy W, Taylor TN, Hass H, Kerp H (1994) Four-hundred-million-year-old vesicular-arbuscular mycorrhizae. Proc Natl Acad Sci USA 91:11841–11843
Ridenour WM, Callaway RM (2001) The relative importance of allelopathy in interference: the effects of an invasive weed on a native bunchgrass. Oecologia 126:444–450
Rillig MC (2004) Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecol Lett 7:740–754
Roberts KJ, Anderson RC (2001) Effect of garlic mustard Alliaria petiolata (Beib. Cavara & Grande) extracts on plants and arbuscular mycorrhizal (AM) fungi. Am Midland Nat 146:146–152
Schlaepfer MA, Sherman PW, Blossey B, Runge MC (2005) Introduced species as evolutionary traps. Ecol Lett 8:241–246
Schwartz MW, Hoeksema JD, Gehring CA, Johnson NC, Klironomos JN, Abbott LK, Pringle A (2006) The promise and the potential consequences of the global transport of mycorrhizal fungal inoculum. Ecol Lett 9:501–515
Sikes BA, Cottenie K, Klironomos JN (2009) Plant and fungal identity determines pathogen protection of plant roots by arbuscular mycorrhizas. J Ecol 97:1274–1280
Smith SE, Read DJ (2008) Mycorrhizal Symbiosis. Academic Press, London, U.K
Stinson KA, Campbell SA, Powell JR, Wolfe BE, Callaway RM, Thelen GC, Hallett SG, Prati D, Klironomos JN (2006) Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLoS Biology 4:727–731
Trouvelot S, van Tuinen D, Hijri M, Gianinazzi-Pearson V (1999) Visualization of ribosomal DNA loci in spore interphasic nuclei of glomalean fungi by fluorescence in situ hyhridization. Mycorrhiza 8:203–206
Tsao R, Yu Q, Friesen I, Potter J, Chiba M (2000) Factors affecting the dissolution and degradation of oriental mustard-derived sinigrin and allyl isothiocyanate in aqueous media. J Agri Food Chem 48:1898–1902
van der Heijden MGA (2004) Arbuscular mycorrhizal fungi as support systems for seedling establishment in grassland. Ecol Lett 7:293–303
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72
van der Putten WH, Klironomos JN, Wardle DA (2007) Microbial ecology of biological invasions. ISME J 1:28–37
van Tuinen D, Jacquot E, Zhao B, Gollotte A, Gianinazzi-Pearson V (1998) Characterization of root colonization profiles by a microcosm community of arbuscular mycorrhizal fungi using 25S rDNA-targeted nested PCR. Mol Ecol 7:879–887
Vaughn SF, Berhow MA (1999) Allelochemicals isolated from tissues of the invasive weed garlic mustard (Alliaria petiolata). J Chem Ecol 25:2495–2504
Wehner J, Antunes PM, Powell JR, Mazukatow J, Rillig MC (2010) Plant pathogen protection by mycorrhizas: diversity takes central role. Pedobiol. 53:197–201
Wolfe BE, Rodgers VL, Stinson KA, Pringle A (2008) The invasive plant Alliaria petiolata (garlic mustard) inhibits ectomycorrhizal fungi in its introduced range. J Ecol 96:777–783
Zar JH (1984) Biostatistical analysis, 2nd Ed edn. Prentice-Hall, Englewood Cliffs, NJ, USA
Zhang Q, Yao LJ, Yang RY, Yang XY, Tang JJ, Chen X (2007) Potential allelopathic effects of an invasive species Solidago canadensis on the mycorrhizae of native plant species. Allelopath J 20:71–77
Acknowledgments
We thank Michael Mucci and Lindsay Wilson for technical assistance. Isabelle Meusnier and the Biodiversity Institute of Ontario helped us with the T-RFLP analysis. A.M.K. was supported by fellowships from the Swiss National Science Foundation (PBLAA-114210 and PAOOA-119519) and the Roche Research Foundation. D.L.M. was supported by grants from National Science Foundation Ecology (0515904) and United States Department of Agriculture—Cooperative State Research, Education, and Extension Service (2005-35320-16267). We also thank the Natural Sciences and Engineering Research Council of Canada for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Alexander M. Koch and Pedro M. Antunes contributed equally to the work.
Rights and permissions
About this article
Cite this article
Koch, A.M., Antunes, P.M., Kathryn Barto, E. et al. The effects of arbuscular mycorrhizal (AM) fungal and garlic mustard introductions on native AM fungal diversity. Biol Invasions 13, 1627–1639 (2011). https://doi.org/10.1007/s10530-010-9920-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-010-9920-7