Abstract
The influence of a water deficit treatment and mycorrhizal inoculation with Pisolithus tinctorius (Pers.) Coker and Couch on the water relations, gas exchange, and plant growth in Arbutus unedo L. plants was studied in order to evaluate the hardening process during the nursery period. The ability to withstand the adverse conditions after transplantation was also studied. Mycorrhizal and non-mycorrhizal seedlings of A. unedo were pot-grown for 4 months in a greenhouse (nursery period), during which time two irrigation treatments, well watered (100% water holding capacity, leaching 20% of the applied water) and deficit irrigation (50% of the well watered), were applied. Subsequently, the plants were transplanted to the field and well irrigated (transplanting period), after which and until the end of the experiment they received no water (establishment period). At the end of the nursery period, both water deficit and mycorrhizae were seen to have altered the plant morphology. Mycorrhizal plants had lower leaf area and improved leaf color parameters, while the water deficit increased root dry weight and the root/shoot ratio. Mycorrhizal plants had higher leaf water potential values than non-inoculated plants. Mycorrhizae increased stomatal conductance and photosynthesis values, especially in stressed plants. Drought led to an osmotic adjustment and a decrease in the leaf water potential values at turgor loss point in the mycorrhizal plants. Cell wall rigidity, measured as increased bulk modulus of elasticity, was decreased by the mycorrhizae effect. After transplanting, no differences were found in the water relations or gas exchange values between treatments. During the establishment period, the plants that had been exposed to both drought and mycorrhizae showed a better water status (higher leaf water and turgor potential values) and higher gas exchange values. In conclusion, water deficit and mycorrhizal inoculation of A. unedo plants in nursery produced changes in tissue water relations, gas exchange, and growth, related with the acclimation process in the seedlings, which could provide better resistance to drought and stress conditions following planting.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The morphological, nutritional, and physiological shoot and root characteristics of plants grown under nursery conditions and their performance following transplantation may be critical for optimizing high-quality plants and essential for their successful establishment and survival (Franco et al. 2006). Numerous reports have determined that deficit irrigation during nursery affects several aspects related to the hardening of ornamental plants, such as reductions in leaf area, changes in root morphology, osmotic adjustment, and stomata closure (De Herralde et al. 1998).
Mycorrhizal symbiosis also affects the root morphology and its functioning, which often causes physiological changes in host plant in both well-watered (Danneberg et al. 1992; Drüge and Schönbeck 1992) and water-stressed (Goicoechea et al. 1995, 1996) conditions. This symbiosis can increase drought resistance of the host, improving the plant water status as a result of changes in water relations due to a mechanism related with water uptake (Ruíz-Lozano and Azcón 1995; Duan et al. 1996; Morte et al. 2000; Dell’Amico et al. 2002) and/or phosphorus nutrition (Fitter 1988). Several authors concluded that increases in stomatal conductance and transpiration of plants infected by mycorrhizae are related to higher leaf P concentrations (Nelsen and Safir 1982; Duan and Augé 1992), while others maintain that these responses are not mediated by the P status (Augé et al. 1986; Augé and Stodola 1990).
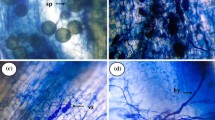
Modifications of host plant–water relations induced by mycorrhizal fungi have been reported for arbuscular mycorrhizal symbiosis, but less information exists about the effects caused by arbutoid mycorrhizae. Pisolithus tinctorius (Pers.) Coker and Couch forms arbutoid mycorrhiza with Arbutus unedo L., characterized by a cruciform structure usually branched in pinnate pattern, a few hyphae along the cruciform roots without forming a true mantle (Navarro et al. 2009). These hyphae penetrate between the epidermical cells, forming the Hartig net, and invade the epidermical cells. A. unedo roots have two layers of cortical cells, and the hyphae penetrate some cells of the first cortical layer but never the endodermis or central cylinder (Navarro et al. 2009).
A. unedo is a sclerophyllus evergreen shrub, characteristic of Mediterranean coastal scrub vegetation, which is of great interest in gardening and landscaping. Its commercial value as a potted plant has also been evaluated (Navarro et al. 2007). It is a species well adapted to Mediterranean climatic conditions, and the effect of drought on water relations, leaf gas exchange, and growth in this species has been widely reviewed (Araújo-Alves et al. 2000; Munné-Bosch and Peñuelas 2004; Serrano et al. 2005). However, the effects of the arbutoid mycorrhiza on A. unedo plants and the combined effect of water deficit and mycorrhiza have not been studied.
Therefore, the purpose of this work was to test the effects of water deficit on the response of A. unedo seedlings associated with P. tinctorius. Changes in growth, ornamental characteristics, nutritional status, water relations, and gas exchange of A. unedo seedlings during the nursery period were evaluated and related with its ability to withstand adverse conditions during establishment in field conditions.
Materials and methods
Plant material
A. unedo (native of SE Spain) seedlings of 2 years from multi-alveolar trays of black polyethylene with dimensions for alveolus of 50 × 50 × 115 mm filled with black peat were acquired. Only seedlings of 20–22 cm height were chosen for transplanting in order to homogenize the plant material used in the experiment.
Fungal inoculum
A spore suspension of P. tinctorius (from ripe sporocarps collected from a Quercus rotundifolia orchard in Campus de Espinardo, University of Murcia, Spain) was used as mycorrhizal inoculum. The spore suspension was prepared according to Castellano and Molina (1989) with a concentration of approximately 107 spores per plant.
