Abstract
Aims
Most plants benefit from mycorrhizal symbiosis through their improved abilities to take up nutrients and water. Information on the interactive effects of fungal inoculation, nitrogen (N) fertilization and drought on water use efficiency (WUE) and productivity of Canadian wheat varieties is scanty.
Methods
In this study, we investigated the effects of arbuscular mycorrhizal fungi (AMF) inoculation, N fertilization and water regime (well-watered (WW) vs water-deficit (WD)) on WUE, phosphorus (P) and N uptake and growth of spring wheat (Triticum aestivum var. Superb) in a greenhouse experiment.
Results
The specific leaf area (SLA) of flag leaves was significantly increased by AMF inoculation under the two water regimes but was lowered under N fertilization and drought conditions. The AMF inoculation significantly enhanced relative water content under WD. The WUE and instantaneous WUE (WUEi) were enhanced by N fertilization and AMF inoculation under both water regimes. Combined N fertilization and AMF inoculation significantly increased N concentrations in stem and grain, plant height, biomass and grain yield under WD. The P concentrations in stem and grain were increased under WD, irrespective of other treatments applied. The WUE and grain N, stem N, and P concentrations were positively correlated.
Conclusions
Plant physiological characteristics were negatively affected by WD, while N fertilization and AMF inoculation enhanced plant performance under WD, including the increase of N and P concentrations in different componments of spring wheat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wheat (Triticum aestivum) is a major crop grown on the prairie in western Canada, a region characterized by short growing seasons and frequent occurrence of drought and heat stress. In Alberta, wheat is mostly grown under rainfed conditions, where drought can occur at any time during the growing season. In addition to drought, deficiency of nutrients, especially nitrogen (N) and phosphorus (P), is also a major concern for producers on the prairie.
The symbiosis between mycorrhizal fungi and plant roots can promote plant uptake of water and nutrients and improve plant growth (Ho and Trappe 1975; Martin and Plassard 2001). This symbiosis often results in altered rates of water movement into, through and out of host plants, with consequent effects on tissue hydration and plant physiology (Augé 2001).
Mycorrhizal fungi can modify water relations in host plants (Zhang et al. 2013; Nelsen 1987). Leaf water potential is often higher in mycorrhizal plants under drought conditions due to higher water uptake (Augé et al. 1987; Subramanian et al. 1995), which allows such plants to maintain higher water content than non-mycorrhizal plants. Arbuscular mycorrhizal fungi (AMF) associated plants often show higher photosynthetic rates than their non-mycorrhizal counterparts: stomatal conductance (gs), transpiration (Tr) and photosynthesis (Pn) were stimulated by AMF symbiosis about as frequently under non-stressed as under drought conditions (Tawaraya 2003). Different AMF fungi have different effects on stomatal conductance and rate of photosynthesis during drought (Dixon et al. 1994). The AMF symbiosis has been reported to increase the number of photosynthetic units (Allen et al. 1981), photosynthetic storage and export rates (Wang et al. 1989), and water use efficiency (Simpson and Daft 1990).
Previous studies have mostly been focused on the direct effects of different fungal inoculation on plant growth and yield (Gholamhoseini et al. 2013; Wu et al. 2008). The interactive effects of fungal inoculation, drought and N fertilization on the water use efficiency of wheat grown on the Canadian prairie have not been studied. In this study, we used gas exchange measurement and carbon isotope discrimination to measure WUE (Farquhar et al. 1989) and to evaluate the influence of AMF on WUEi/WUE and N, P uptake of spring wheat under two levels of N fertilization and two levels of water regime in a greenhouse experiment.
Materials and methods
Plant material and fungal inoculation
Spring wheat variety Superb, a semi-dwarf high yielding Canada Western Red Spring Wheat variety with an awny head, short and strong straw, large kernel weight, and late maturing, was used for this study.
The mycorrhizal species used in this study was Glomus intraradices (Mix MYKE® PRO PS3). The mycorrhizal species was obtained from Premier Tech Biotechnologies (Québec, Canada). The inoculum (1600 propagules.g−1) was mixed with wheat seed at the rate of 10 mL/kg pre-moistened seeds.
