Abstract
Under laboratory conditions, spores of ectomycorrhizal fungi usually germinate very poorly or not at all. In a previous study, we showed that spores of the ectomycorrhizal fungus Suillus bovinus germinated through the combination of activated charcoal treatment of media and co-culture with seedlings of Pinus densiflora, which suggested that some substances contained in root exudates induced the germination. Among the compounds reported from root exudates, flavonoids have been elucidated to play various and substantial roles in plant–microbe interactions; we therefore investigated the effects of flavonoids on basidiospore germination of S. bovinus by the diffusion gradient assay on water agar plates pretreated with charcoal powder. Seven out of the 11 flavonoids tested, hesperidin, morin, rutin, quercitrin, naringenin, genistein, and chrysin, had greater effects than controls, whereas flavone, biochanin A, luteolin, and quercetin showed no positive effects. The effective concentration presumably corresponded to several micromolar levels, which was equivalent to those effective for pollen development, nod gene induction, and spore germination of F. solani f. sp. pisi and AM fungi. The results suggest that flavonoids play a role as signaling molecules in symbiotic relationships between woody plants and ectomycorrhizal fungi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Propagation of ectomycorrhizal fungi occurs primarily by spore dispersal and vegetative growth of mycelia. Since the last decade, with the development of polymerase chain reaction-based DNA analysis, molecular ecological studies have been intensively conducted and have revealed mainly from clone sizes that their relative importance depends on the species studied. It has been concluded that basidiospores are the main means for dispersal and initiation of infections in some ectomycorrhizal species of relatively small genet sizes (Gryta et al. 1997; Gherbi et al. 1999; Fiore-Donno and Martin 2001; Redecker et al. 2001; Huai et al. 2003; Liang et al. 2005; Bergemann and Miller 2002). However, under laboratory conditions, spores of ectomycorrhizal fungi usually germinate very poorly or not at all (Bowen and Theodorou 1973; Fries 1984), and thus, comprehensive explanations of life cycles of ectomycorrhizal fungi in forests cannot be based solely on clone sizes.
Although spores are assumed to require stimulatory factors for germination, these conditions are still unknown in most ectomycorrhizal species. Among ectomycorrhizal basidiomycetes, three main types of germination activators have been reported (Fries 1987): (1) various types of non-ectomycorrhizal microorganisms such as colonies of the yeast Rhodotorula glutinis (Frensen.) Harrison (Fries 1976, 1978), the filamentous fungus Ceratocystis fagacearum Brents (Hunt; Oort 1974), and some bacterial isolates obtained from sporophores, mycorrhizae, or soil (Ali and Jackson 1988); (2) a mycelium of the same species as the spores (Fries 1978, 1983a; Iwase 1992); and (3) roots of higher plants (Melin 1962; Fries and Birraux 1980; Birraux and Fries 1981; Kope and Fortin 1990; Fries 1989). Generally, the effects are caused by exudates from various microorganisms or root exudates of plants, and these exudates presumably contain compounds possessing the capacity to trigger spore germination. However, only a few of the chemical substances active in induction of basidiospore germination in ectomycorrhizal fungi have been identified. They include n-butyric acid and related compounds for Tricholoma matsutake (Ito & Imai) Singer and several other species (Ohta 1986, 1988), gluconic acid for T. robustum (Alb. & Schwein.) Ricken (Iwase 1992), and abietic acid and some resin acids for Suillus species (Fries et al. 1987; Fries 1988).
In a previous study, we showed that spores of Suillus bovinus (Pers.) Roussel germinated only by the combination of activated charcoal treatment of media and co-culture with seedlings of Pinus densiflora Sieb., but that abietic acid, a genus-specific inducer of spore germination for Suillus species (Fries 1988), showed no positive effect; this result suggested that some substances other than abietic acid contained in root exudates induced germination of basidiospores of S. bovinus (Kikuchi et al. 2006).