Plant growth conditions
Seedlings were transplanted into 2.5-l plastic pots filled with a mixture of clay-loam soil, vermiculite, and black peat (5:3:2, v/v/v) previously sterilized by autoclaving (115°C, 60 min) on three alternate days and amended with 2 g l−1 substrate of osmocote plus (10:11:18 N, P, K + microelements). The experiment was conducted during 2004 in Santomera (Murcia, Spain) in a plastic greenhouse equipped with a cooling system. The plants were irrigated three to five times per week, depending on the evapotranspirational demand, using a drip irrigation system. The micro-climatic conditions were registered with an Escort Junior Data Logger (Escort Data Loggers, Inc., Buchanan, VA, USA). The maximum/minimum average temperatures in the greenhouse were 33/18°C and the relative humidity ranged between 55% and 80%. The average maximum photosynthetically active radiation was 960 μmol m−2 s−1.
At 2 months after transplanting (26 February 2004), 120 plants (30 per treatment) were subjected to four treatments lasting 4 months (from 26 February to 30 June 2004): two inoculation treatments (mycorrhizal, M and non-mycorrhizal, NM) by two irrigation treatments (deficit, D and well-watered, WW). Mycorrhizal plants were inoculated with a spore suspension of P. tinctorius by placing inoculum close to the roots. The remaining plants were not inoculated. Well-watered irrigation treatment plants were watered to 100% water holding capacity (leaching 20% of the applied water), while deficit irrigation plants received 50% of the well-watered irrigation.
On 30 June 2004 (end of nursery period), 15 plants per treatment were transplanted to the field. Prior to transplanting, the plants were watered abundantly and after transplanting were well irrigated by drip using one emitter per plant, each delivering 1 l h−1 until 27 July 2004 (transplanting period), after which and until the end of the experiment (2 September 2004) they received no water (establishment period).
Fungal colonization assessment
At harvest (end of the nursery period), root samples were collected from five root systems per treatment and examined for mycorrhizal colonization. Roots were observed by stereomicroscope (Olympus SZH) to determine the pattern of the mycorrhizal system and the presence of a mantle along the mycorrhizal roots.
To quantify the intracellular P. tinctorius colonization, the root samples were cleared and trypan blue-stained according to Phillips and Hayman (1970) but using lactic acid instead of lactophenol, and 100 root segments per plant were mounted on slides, squashed by pressing on the cover glasses and quantified for colonization according to McGonigle et al. (1990). This quantification was carried out 1 month after mycorrhizal application and at the end of the nursery period to check the presence or absence of mycorrhiza.
Growth and ornamental characters
At the end of the nursery period, the relative chlorophyll content (RCC) and leaf color were determined in three leaves per plant and ten plants per treatment. RCC was measured at the midpoint of mature leaves with a Minolta SPAD-502 chlorophyll meter (Konica Minolta Sensing Inc., Osaka, Japan). Leaf color was measured with a Minolta CR-10 colorimeter (Konica Minolta Sensing Inc.), giving the color coordinates lightness (L*), chroma (C*), and hue angle (h°) (McGuire 1992).
The substrate was gently washed from the roots, ten plants per treatment were divided into leaves, stems, and roots, and the fresh weights were measured. Leaf area and leaf number were measured using a Delta-T Leaf Area Meter (Device Ltd., Cambridge, UK). Later, the different parts of the plants were oven-dried at 70°C until they reached a constant mass to measure the respective dry weights. With these data, the root/shoot ratio was determined.
Mineral content
At the end of the nursery period, B, Ca, Cu, Fe, K, Mg, Mn, N, Na, P, S, and Zn were determined in leaves and roots of ten plants per treatment. The determination of the mineral content was carried out on dry material, which was milled to a particle size able to pass through a 0.5-mm-diameter mesh and stored in plastic containers until later chemical analysis. B, Ca, Cu, Fe, K, Mg, Mn, Na, P, S, and Zn (mg g−1) were determined in the extract, obtained after nitric perchloric digestion (HNO3:HCLO4, 2:1, v/v) of dry vegetable powder, with an atomic absorption spectrophotometer (Perkin-Elmer mod. 5500, USA). Plant tissue N was determined using an elemental analyzer (Thermo Finnigan Flash EA 1112 Series, USA).
Water relations
Daily evolutions of leaf water (Ψ l), leaf osmotic (Ψ s), and leaf turgor (Ψ t) potential were registered on 30 June (end of the nursery period), 27 July (end of transplanting period), and 2 September 2004 (1 month after establishment) in five plants per treatment from dawn to sunset at 2-hour intervals. To measure Ψ l, the sampled leaves were enclosed in a plastic bag (Turner 1988), which was immediately placed in a pressure chamber (Soil Moisture Equipment Co., Santa Barbara, CA, USA) according to Scholander et al. (1965), raising the pressure at a rate of 0.02 MPa s−1 using nitrogen gas. Ψ s was estimated using a vapor pressure osmometer (Wescor 5520, Wescor Inc., Logan, UT, USA) in excised leaves harvested and immediately frozen in liquid nitrogen (−170°C) and stored at −30°C. Before the measurements, samples were thawed and leaf sap was extracted for immediate Ψ s determination according to Gucci et al. (1991). Ψ t was estimated as the difference between Ψ l and Ψ s.