Experimental design and growth conditions
The experimental treatments were made up of two levels of N fertilization (0 kg/ha and 180 kg/ha), two soil water regimes (well-watered, WW, where soil water content was maintained at about 95 % field capacity (FC), and water-deficit, WD, where soil water content was maintained at about 40 % FC) and two mycorrhizal inoculations (Glomus intraradices inoculation and non-mycorrhizal inoculation). The treatment combinations were arranged in a completely randomized block design with 4 replicates per treatment. Thus, four treatments were set up under WW or WD conditions: control (CK): no N fertilization and non-mycorrhizal inoculation, M: mycorrhizal inoculation with no N fertilization, N: 180 kg/ha N fertilization with no mycorrhizal inoculation, MN: 180 kg/ha N fertilization and mycorrhizal inoculation.
The experiment was conducted in the greenhouse at the Alberta Research Council (now part of Alberta Innovates - Technology Futures) in Vegreville. Plants were grown in pots (21 cm tall and 21 cm diameter) filled with 3.5 kg of soil mix containing field topsoil and peat moss in a 1:3 ratio (v:v). All pots were flushed with 2 L tap water and drained overnight to determine field capacity before seeding.
Eight seeds were sown per pot and were thinned to four seedlings per pot after emergence. After thinning, the surface of each pot was covered with a 2 cm layer of perlite to minimize soil surface evaporation. All pots were then weighed and kept well-watered until the stem elongation stage (BBCH 30, Lancashire et al. 1991) when water-deficit was imposed by withholding watering until the soil moisture content was 40 % FC. Soil moisture content of pots in the well-watered treatment was kept at 95 % FC. The target soil moisture levels were then maintained for the well-watered and water-deficit treatments until plant maturity.
Pots were weighed every 1–2 days to monitor water use and adjust watering as plant demand increased. Four pots without plants were used to determine the evaporation rate. Fertilizer treatments were carried out after emergence of seedlings by adding N in form of urea at the rate of 180 kg/ha (0.29 g urea per pot) to pots assigned to the N fertilization treatment.
All pots were placed in a greenhouse with a photoperiod of 16 h. Day and night temperatures in the greenhouse were programmed at 25 and 15 °C, respectively. The experiment was seeded at the end of April and harvested at the end of July in 2009.
Physiological measurements
Specific leaf area (SLA) and relative water content (RWC)
The width and length of flag leaves (5 leaves per pot) were measured and used for leaf area calculation at flowering stage. The leaves were then oven dried at 70 °C to a constant weight (about 48 h). The RWC was determined using the flag leaves. Five leaves per pot were cut and weighed immediately and recorded as fresh weight (FW). Then the leaves were placed in a tray lined with moist filter papers and left in the dark in the laboratory over night, they were blotted dry and re-weighed to obtain turgid weight (TW). The leaves were then dried overnight at 70 °C and weighed (dry weight, DW). The SLA and RWC were calculated as (Rebetzke and Richards 1999):
Instantaneous water use efficiency (WUEi)
Leaf photosynthesis (Pn) and transpiration (Tr) rates were measured on the flag leaf at the flowering stage using a portable LI-6400 photosynthesis system (Li-Cor, Lincoln, USA) with the following settings: flow rate 500 μmol/s, leaf/block temperature 20 °C, photosynthetically active radiation (PAR) 1000 μmol/(m2 s) and ambient CO2 concentration 400 mg/kg (ppm). The leaf area was manually entered for each measurement. Six measurements were taken per leaf of all 6 replicates at 15 s intervals after the CO2/H2O parameters became stable. The WUEi was calculated as Pn/Tr, the ratio of photosynthetic rate to transpiration rate (Anyia et al. 2007).
Carbon isotope discrimination
Five leaves (penultimate and flag) were randomly collected per pot and bulked to form a composite sample to measure leaf carbon isotope composition (δ13C). All samples were oven dried at 70 °C for 48 h. Dried leaf samples were ground with a Spex Sample Prep 8000D ball mill (Metuchen, NJ, USA) to fine power and analyzed for stable carbon isotope composition using an Iso Prime continuous-flow stable isotope ratio mass spectrometer (GV Instruments Ltd, Manchester, UK) in the Department of Renewable Resources, University of Alberta. Carbon isotope discrimination (Δ13C) of leaves was calculated assuming an isotope composition in ambient air of −8.0‰, using the following equation as defined by Farquhar et al. (1989)
Where δa and δp are the isotope composition of atmospheric CO2 and plant samples.