Root exudates contain a wide variety of compounds, including sugars, amino acids, organic acids, fatty acids, sterols, flavonoids, growth factors, and enzymes (Uren 2001). Among them, amino acids show some positive effects on germination of basidiospores of Laccaria laccata (Scop.) Fr. and Suillus species (Fries 1976, 1983a), although more effective treatments were reported later for both species: Co-culture with conspecific mycelium or seedlings of host plants stimulated germination (Fries 1978, 1983a, 1983b). Certain flavonoids induce transcription of nodulation genes in Rhizobium (Peters et al. 1986; Redmond et al. 1986) and promote spore germination and hyphal growth of arbuscular-mycorrhizal (AM) fungi (Gianinazzi-Pearson et al. 1989; Vierheilig et al. 1998). Flavonoids have been elucidated to enhance growth rates of R. meliloti (Hartwig et al. 1991). In two ectomycorrhizal fungi, Pisolithus tinctorius (Pers.) Cocker & Couch and S. bovinus, the flavonol rutin is effective at concentrations as low as 1 pM for their growth (Lagrange et al. 2001).
By analogy of the roles of flavonoids in the two major plant–microbe symbioses, i.e., legume–rhizobia and herbs–AM fungi, it is possible that flavonoids play roles in another major symbiosis between woody plants and ectomycorrhizal fungi. In this study, we investigated the effect of flavonoids on spore germination of the ectomycorrhizal fungus S. bovinus.
Materials and methods
Spore collection and seedlings of P. densiflora
Fruit bodies of S. bovinus were collected in a P. densiflora stand located in Kake, Hiroshima Prefecture, Japan in October 2005. Spore prints were obtained by fastening pieces of a cap under the lid of a sterilized plastic petri dish with vaseline so that the spores were cast into the bottom of the dish. After 2 to 3 h, the lid was replaced by another sterile lid, and several spore prints from the same fruit body were collected in separate petri dishes. The spore prints were stored in darkness at 4°C until use.
Seeds of P. densiflora were surface sterilized by 30% H2O2 for 5 min, washed with sterilized distilled water, and placed on one fifth strength Hamada medium [0.4% glucose, 0.04% yeast extract, 1.0% agar (w/v)].
Chemicals
Eleven flavonoids, specifically three flavones (flavone, luteolin, chrysin), four flavonols (quercetin, quercitrin, rutin, morin), two flavanones (naringenin, hesperidin), and two isoflavones (genistein, biochanin A), and abietic acid were tested to determine their effect on the germination of S. bovinus basidiospores. The flavonoids were selected among those investigated previously for their effects on spore germination of AM fungi (Gianinazzi-Pearson et al. 1989; Vierheilig et al. 1998) or hyphal growth of ectomycorrhizal fungi (Lagrange et al. 2001). All investigated flavonoids and abietic acid were available commercially: flavone, morin, narigenin, hesperidin were obtained from Sigma Aldrich (USA), luteolin, quercetin, quercitrin, rutin, biochanin A, and abietic acid from Wako Pure Chemical (Japan), and chrysin and genistein from Funakoshi (Japan).
Abietic acid and all flavonoids except for hesperidin were dissolved in absolute ethanol (Wako Pure Chemical) to provide 2 mM stock solutions. Hesperidin was dissolved in 0.1 N KOH to provide 2 mM stock solutions.
Pretreatment of water agar plates with charcoal powder
As reported by Bjurman (1984), an organic acid inhibitory to spore germination of ectomycorrhizal fungi is formed during autoclaving, and pretreatment of solidified agar with charcoal powder is effective for removal of the inhibitory factor.
Autoclaved (121°C, 10 min), molten 1% (w/v) water agar was poured into plastic petri dishes (φ90 mm), 15 ml per dish. When the agar had solidified, its surface was covered with a thin cellophane disk autoclaved (121°C, 15 min) in distilled water, and a thin layer of sterilized (autoclaved at 121°C for 30 min) activated charcoal powder was sprinkled over the cellophane. After 2 weeks, the cellophane and the overlying charcoal powder were removed.
Effects of flavonoids on spore germination
Effects of flavonoids on spore germination were evaluated by the diffusion gradient assay (Bjurman and Fries 1984) with slight modification. A hole, approximately 2 mm in diameter, was punched in the center of each plate with a sterilized Pasteur pipette; the pipette tip was inserted into the agar, the contents were removed by suction (Nagahashi and Douds 1999), and then the flavonoid or control solution was injected into the hole.