Estimates of the bulk modulus of elasticity (ε) at 100% RWC, relative water content at zero turgor (RWC0), osmotic potential at full turgor (Ψ 100s), and zero turgor (Ψ 0s) were obtained at the end of the nursery period in four leaves per plant and five plants per treatment via pressure–volume analysis of leaves, as outlined by Hinckley et al. (1980). Bulk modulus of elasticity (ε) at 100% RWC was calculated using the formula:
where ε is expressed in megapascals, Ψ 100s is the osmotic potential at full turgor (MPa), and RWC0 is the relative water content at zero turgor.
Leaves were excised in the dark, placed in plastic bags, and allowed to reach full turgor by dipping the petioles in distilled water overnight (Davis and Mooney 1986; Yoon and Richter 1990). Pressure–volume curves were obtained from periodic measurements of leaf weight and balance pressure as leaves dried on the bench at constant temperature of 20°C. The leaf drying period in each curve was about 3–5 h.
Soil and substrate water content
Substrate and soil water content were measured each month in the pots and the field by measuring the volumetric content of water in the substrate and soil (θ v) in the top 15 cm using time domain reflectometry probes (TDR) (Topp et al. 1980, 1982) attached to a TEKTRONIX 1502C in five replicates per treatment.
Gas exchange
Net photosynthesis (P n) and stomatal conductance (g s) were determined on the same days and in the same plants as Ψ l using a portable gas-exchange system (LI-6400, LI-COR Inc., Lincoln, NE, USA). Measurements were made from sunrise to sunset, at 2-hourly intervals on the abaxial surface of well-developed, sun-exposed leaves.
Experimental design and statistical analyses
In the nursery period, each treatment comprised 30 plants divided into three randomly distributed blocks of ten plants. In the transplanting and establishment period, each treatment comprised 15 plants divided into three randomly distributed blocks of five plants.
The data were analyzed by two-way analyses of variance using Statgraphics Plus 5.1 for Windows. Treatment means were separated with Duncan’s multiple range test (P < 0.05). Ratio and percentage data were arcsine-transformed before statistical analysis to ensure homogeneity of variance.
Results
Nursery period
Mycorrhizal colonization reached values of 34% (Table 1), which neither the drought effect nor the interaction between drought and mycorrhizae influenced. The water deficit treatment significantly increased root dry weight (100%) and root/shoot ratio (59%), but did not affect leaf area or leaf number. While leaf area of mycorrhizal plants decreased, the root/shoot ratio increased. Growth parameters were statistically unaffected by the interaction of both factors (Table 1).
Changes in RCC and in color characteristics were unaffected by the water deficit. However, M plants, independently of soil water conditions, had higher chroma and lightness values (Table 2).
With respect to the mineral nutrition, the leaves of M plants showed higher concentrations of B, K, and Mn than non-mycorrhizal (NM) plants (Table 3), with increases of 17%, 13%, and 60%, respectively, but a lower Zn concentration (11%). The deficit irrigation induced lower Cu, K, and Na concentrations and higher S and Zn concentrations (Table 3). This effect was particularly pronounced in the K and Na concentrations, which decreased by 46% and 51%. The roots of M plants had higher Cu, K, Mg, Mn, and Zn concentrations than the NM plants (Table 3). Deficit irrigation decreased the Ca concentration by 13% and increased K and P by 126% and 39%, respectively, compared with the well-watered irrigation treatment (Table 3).
The soil water content decreased in deficit irrigation treatments, although the differences between M and NM plants were not evident until the end of the nursery period (June), coinciding with high temperatures (Fig. 1a).
Seasonal patterns of substrate and soil water content (TDR volumetric percentage) during the nursery period (a) and during the transplanting and establishment periods (b) in well-watered irrigation mycorrhizal (WWM), well-watered irrigation non-mycorrhizal (WWNM), deficit irrigation mycorrhizal (DM), and deficit irrigation non-mycorrhizal (DNM) treatments. Values are means (n = 5) and vertical bars indicate standard errors
The water deficit and mycorrhizal colonization caused significant differences in the water relations of the A. unedo plants (Figs. 2 and 3). The diurnal patterns of leaf water potential (Ψ l) and turgor potential (Ψ t) showed the highest values at early morning and the lowest at midday (Fig. 2a, b), after which the values recovered. Significant differences in Ψ l levels were noted between treatments. The values of Ψ l were, in general, always higher in the inoculated well-watered plants and lower in the non-inoculated water deficit plants. The well-watered NM plants and the water deficit M plants had similar values. The differences between treatments for Ψ t values were lower than those observed for Ψ l (Fig. 2b).