Crop biomass and grain yield
Two plants per pot were harvested at maturity at which time plant height, aerial biomass (biomass) and grain yield were measured, while WUE was determined (WUE was calculated as aerial biomass/total water use from flowering stage to maturity). The grain was also collected and ground for measuring N and P concentrations (described below).
Nitrogen and P concentrations
The grains and stem samples were ground and then digested using the sulfuric acid-hydrogen peroxide digestion procedure. The N and P concentrations in the digest were determined using the Kjeldahl N determination method and phosphomolybdenum blue photometric method, respectively (Perrin 1953).
Statistical analysis of data
All collected data under WW and WD conditions were subjected to analysis of variance using the General Linear Model procedure in SPSS (SPSS software version 16.0, Chicago, Illinois, USA). Differences between means were compared by Tukey’s HSD test at P < 0.05. The correlations between parameters were analyzed using the Pearson’s simple correlation test function in SPSS.
Results
Relative water content and specific leaf area
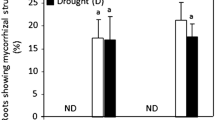
Nitrogen fertilization and mycorrhizal inoculation significantly increased (12.9 %) the RWC of flag leaves under WW while also significantly increased under WD 17.3 % (Table 1 and Fig. 1a). With regards to the SLA of flag leave, there were significant effects of N fertilization and mycorrhizal inoculation and water regime (Table 1). The SLA was increased by AMF inoculation while it was lowered under N fertilization and drought (Fig. 1b).
Effects of N fertilization and mycorrhizal inoculation under Well-waterd (WW) and Water-deficit (WD) conditions on a) relative water content (RWC); b) specific leaf area (SLA) of flag leaves at flowering stage. CK: no N fertilization and non-mycorrhizal inoculation; M: mycorrhizal inoculation with no N fertilization; N: 180 kg/ha N fertilization with no mycorrhizal inoculation; MN: 180 kg/ha N fertilization and mycorrhizal inoculation. Error bars were standard errors
Leaf photosynthesis (Pn), Transpiration (Tr), Water use efficiency (WUE) and carbon isotope discrimination (Δ13C)
At the flowering stage, the leaf Pn and Tr were higher under WW than under WD treatment (Table 2). Nitrogen fertilization and mycorrhizal inoculation significantly increased Pn and Tr under WW (Pn increased by 34 % and Tr by 5.1 %, as compared with the control treatments). Similarly, under WD, Pn of leaves was increased by 31 % and Tr by 20.2 % for treatments receiving combined N fertilization and mycorrhizal inoculation as compared with the controls. From flowering to maturity stage, WUE was enhanced under N fertilization and WD with no significant effect from mycorrhizal inoculation (Table 2). Leaf Δ13C was slightly lowered under WD, irrespective of mycorrhizal inoculation or N fertilization.
Nitrogen and P concentrations in stem and grain
Stem and grain N concentrations were significantly increased by N fertilization and mycorrhizal inoculation under WW (Table 3). Combined N fertilization and AMF inoculation significantly increased stem and grain N concentrations under WD (Fig. 2a). Nitrogen fertilization significantly increased stem and grain P concentrations under WD while AMF inoculation increased grain P concentration under both water regimes (Table 3). Stem and grain P concentrations were higher under WD than under WW irrespective of other treatments (Fig. 2b).
Effect of N fertilization and mycorrhizal inoculation under Well-waterd (WW) and Water-deficit (WD) conditions on a) stem and grain N concentration; b) stem and grain P concentration at maturity stage.CK: no N fertilization and non-mycorrhizal inoculation; M: mycorrhizal inoculation with no N fertilization; N: 180 kg/ha N fertilization with no mycorrhizal inoculation; MN: 180 kg/ha N fertilization and mycorrhizal inoculation. Error bars were standard errors
Stem N, grain N and grain P concentrations were significantly correlated under different N, water and mycorrhizal inoculation treatments (data not shown). There were positive correlations between grain P and N concentrations (r = 0.50, P < 0.05) and between grain P and stem N concentrations (r = 0.68, P < 0.01).
Plant height, biomass and grain yield
Nitrogen fertilization and mycorrhizal inoculation all significantly increased plant height under both water regimes with no interaction among the factors (Table 4). Combined N fertilization and AMF inoculation increased plant height, biomass and grain yield under two water regimes (Fig. 3).