In the experiments, controls were injected with equal volumes of absolute ethanol (for chemicals other than hesperidin) or 0.1 N KOH solution (for hesperidin) into the hole made on non-pretreated and pretreated plates [water agar plates (WA) and water agar plates pretreated with charcoal powder (WAC), respectively]. As positive controls, seedlings of P. densiflora were placed on pretreated plates, one seedling per plate (referred to as Pd).
After several hours, the solution dried up, and a suspension of spores containing ca 4 × 106 spores per milliliter in sterilized distilled water was spotted at 0.1 ml per plate and spread over the plate with a glass rod. The petri dishes were sealed with parafilm and incubated at 23°C in the dark.
Experiment I. Effects of different flavonoids on spore germination
The effect of each flavonoid on germination was evaluated by injecting 20 μl of 2 mM solution of each flavonoid. From preliminary experimentation, it was determined that 20 μl was an adequate volume for showing clear effects among injection volumes of 5, 10, 20, and 40 μl.
Experiment II. Effects of different volumes of injected 2 mM morin solution on spore germination
To find an optimal flavonoid concentration for germination, effects of different volumes of flavonoids on germination were evaluated by injecting 2, 5, 10, 20, 40, and 100 μl of 2 mM solution of morin. Morin was selected because it produced the largest levels of spore germination in the preliminary experiment.
Spore collections from five fruiting bodies were used due to limited spore quantities from each collection; one collection was used in both experiments, and two additional collections were used in each experiment. Five plates were prepared for each treatment.
Germination rates
Germination rates were scored once a week, via photomicrographs, in four directions perpendicular to each other across a hole made by a Pasteur pipette. In the Pd treatment, photomicrographs were taken and germination was measured along two perpendicular lines at the middle and lower one fourth part of the root of each seedling. As each direction was considered independent, even on the same plate, the number of replicates was 20 (five plates × four directions) per treatment. Unfortunately, as a result of contamination or death of pine seedlings, the number of plates of Pd examined in experiments I and II (two and three, respectively) and the number of repeats (8 and 12, respectively) were low.
Experimental data were analyzed by analysis of variance, and a multiple comparison was conducted with Holm’s method using the software R ver.2.3.1 (R Development Core Team 2005).
Results
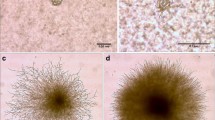
Observations were made up to 2 weeks because on the plates of treatments that showed positive effects, the germ tubes grew so long that it became difficult to distinguish among germinating spores after 2 weeks (Fig. 1). In both experiments, no spores were observed to germinate on WA plates, whereas spontaneous germination was observed on WAC plates (Figs. 2 and 3).
Effects of flavonoids on spore germination of S. bovinus, 2 weeks after inoculation. Boxes with different letters indicate significantly different germination rates (p < 0.05). Closed diamonds represent the average germination rates. Pd Co-culture with a seedling of P. densiflora, WA water agar plate, WAC water agar plate pretreated with charcoal powder
Effects of injection volumes of morin solution (2 mM in ethanol) on spore germination of S. bovinus 2 weeks after inoculation. Boxes with different letters indicate significantly different germination rates (p < 0.05). Closed diamonds represent average germination rates. Pd Co-culture with a seedling of P. densiflora, WA water agar plate, WAC water agar plate pretreated with charcoal powder
Here, spore collections from five fruiting bodies were used: One collection was used in both experiments and two other collections in each experiment. However, in each experiment, the spores of only one of the additional spore collections germinated. Therefore, the results for experiments I and II are based on two different spore collections.
Experiment I. Effects of different flavonoids on spore germination
Germination rates observed in experiment I are shown in Fig. 2. The two control treatments, i.e., the ethanol treatment and the 1 N KOH treatment, had similar effects (data not shown). Thus, we included only the values of the ethanol treatment in Fig. 2.