Diurnal patterns of leaf water potential (Ψ l; a), leaf turgor potential (Ψ t; b), net photosynthetic (P n; c), and stomatal conductance (g s; d) at the end of the nursery period in A. unedo seedlings with well-watered irrigation mycorrhizal (WWM), well-watered irrigation non-mycorrhizal (WWNM), deficit irrigation mycorrhizal (DM), and deficit irrigation non-mycorrhizal (DNM) treatments. Values are means (n = 5) and vertical bars indicate standard errors
Osmotic potential at full turgor (Ψ 100s; a) and osmotic potential at zero turgor (Ψ 0s; b) in A. unedo seedlings at the end of nursery period with well-watered irrigation mycorrhizal (WWM), well-watered irrigation non-mycorrhizal (WWNM), deficit irrigation mycorrhizal (DM), and deficit irrigation non-mycorrhizal (DNM) treatments. Each histogram represents the mean of five values and the vertical bars indicate standard errors
The highest values of g s and P n corresponded to the well-watered inoculated plants, as did Ψ l. Both g s and P n decreased to a similar extent in both water deficit treatments, but more significantly in NM plants (Fig. 2c, d). In all treatments, a marked decrease in g s and P n values was observed in the afternoon. From this moment, there were no differences between treatments in these parameters.
Parameters derived from the pressure–volume curves are shown in Fig. 3. The leaf osmotic potential at full turgor (Ψ 100s) decreased in the water deficit inoculated plants (Fig. 3a), indicating leaf osmotic adjustment. In these same plants, leaf potential at zero turgor (Ψ 0s) was lower, showing values of −3.34 MPa (Fig. 3b). The bulk modulus of elasticity (ε) decreased from 12.58 to 9.76 MPa (data not shown) as a result of mycorrhizal colonization.
Transplanting and establishment period
After the nursery period, when all the plants had been transplanted and were well irrigated, the differences in the soil water content between irrigation treatments diminished, with similar recorded in all treatments (19%) except for the DM treatment, where it was about 15% (Fig. 1b). These soil conditions induced similar Ψ l and Ψ t diurnal patterns in all treatments. High and similar values of Ψ l and Ψ t at dawn and minimum values at midday were observed in all the treatments (Fig. 4a, b). The diurnal patterns of g s and P n (Fig. 4c, d) showed much greater variability, although inoculated plants tended to have the highest values. At the end of the experiment (establishment period), a significant decrease in the soil water content was observed and no differences between treatments were found (Fig. 1b) since irrigation was withheld from all the plants for 1 month. In general, Ψ l and Ψ t reflected the dehydration suffered during the field conditions (Fig. 5a, b), both showing lower values than during the transplanting period. Nevertheless, the highest values of Ψ l during the first hours of the day were for the plants that had been exposed to deficit irrigation during nursery, concretely when combined with mycorrhizae (Fig. 5a). As the day progressed, lower values of Ψ l and Ψ t were seen coinciding with increasing vapor pressure deficit, after which these values were not recovered in the NM plants (Fig. 5a, b). In this period, although the values of both gas exchange parameters were lower than those for the transplanting period in all treatments, the lowest values of P n and g s were for the plants that had been well irrigated and non-inoculated during nursery period (Fig. 5c, d).
Diurnal patterns of leaf water potential (Ψ l; a), leaf turgor potential (Ψ t; b), net photosynthetic (P n; c), and stomatal conductance (g s; d) after transplanting in A. unedo seedlings with well-watered irrigation mycorrhizal (WWM), well-watered irrigation non-mycorrhizal (WWNM), deficit irrigation mycorrhizal (DM), and deficit irrigation non-mycorrhizal (DNM) treatments. Values are means (n = 5) and vertical bars indicate standard errors
Diurnal patterns of leaf water potential (Ψ l; a), leaf turgor potential (Ψ t; b), net photosynthetic (P n; c), and stomatal conductance (g s; d) during establishment in A. unedo seedlings with well-watered irrigation mycorrhizal (WWM), well-watered irrigation non-mycorrhizal (WWNM), deficit irrigation mycorrhizal (DM), and deficit irrigation non-mycorrhizal (DNM) treatments. Values are means (n = 5) and vertical bars indicate standard errors
Discussion
Mycorrhizal colonization percentage in A. unedo (34%) was similar to those obtained in eucalyptus (28%; Mason et al. 2000) and in pine (22%; Walker 2001) with the same fungal species P. tinctorius. The host–fungus compatibility between A. unedo and P. tinctorius was previously demonstrated (Navarro et al. 2009). Moreover, deficit irrigation did not affect the mycorrhizal colonization percentage. Previous studies have shown that the percentage of roots colonized by mycorrhizal fungi is not affected by water deficit (Nelsen and Safir 1982; Davies et al. 1992; Bryla and Duniway 1997; Morte et al. 2000).
The plants subjected to deficit irrigation showed a different distribution of biomass between the aerial part and the root system. Significant increases in root growth and the root/shoot ratio were seen in response to drought (Table 1), responses that have been observed by several authors in different species (Molyneux and Davies 1983; Khalil and Grace 1992; Palta and Gregory 1997). Under drought conditions, this behavior may improve the ability of a plant to obtain water and to acquire nutrients. The morphological characteristics of plant growth in nursery conditions may determine its ability to survive and grow following transplantation in different environmental conditions (Leskovar 1998). The morphology of A. unedo changed when combined with mycorrhizae (Table 1). P. tinctorius has been associated with significant growth reductions in the host plant (Tonkin et al. 1989; Eltrop and Marschner 1996), as was observed in the aerial part (leaf area) in our conditions. It also represents an important adaptation to drought since it reduces the transpiration demand (Sánchez-Blanco et al. 2004a). Also, mycorrhizal colonization induced morphological changes to the root system of the plants, which must be considered positive; this has been seen to affect the successful establishment of many ornamental woody species, particularly in adverse environmental conditions (Hooker et al. 1992; Maestre et al. 2002; Goicoechea et al. 2004). However, other authors observed that P. tinctorius has no effect in plant development in other host species (Svenson et al. 1991; Mason et al. 2000; Walker 2001).