Effect of fertilization and mycorrhizal inoculation under Well-waterd (WW) and Water-deficit (WD) conditions on a) plant height; b) biomass; c) yield at maturity stage. CK: no N fertilization and non-mycorrhizal inoculation; M: mycorrhizal inoculation with no N fertilization; N: 180 kg/ha N fertilization with no mycorrhizal inoculation; MN: 180 kg/ha N fertilization and mycorrhizal inoculation. Error bars were standard errors
Relationships between WUE and stem and grain N and P concentrations
The WUE and grain N, stem N and P concentrations were significantly positively correlated (r = 0.43, 0.59, 0.49, respectively; P < 0.05) (Table 5). Leaf Δ13C was significantly negatively correlated with grain and stem N and P concentrations (r = −0.60, -0.51, −0.49, −0.46, respectively; P < 0.05), while WUEi was only significant correlated with grain P concentration (r = 0.53, P < 0.05) (Table 5).
Discussion
Plant growth and production
Under drought condition, the effect of AMF inoculation and N fertilization on plant growth and biomass production was linked with plant morphology. Plant growth and crop yield are particularly dependent on water availability (Omirou et al. 2013), and as expected WD had a detrimental effects on wheat growth and yield in this study (Tables 1 and 3, Figs. 1 and 3). The WD treatment reduced biomass production and grain yield through affecting several physiological parameters. The canopy absorption of photosynthetically active radiation (PAR) may be reduced by a drought-induced limitation of the leaf area expansion (Guo et al. 1998), which in turn reduces new dry matter production and limit the yield (Stone et al. 2001). The decreased in SLA and RWC by WD (Fig. 1) in this study is consistent with other research on wheat (Zhang et al. 2010). The principal reason for reduced leaf expansion under drought stress is the reduction in leaf cell hydraulic conductivity, which, in turn, decreases water transport and hinders both cell division and enlargement, and leaf development (Gholamhoseini et al. 2013).
Subramanian and Charest (1995) showed that leaf area of corn under AMF inoculation was higher than that without inoculation, consistent with the higher SLA under AMF inoculation in this study (Fig. 1b). Plant water content can be increased by AMF inoculation (Morte et al. 2000). In this study, AMF inoculation resulted in a 10 % increase in leaf RWC (Fig. 1a). We also observed positive effects of AMF inoculation on the biomass and yield of wheat under WW and WD conditions. The positive effects are likely due to improved N nutrition (Bethlenfalvay et al. 1988; Sweatt and Davies 1984), increased uptake of water by hyphae (Faber et al. 1991) and overall enhancement of plant growth (Bryla and Duniway 1997).
Water use efficiency
The WUE and WUEi of spring wheat were greater under WD than under WW (Table 2), consistent with results on cowpea (Anyia and Herzog 2004) and barley (Anyia et al. 2007; Chen et al. 2013). Under WD, gs and leaf Tr were markedly reduced but leaf Pn was slightly decreased and WUE increased.
Under WD, WUE was significantly higher in inoculated plants, indicating that AMF inoculation was effective in improving plant performance under a drought condition. Mycorrhizal inoculation of plants has been previously reported to increase the ability of crops to mitigate the negative effects of water stress by increasing WUE (Kaya et al. 2003). Similarly, in the current study, WUE increased (P < 0.05) by 10 % in inoculated than in non-inoculated plants grown under water stress (Table 2). AMF inoculation helps to improve water uptake by roots through the formation of extensive network of hyphae on roots.
Drought stress decreased leaf Δ13C while AMF inoculation increased leaf Δ13C (Table 2). Yasira et al. (2013) also reported a decrease in Δ13C under limited irrigation in 49 different wheat varieties. Significant negative association between Δ13C and WUE was observed in our experiment (Table 5), consistent with the theory of Farquhar et al. (1982) that stated that Δ13C and WUE are negatively correlated in C3 plants.
Plant uptake of N and P
The AMF inoculation increased stem and grain N and P concentrations, consistent with the results of Attia and Nemat (2003). In this study, inoculation of plants with AMF under WD increased N and P concentrations in stem and grain (Fig. 2). Increased transport of N and P by external AM fungal hyphae to maize has been reported under drought condition (Subramanian and Charest 1999). Similar to past studies, N and P acquisition and assimilation were affected after AMF inoculation regardless of N addition in our research. The significant positive correlation between P concentration in stem and N concentration in stem and grain likely reflected the synergistic effect of AMF on N and P uptakes (Tawaraya et al. 2007).