Except for flavone, biochanin A, luteolin, and quercetin germination rates were significantly higher than the control ethanol treatment. Abietic acid showed no positive, rather an inhibitory effect. Among the flavonoids tested, hesperidin and morin showed the considerable effects on germination, which was in accordance with the result of preliminary experiments in which two spore collections were used (data not shown).
Experiment II. Effects of different volumes of injected 2 mM morin solution on spore germination
Germination rates observed in experiment II are shown in Fig. 3. In the control ethanol treatment, volumes of ethanol applied did not significantly affect germination rates (data not shown). Therefore, we included only the results of the 20-μl injection in Fig. 3 as the control.
When 20, 40, or 100 μl of 2 mM morin solution were applied, overall germination rates were significantly higher than those in the control ethanol treatment, but they were not significantly different among the three volumes injected. This suggested the existence of a plateau in the relationships between concentration of flavonoids and germination rates and justified the volumes of morin solution applied in experiment I. When 2, 5, or 10 μl of the flavonoid solution were injected, although more basidiospores were observed to germinate at higher rates than in the control ethanol treatments (data not shown), germination rates were not significantly different among them.
Discussion
In this study, 7 out of 11 flavonoids investigated were effective in stimulating basidiospore germination of the ectomycorrhizal fungus S. bovinus. Including the results of the preliminary study, in which spore collections from two fruiting bodies were used, positive effects of flavonoids were shown in four spore collections. To our knowledge, this is the first report of a positive effect of flavonoids on the germination of basidiospores and spores of an ectomycorrhizal fungus.
Flavonoids as signaling molecules
Flavonoids are secondary plant products that include pigments (anthocyanins) and colorless or yellow compounds (flavanones, flavones, and flavonols) involved in attracting animal vectors for pollination and seed dispersal (e.g., flower color), UV light protection, stress protection, and signaling in plant reproduction and plant–microbe associations (Gould and Lister 2005).
In plants, flavonols have a strong stimulatory effect on in vitro pollen development, pollen germination, and pollen tube growth in tobacco, maize, and petunia and are effective at concentrations in the micromolar range (Ylstra et al. 1992; Mo et al. 1992).
In plant–microbe interactions, detection of specific plant molecules by microbes is critical for recognition of hosts and subsequent colonization in a complex environment like soil. An early and essential event in most plant–microbe interactions involves chemotaxis toward plant root exudates to find infection zones. In members of the Rhizobiaceae, expression of nodulation (nod) genes is induced by flavonoids or isoflavonoids specific to the particular hosts at 1 to 0.1 μM and induction of the nod genes leads to production of a lipopolysaccharide (Nod factor), which triggers the formation of nodulation structure by plants (Spaink et al. 1998). Rhizobia are attracted to a wide range of chemicals such as sugars and amino acids in the milli- to micromolar range (Burg et al. 1982; Armitage et al. 1988), whereas several studies have shown that flavonoids also serve as chemoattractants in the micro- to nanomolar range for these bacterial symbionts (Aguilar et al. 1988; Armitage et al. 1988; Caetano-Anollés et al. 1988; Dharmatilake and Bauer 1992), with the exception of Bradyrhizobium japonicum (Parke et al. 1985; Kape et al. 1991; Barbour et al. 1991). It should be noted that concentrations of the attractants required for elicitation of chemotaxis in rhizobia are about 100- to 1,000-fold lower than those for inducing nod genes (Bauer and Caetano-Anollés 1990).
As for fungi, zoospores of most Phytophthora species are attracted to a variety of sugars and amino acids in the micromolar range and ethanol in the 0.2 to 20 mM range (Carlile 1983). In some plant-pathogenic oomycetes, zoospores exhibit positive chemotaxis in response to specific plant signals, mainly by isoflavonoids in the nanomolar range (Yokosawa et al. 1986; Sekizaki and Yokosawa 1988; Sekizaki et al. 1993; Horio et al. 1992; Morris and Ward 1992).