Deficit irrigation did not cause changes in RCC or the color characteristics of A. unedo (Table 2), which is an important observation since it is used as an ornamental species. The leaf color of M plants was lighter (increased lightness) and gained in saturation or vividness (increased chroma) compared with the NM plants (Table 2), thus improving their ornamental quality. This suggests that the benefit granted by P. tinctorius inoculation would affect aerial part quality rather than quantity.
Changes in plant growth were associated with root colonization by P. tinctorius, as a response that has frequently been correlated with increases in phosphorus nutrition of host plants (Rousseau et al. 1992; Burgess et al. 1993; Thomson et al. 1994), although this was not observed in our experiment. In contrast, increases in the Mn, K, and Zn concentrations of roots and leaves were detected (Table 3). P. tinctorius therefore seems to participate in mineral transformations that increase the availability of some nutrients in the soil (Cairney and Ashford 1989). There is direct evidence that P. tinctorius increased Mn accumulation in Pinus virginiata plants (Miller and Rudolph 1986) and may displace K from the soil and make it more available to the plants, probably through secretion of oxalate (Paris et al. 1995, 1996). Increases in K and Mg (Hall 1978; Azcón and Ocampo 1981), Ca (Pai et al. 1994), Cu, and Mn (Sylvia et al. 1993) have also been observed in arbuscular mycorrhizal plants. Indirectly, these increments may have an impact on host plant drought resistance.
Under drought conditions, the availability of nutrients for the plant is limited, fundamentally due to the low mobility of mineral ions in the soil (Subramanian 1997). This was seen from the Cu, K, and Na leaf concentrations, which may participate in the enzymatic processes involved in amino acid transformation in plants and improve the protection of plants when they are subjected to unfavorable environmental situations. The contrary occurred for Zn and S concentrations.
The K concentration of plants was increased by the effect of mycorrhizal colonization (Table 3). Potassium plays an important role in the drought tolerance of plants and is related to stomatal movement in response to the changes produced in leaf water status (Premachandra et al. 1993). Ruíz-Lozano et al. (1995) observed that the protection of M plants against water stress is partially related to K uptake. The significant increase in P levels observed in the roots of non-mycorrhizal plants subjected to deficit irrigation should be considered as a positive effect for maintaining root growth and prolonging the period of root development (Li et al. 2001).
Osmotic adjustment plays an important role in the response of plant to hardening (Bañón et al. 2006). This mechanism was developed by A. unedo in response to a combination of mycorrhiza and water deficit conditions and these plants therefore presented higher leaf turgor values (Fig. 3b). This was accompanied by lower Ψ 0s values, which indicates that the turgor loss point is reached at lower leaf water potential. These same plants exhibited Ψ l values that were higher throughout the day than in NM plants under water deficit conditions, which were also observed by Subramanian et al. (1995). The higher Ψ l values in mycorrhizal plants may be due to a higher water uptake capacity by the roots as a result of better hydraulic conductivity in the root system (Sánchez-Blanco et al. 2004b). This was also reported in this same species by Navarro et al. (2009).
Turgor can also be maintained by changes in the elasticity of the cellular walls (Radin 1983). In this sense, mycorrhizal colonization decreased the bulk modulus of elasticity in the A. unedo plants, independently of the substrate water conditions (data not shown), and seems to induce an increase in the elasticity of the cell walls. Similar results but in alfalfa plants inoculated with Glomus fasciculatum and/or Rhizobium meliloti were found by Goicoechea et al. (1997).
As many other Mediterranean shrubs, A. unedo shows a conservative strategy in the use of water (Tenhunen et al. 1990), mainly based on the avoidance of drought stress through reducing stomatal conductance, as was observed in water deficit (M and NM) plants (Fig. 2d). M plants, in general, had higher gas exchange levels that NM plants. This effect has also been observed in other species (Augé et al. 1992; Ebel et al. 1997; Morte et al. 2001) and could be related to the better water status since it has been associated with altered root or whole plant hydraulic conductivity (Allen et al. 1981). Besides, mycorrhizal colonization induced plants subjected to the deficit irrigation to show higher stomatal conductance than NM plants. This effect has been reported in cultivated pot plants and in field conditions (Thomas et al. 1976; Ackerson 1980; Augé et al. 1986; Sánchez-Blanco et al. 2004b).
After the plants had left the nursery and after rewatering (transplanting period), all plant showed a similar water status, although the Ψ l values at midday were lower than in the nursery period due the higher evaporative demand in the transplanting period. During the drought period (establishment), the mycorrhizal plants showed a significantly better water status and the water deficit treatment produced a g s above that of the well-watered condition (Fig. 5d), that is, they showed a higher degree of stomatal opening than non-hardened plants (Spence et al. 1986). The parallel behavior of P n and g s during the experiment explains why the reduction in photosynthetic rates under drought has been mainly attributed to stomatal conductance reductions (De Herralde et al. 1998).
Conclusions
A. unedo plants responded to deficit irrigation through a water conservation strategy, which involved reducing stomatal conductance and the increasing root/shoot ratio. The mycorrhizal symbiosis had a beneficial effect on plant water status and increased photosynthetic activity.