Conclusions
As water increasingly becomes a limiting factor in crop production in many regions, AMF inoculation is a promising strategy to improve wheat production under rainfed conditions. In this study, AMF inoculation enhanced N and P uptake and increased WUE and yield of wheat under WD in a greenhouse experiment. The WUE of plants grown under WD was significantly higher in inoculated plants, indicating that AMF inoculation was effective in improving plant performance under WD. The positive correlations between WUE and grain N, stem N and stem P concentrations suggests that these traits are closely related and yield enhancement by N fertilization and AMF inoculation was likely a combination of positive effects on WUE and nutrient uptake. However, further research under field conditions and with more genotypes will be needed to evaluate and extend the results.
References
Allen MF, Smith WK, Moore TS, Christensen M (1981) Comparative water relations and photosynthesis of mycorrhizal and non-mycorrhizal Bouteloua gracilis H.B.K. New Phytol 88:683–693
Anyia AO, Herzog H (2004) Water-use efficiency, leaf area and leaf gas exchange of cowpeas under mid-season drought. Eur J Agron 20:327–339
Anyia AO, Slaski JJ, Nyachiro JM, Archambault DJ, Juskiw P (2007) Relationship of carbon isotope discrimination to water use efficiency and productivity of barley under field and greenhouse conditions. J Agron Crop Sci 193:313–323
Attia M, Nemat AM (2003) Assessment the impact of certain growth promoting rhizobacteria strains on symbiotic effectiveness of arbuscular mycorrhizal fungi. Egypt J Microbiol 38:75–88
Augé RM, Schekel KA, Wample RL (1987) Rose leaf elasticity changes in response to mycorrhizal colonization and drought acclimation. Plant Physiol 70:175–182
Augé RM (2001) Water relations, drought and vesicular arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Bethlenfalvay GJ, Brown MS, Ames RN, Thomas RS (1988) Effects of drought on host and endophyte development in mycorrhizal soybeans in relation to water use and phosphate uptake. J Plant Physiol 72:565–71
Bryla DR, Duniway JM (1997) Effects of mycorrhizal infection on drought tolerance and recovery in safflower and wheat. Plant Soil 97:95–103
Chen J, Chang SX, Anyia OA (2013) Physiological characterization of recombinant inbred lines of barley with contrasting levels of carbon isotope discrimination. Plant Soil 369:335–349
Dixon RK, Rao MV, Garg VK (1994) Water relations and gas exchange of mycorrhizal Leucaena leucocephala seedlings. J Trop For Sci 6:542–552
Faber BA, Zasoske RJ, Munns DN, Shackel K (1991) A method for measuring hyphal nutrition and water uptake in mycorrhizal plants. Can J Bot 69:87–94
Farquhar GD, O’Leary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9:121–137
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Ann Rev Plant Physiol 40:503–537
Gholamhoseini M, Ghalavand A, Dolatabadian A, Jamshidi E, Khodaei-Joghan A (2013) Effects of arbuscular mycorrhizal inoculation on growth, yield, nutrient uptake and irrigation water productivity of sunflowers grown under drought stress. Agric Water Manag 117:106–114
Ho I, Trappe JM (1975) Nitrate reducing capacity of two vesicular-arbuscular mycorrhizal fungi. Mycologia 67:886–888
Kaya C, Higgs D, Kirnak H, Tas I (2003) Mycorrhizal colonization improves fruit yield and water use efficiency in watermelon (Citrullus lanatus Thumb) grownunder well-watered and water-stressed conditions. Plant Soil 253:287–292
Lancashire PD, Bleiholder H, Langelüddeke P, Strauss R, Van den Boom T, Weber E, Witzenberger A (1991) A uniform decimal code for growth stages of crops and weeds. Ann Appl Biol 119:561–601
Martin FM, Plassard C (2001) Nitrogen assimilation by ectomycorrhizal symbiosis. In: Morot- Gaudry J-F (ed) Nitrogen assimilation by plants: physiological, biochemical and molecular aspects. Science Publishers, Inc, Enfield, pp 169–183