The combined effect of mycorrhizal inoculation and drought in the nursery produced a hardening process in A. unedo seedlings through osmotic adjustment and stomatal regulation. This improved the water status of plants (higher leaf water and turgor potential) and induced a lower turgor loss point, which could provide better resistance to drought and stress conditions following planting.
References
Ackerson RC (1980) Stomatal response of cotton to water stress and abscisic acid as affected by water stress history. Plant Physiol 65:455–459
Allen MF, Smith WK, Moore TS, Christensen M (1981) Comparative water relations and photosynthesis of mycorrhizal and non-mycorrhizal Bouteloua gracilis H.B.K. Lag. ex Steud. New Phytol 88:683–693
Araújo-Alves JP, Torres-Pereira JM, Biel C, De Herralde F, Savé R (2000) Effects of minimum irrigation technique on ornamental parameters of two Mediterranean species used in xerigardening and landscaping. Acta Horticulturae 541:353–358
Augé RM, Stodola AJW (1990) An apparent increase in symplastic water contributes to greater turgor in mycorrhizal roots of droughted Rosa plants. New Phytol 115:285–295
Augé RM, Schekel KA, Wample RL (1986) Osmotic adjustment in leaves of VA mycorrhizal and non mycorrhizal rose plants in response to drought stress. Plant Physiol 82:765–770
Augé RM, Brown MS, Bethlenfalvay GJ, Stodola AJW (1992) Stomatal response of mycorrhizal cowpea and soybean to short-term osmotic stress. New Phytol 120:117–125
Azcón R, Ocampo JA (1981) Factors affecting the vesicular-arbuscular infection and mycorrhizal dependency of thirteen wheat cultivars. New Phytol 87:677–685
Bañón S, Ochoa J, Franco JA, Alarcón JJ, Sánchez-Blanco MJ (2006) Hardening of oleander seedlings by deficit irrigation and low air humidity. Environ Exp Bot 56(1):36–43
Bryla DR, Duniway JM (1997) Growth, phosphorus uptake, and water relations of safflower and wheat infected with an arbuscular mycorrhizal fungus. New Phytol 136:581–590
Burgess T, Malajczuk N, Grove TS (1993) The ability of 16 ectomycorrhizal fungi to increase growth and phosphorus uptake by Eucalyptus globulus Labill and E. diversicolor F. Muell. Plant Soil 153:155–164
Cairney JWG, Ashford AE (1989) Reducing activity at the root surface in Eucalyptus pilularis–Pisolithus tinctorius ectomycorrhizas. Aust J Plant Physiol 16:99–105
Castellano MA, Molina R (1989) Mycorhizae. In: Landis TD, Tinus TD (eds) The biological component: nursery pest and mycorrhizae. Agricultural Handbook 674. USDA Forest Service, Washington, pp 101–171
Danneberg G, Latus C, Zimmer W, Hundeshagen B, Schneider-Poetch HJ, Bothe H (1992) Influence of vesicular–arbuscular mycorrhiza on phytohormone balances in maize (Zea mays L.). J Plant Physiol 141:33–39
Davies FT Jr, Potter JR, Linderman PG (1992) Mycorrhiza and repeated drought exposure effect drought resistance and extraradical hyphae development of pepper plants independent of plant size and nutrient content. J Plant Physiol 139:289–294
Davis SD, Mooney HM (1986) Tissue water relations of four co-occurring chaparral shrubs. Oecologia 70:527–535
De Herralde F, Biel C, Savé R, Morales MA, Torrecillas A, Alarcón JJ, Sánchez-Blanco MJ (1998) Effect of water and salt stresses on the growth, gas exchange and water relations in Argyranthemum coronopifolium plants. Plant Sci 139:9–17
Dell’Amico J, Torrecillas A, Rodríguez P, Morte A, Sánchez-Blanco MJ (2002) Responses of tomato plants associated with the arbuscular mycorrhizal fungus Glomus clarum during drought and recovery. J Agr Sci 138:387–393
Drüge U, Schönbeck F (1992) Effect of vesicular–arbuscular mycorrhiza infection on transpiration, photosynthesis and growth of flax (Linum usitatissimum L.) in relation to cytokinin levels. J Plant Physiol 141:40–48
Duan X, Augé RM (1992) Stomatal response to short-term osmotic stress of cowpea leaves given varying phosphorus fertilization. J Plant Nutr 15:265–274
Duan X, Neuman DS, Reiber JM, Green CD, Saxton AM, Augé RM (1996) Mycorrhizal influence on hydraulic and hormonal factors involved in the of stomatal conductance during drought. J Exp Bot 47:1541–1550
Ebel RC, Duan X, Still DW, Augé RM (1997) Xylem sap abscisic acid concentration and stomatal conductance of mycorrhizal Vigna unguiculata in drying soil. New Phytol 135:755–761
Eltrop L, Marschner H (1996) Growth and mineral nutrition of non-mycorrhizal and mycorrhizal Norway spruce (Picea abies) seedlings grown in semi-hydroponic sand culture. I. Growth and mineral nutrient uptake in plants supplied with different forms of nitrogen. New Phytol 133:469–478
Fitter AH (1988) Water relations of red clover Trifolium pratanse L. as affected by VA mycorrhizal infection and phosphorus supply before and during drought. J Exp Bot 39:599–608
Franco JA, Martínez-Sánchez JJ, Fernández JA, Bañón S (2006) Selection and nursery production of ornamental plants for landscaping and xerogardening in semi-arid environments. J Hort Sci Biotechnol 81(1):3–17 (review article)
Goicoechea N, Dolezal K, Antolin MC, Strnad M, Sánchez-Díaz M (1995) Influence of mycorrhizae and Rhizobium on cytokinin content in drought-stressed alfalfa. J Exp Bot 46:1543–1549
Goicoechea N, Antolín MC, Strnad M, Sánchez-Díaz M (1996) Root cytokinins, acid phosphatase and nodule activity in drought-stressed mycorrhizal or nitrogen fixing alfalfa plants. J Exp Bot 47:683–686
Goicoechea N, Antolín MC, Sánchez-Díaz M (1997) Influence of arbuscular mycorrhizae and Rhizobium on nutrient content and water relations in drought stressed alfalfa. Plant Soil 192:261–268
Goicoechea N, Merino S, Sánchez-Díaz M (2004) Management of phosphorous and nitrogen fertilization to optimize Anthyllis–Glomus–Rhizobium symbiosis for revegetation of desertified semiarid areas. J Plant Nutr 27:1395–1413
Gucci R, Xiloyannis C, Flore JA (1991) Gas exchange parameters water relations and carbohydrate partitioning in leaves of field-grown Prunus domestica following fruit removal. Physiol Plant 83:497–505
Hall JR (1978) Effect of vesicular–arbuscular mycorrhizas on two varieties of maize and one of sweet corn. New Zeal J Agric Res 21:517–519
Hinckley TM, Duhme F, Hinckley AR, Richter H (1980) Water relations of drought hardy shrubs: osmotic potential and stomatal reactivity. Plant Cell Environ 3:131–140
Hooker JE, Munro M, Atkinson D (1992) Vesicular–arbuscular fungi induced alteration in poplar root system morphology. Plant Soil 145:207–214
Khalil AAM, Grace J (1992) Acclimation to drought in Acer pseudoplatanus L. (sycamore) seedlings. J Exp Bot 43:1591–1602
Leskovar DI (1998) Transplant production and performance. Root and shoot growth modification by irrigation. HortTechnology 8(4):510–514
Li FM, Song QH, Liu XL, Li FR, Liu HS (2001) Effects of pre-sowing irrigation and phosphorus application on water use and yield of spring wheat under semi-arid conditions. Agric Water Manage 49:173–183
Maestre FT, Bautista S, Cortina J, Díaz G, Honrubia M, Vallejo R (2002) Microsite and mycorrhizal inoculum effect on the establishment of Quercus coccifera in semi-arid degraded steppe. Ecol Eng 19:289–295
Mason PA, Ibrahim K, Ingleby K, Munro RC, Wilson J (2000) Mycorrhizal development and growth of inoculated Eucalyptus globulus (Labill.) seedlings in wet and dry conditions in the glasshouse. For Ecol Manag 128:269–277
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular–arbuscular mycorrhizal fungi. New Phytol 115:495–501
McGuire RG (1992) Reporting of objective colour measurements. HortScience 27:1254–1255
Miller FA, Rudolph ED (1986) Uptake and distribution of manganese and zinc in Pinus virginiata seedlings infected with Pisolithus tinctorius. Ohio J Sci 86:22–25
Molyneux DE, Davies WJ (1983) Rooting pattern and water relations of three pasture grasses growing in drying soil. Oecologia 58:220–224
Morte A, Lovisolo C, Schubert A (2000) Effect of drought stress on growth and water relations of the mycorrhizal association Helianthemum almeriense–Terfezia claveryi. Mycorrhiza 10:115–119
Morte A, Díaz G, Rodríguez P, Alarcón JJ, Sánchez-Blanco MJ (2001) Growth and water relations in mycorrhizal and nonmycorrhizal Pinus halepensis plants in response to drought. Biol Plant 44(2):263–267
Munné-Bosch S, Peñuelas J (2004) Drought-induced oxidative stress in strawberry tree (Arbutus unedo L.) growing in Mediterranean field conditions. Plant Sci 166:1105–1110
Navarro A, Sánchez-Blanco MJ, Bañón S (2007) Influence of paclobutrazol on water consumption and plant performance of Arbutus unedo seedlings. Sci Hort 111(2):133–139
Navarro A, Sánchez-Blanco MJ, Morte A, Bañón S (2009) The influence of mycorrhizal infection on water and nutritional status of Arbutus unedo treated and not treated with paclobutrazol. Environ Exp Bot 66:362–371
Nelsen CE, Safir GR (1982) Increased drought tolerance of mycorrhizal onion plants caused by improved phosphorus nutrition. Planta 154:407–413
Pai G, Bagyaraj DJ, Padrnavathi RT, Prasad TG (1994) Calcium uptake by cowpea as influenced by mycorrhizal colonization and water stress. Curr Sci 66:444–445
Palta JA, Gregory PJ (1997) Drought affects the fluxes of carbon to roots and soil in 13C pulse-labeled plants of wheat. Soil Biol Chem 29:1395–1403
Paris F, Bonnaud P, Ranger J, Lapeyrie F (1995) In vitro weathering of phlogopite by ectomycorrhizal fungi. I. Effect of K and Mg deficiency on phyllosilicate evolution. Plant Soil 177:191–201
Paris F, Botton B, Lapeyrie F (1996) In vitro weathering of phlogopite by ectomycorrhizal fungi. II. Effect of K+ and Mg2+ deficiency and N sources on accumulation of oxalate and H+. Plant Soil 179:141–150