Morte A, Lovisolo C, Schubert A (2000) Effect of drought stress on growth and water relations of themycorrhizal association Helianthemum almeriense-Terfezia claveryi. Mycorrhiza 10:115–119
Nelsen CE (1987) The water relations of vesicular-arbuscular mycorrhizal systems. In: Safir GR (ed) Ecophysiology of VA mycorrhizal plants. CRC, Boca Raton, pp 71–91
Omirou M, Ioannides MI, Ehaliotis C (2013) Mycorrhizal inoculation affects arbuscular mycorrhizal diversity in water melon roots, but leads to improved colonization and plant response under water stress only. Appl Soil Ecol 63:112–119
Perrin CH (1953) Rapid modified procedure for determination of Kjeldahl Nitrogen. Anal Chem 25:968–971
Rebetzke GJ, Richards RA (1999) Genetic improvement of early vigour in wheat. Aust J Agric Res 50:291–301
Simpson D, Daft MJ (1990) Interactions between water-stress and different mycorrhizal inocula on plant growth and mycorrhizal development in maize and sorghum. Plant Soil 121:179–186
Stone PJ, Wilson DR, Reid JB, Gillespie GN (2001) Water deficit effects on sweetcorn: I. Water use, radiation use efficiency, growth, and yield. Aust J Agric Res 52:103–113
Subramanian KS, Charest C, Dwyer LM, Hamilton RI (1995) Arbuscular mycorrhizas and water relations in maize under drought stress at tasseling. New Phytol 129:643–650
Subramanian KS, Charest C (1999) Acquisition of N by external hyphae of an arbuscular mycorrhizal fungus and its impact on physiological responses in maize under drought-stressed and well-watered conditions. Mycorrhiza 9:69–75
Sweatt MR, Davies FT (1984) Mycorrhizae, water relations, growth, and nutrient uptake of geranium grown under moderately high phosphorus regimes. J Am Soc Horti Sci 109:210–213
Tawaraya K (2003) Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Sci J Plant Nutr 49:655–668
Tawaraya K, Turjaman M, Ekamawanti HA (2007) Effect of arbuscular mycorrhizal colonization on nitrogen and phosphorus uptake and growth of Aloe vera L. Hortscience 42:1737–1739
Wang GM, Coleman DC, Freckman DW, Dyer MI, McNaughton SJ, Acra MA, Goeschl JD (1989) Carbon partitioning patterns of mycorrhizal versus non-mycorrhizal plants: real time dynamic measurements using CO2. New Phytol 112:489–493
Wu QS, Xiao RX, Zou YN (2008) Improved soil structure and citrus growth after inoculation with three arbuscular mycorrhizal fungi under drought stress. Eur J Soil Biol 44:122–128
Guo XS, Sinclair TR, Ray JD (1998) Effect of drought history on recovery of transpiration, photosynthesis, and leaf area development in maize. Soil Crop Sci Soc Fl 57:83–87
Yasira TA, Min DH, Chen XJ, Condon AG, Hua YG (2013) The association of carbon isotope discrimination (Δ) with gas exchange parameters and yield traits in Chinese bread wheat cultivars under two water regimes. Agric Water Manag 119:111–120
Zhang BB, Liu WZ, Chang XS, Anyia OA (2010) Water-deficit and high temperature affected water use efficiency and arabinoxylan concentration in spring wheat. J Cereal Sci 52:257–263
Zhang BB, Liu WZ, Chang SX, Anyia AO (2013) Phosphorus fertilization and fungal inoculations affected the physiology, phosphorus uptake and growth of spring wheat under rainfed conditions on the Canadian prairies. J Agron Crop Sci 199:85–93
Acknowledgments
This work was supported by the China Scholarship Council, Alberta Crop Industry Development Fund Ltd., Alberta Innovates-Technology Futures, the Natural Sciences and Engineering Research Council (NSERC) of Canada, the State Key Laboratory of Soil Erosion and Dryland farming on Loess Plateau (No. K318009902-1403), and Research Program of Baoji University of Arts and Science (No. ZK15035).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Peter Christie.
Rights and permissions
About this article
Cite this article
Zhang, B., Chang, S.X. & Anyia, A.O. Mycorrhizal inoculation and nitrogen fertilization affect the physiology and growth of spring wheat under two contrasting water regimes. Plant Soil 398, 47–57 (2016). https://doi.org/10.1007/s11104-015-2635-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2635-x