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Premachandra GS, Saneoka H, Fujita K, Ogata S (1993) Water stress and potassium fertilization in field grown maize (Zea mays L.): effects on leaf water relation and leaf rolling. J Agron Crop Sci 170:195–201
Radin JW (1983) Physiological consequences of cellular water deficits: osmotic adjustment. In: Taylor HM, Jordan WR, Sinclair JR (eds) Limitation to efficient water use in crop production. American Society of Agronomy, Madison, pp 267–276
Rousseau JVD, Reid CPP, English RJ (1992) Relationship between biomass of the mycorrhizal fungus Pisolithus tinctorius and phosphorus uptake in loblolly pine seedlings. Soil Biol Biochem 24:183–184
Ruíz-Lozano JM, Azcón R (1995) Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiol Plant 95:472–478
Ruíz-Lozano JM, Azcón R, Gómez M (1995) Effects of arbuscular-mycorrhizal Glomus species on drought tolerance: physiological and nutritional plant responses. App Environ Microb 61:456–460
Sánchez-Blanco MJ, Ferrández T, Navarro A, Bañón S, Alarcón JJ (2004a) Effects of irrigation and air humidity preconditioning on water relations, growth and survival of Rosmarinus officinalis plants during and after transplanting. J Plant Physiol 161:1133–1142
Sánchez-Blanco MJ, Ferrández T, Morales MA, Morte A, Alarcón JJ (2004b) Variations in water status, gas exchange and growth in Rosmarinus officinalis plants infected with Glomus deserticola under drought conditions. J Plant Physiol 161:675–682
Scholander PF, Hammel HT, Bradstreet ED, Hemmingsen EA (1965) Sap pressure in vascular plants. Science 148:339–346
Serrano L, Peñuelas J, Ogaya R, Savé R (2005) Tissue–water relations of two co-occurring evergreen Mediterranean species in response to seasonal and experimental drought conditions. J Plant Res 118:263–269
Spence RD, Wu PJH, Clark KG (1986) Water stress effect on guard cell anatomy and the mechanical advantage of the epidermal cells. Plant Cell Environ 9:197–202
Subramanian KS (1997) Role of arbuscular mycorrhizal fungus (Glomus intraradices Schenck & Smith) colonization in drought tolerance of maize (Zea mays L.). PhD thesis. School of Graduate Studies and Research, University of Ottawa, Canada
Subramanian KS, Charest C, Dwyer LM, Hamilton RI (1995) Arbuscular mycorrhizas and water relations in maize under drought stress at tesselling. New Phytol 129:643–650
Svenson SE, Davies FT Jr, Meier CE (1991) Ectomycorrhizae and drought acclimation influence water relations and growth of loblolly pine. HortScience 26(11):1406–1409
Sylvia DM, Hammond LC, Bennet JM, Hass JH, Linda SB (1993) Field response of maize to a VAM fungus and water management. Agron J 85:193–198
Tenhunen JD, Sala Serra A, Harley PC, Dougherty RL, Reynolds JF (1990) Factors influencing carbon fixation and water used by Mediterranean sclerophyll shrubs during summer drought. Oecologia 82:381–393
Thomas JC, Brown KW, Jordan WR (1976) Stomatal response to leaf water potential as affected by preconditioning in the field. Agron J 68:706–708
Thomson BD, Grove TS, Malajczuk N, GEStJ H (1994) The effectiveness of ectomycorrhizal fungi in increasing the growth of Eucalyptus globulus Labill. in relation to root colonization and hyphal development in soil. New Phytol 126:517–524
Tonkin CM, Malajczuk N, McComb JA (1989) Ectomycorrhizal formation by micropropagated clones of Eucalyptus marginata inoculated with isolates of Pisolithus tinctorius. New Phytol 111:209–214
Topp GC, Davis JL, Annan AP (1980) Electromagnetic determination of soil water content: measurements in coaxial transmission lines. Water Resour Res 16(3):574–582
Topp GC, Davis JL, Annan AP (1982) Electromagnetic determination of soil water content using TDR: II. Evaluation of installation and configuration of parallel transmission lines. Soil Sci Soc Am J 46:678–684
Turner NC (1988) Measurement of plant water status by the pressure chamber technique. Irrig Sci 9:289–308
Walker RF (2001) Growth and nutritional responses of containerized sugar and Jeffrey pine seedlings to controlled release fertilization and induced mycorrhization. For Ecol Manag 149:163–179
Yoon TM, Richter H (1990) Seasonal changes in stomatal responses of sweet cherry and plum to water status in detached leaves. Physiol Plant 80:520–526
Acknowledgments
This work was supported by the projects CICYT (AGL 2005-05588-C02-1-2), CDTI (IDI-20070868), Fundación Seneca (05660/PI/07), and CGL 2007-61175.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Navarro García, A., del Pilar Bañón Árias, S., Morte, A. et al. Effects of nursery preconditioning through mycorrhizal inoculation and drought in Arbutus unedo L. plants. Mycorrhiza 21, 53–64 (2011). https://doi.org/10.1007/s00572-010-0310-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-010-0310-x