Flavonoids have been reported to play roles in the germination of propagules of some fungal species. Germination of macroconidia of the soilborne fungus Fusarium solani f. sp. pisi Snyder & Hansen (teleomorph Nectria haematococca Berk. & Broome) is strongly stimulated by some flavones and flavanones at micromolar levels, but not by flavanols or isoflavones (Ruan et al. 1995). Chlamydospore germination of F. solani is also stimulated by the same flavonoids effective in macroconidia germination, although the effect is smaller and variable (Ruan et al. 1995). Germination of spores of AM fungi has been reported to be stimulated or inhibited by flavonoids (Vierheilig et al. 1998), as two flavanones and one flavone were shown to stimulate germination of Gigaspora margarita Becker & Hall spores at 0.15 to 1.5 μM (Gianinazzi-Pearson et al. 1989).
Although previous studies have elucidated the roles of flavonoids as signaling molecules in plant–microbe interactions such as chemotaxis, growth promotion, and germination of propagules, to our knowledge, this is the first report of a positive effect of flavonoids on the germination of basidiospores and spores of an ectomycorrhizal fungus. In the case of spores of F. solani f. sp. pisi and AM fungi, spontaneous germination occurs commonly at high frequency. Germination rates of controls usually reach 30–40% or more (Ruan et al. 1995; Gianinazzi-Pearson et al. 1989; Scervino et al. 2005). These rates are much higher than those observed in ectomycorrhizal fungi, which are usually less than 0.1% on laboratory synthetic media (Bowen and Theodorou 1973) and, in our study, approximately 0.5 and 0.02% on WAC plates in experiments I and II, respectively (Figs. 2 and 3).
The effective concentrations reported previously for basidiospore germination of ectomycorrhizal fungi are as follows: 544.8 μM butyric acid for T. matsutake (0.005% v/v; Ohta 1986, 1988), 509.8 μM gluconic acid for T. robusum (0.01% w/v; Iwase 1992), and 0.33 μM, 330 μM, and 3.3 mM abietic acid for Suillus granulatus (L.) Roussel, S. luteus (L.) Roussel, and S. variegatus (Sw.) Kuntze, respectively (Fries 1988). In contrast to a report by Fries (1988) that abietic acid at 330 μM resulted in poor germination of spores of S. bovinus, our study showed that the addition of 20 μl of 2 mM flavonoid solution (i.e., 40 nmol in 15 ml water agar), which presumably corresponds to several micromolar levels, was sufficient for inducing germination of S. bovinus spores. This concentration was equivalent to those effective for pollen development, nod gene induction, and spore germination of F. solani f. sp. pisi and AM fungi. Therefore, the result suggests a possible role of flavonoids as signaling molecules in basidiospore germination of S. bovinus.
In experiment II, germination rates were lower than those in experiment I, and co-culture with P. densiflora seedlings was the most effective treatment, in contrast to experiment I (Figs. 2 and 3), although morin showed a significant positive effect on germination in both experiments. Spore collections from different basidiocarps within the same species often differ markedly in the duration of germinability and germination frequency (Fries 1976, 1983a, 1984). Moreover, in some cases, the rate of germination may vary not only among different basidiocarps from the same species, but also among different parts of the hymenium in the same basidiocarp (Fries 1984). Miller et al. (1993) assessed germinability and viability of spores of ectomycorrhizal species by spore inoculation to pine seedlings and vital staining of spores, respectively. Based on their results, Miller et al. (1993) suggested that different basidiomes and different populations of the same ectomycorrhizal species might express various abilities or strategies of dormancy, activation, and germination of spores. Therefore, the contradictory results from experiments I and II might be explained by intraspecific physiological variation and imply that the effective flavonoid(s) contained in P. densiflora root exudates is not morin. Further investigation of flavonoid profiles in root exudates will clarify the cause of this contradiction.
Flavonoids as a determinant of host specificity
Contrasts in responsiveness to various flavonoids at inter- or intraspecific levels have been reported for rhizobia (Rolfe 1988), chemoattraction of zoospores (Yokosawa et al. 1986; Morris and Ward 1992), and germination of macroconidia of F. solani (Ruan et al. 1995) and spores of AM fungi (Scervino et al. 2005; Vierheilig et al. 1998), suggesting that flavonoids play a role in determining host ranges.
In basidiospore germination of ectomycorrhizal fungi, host-specific effects of root exudates have been reported. Basidiospores of Thelephora terrestris, Rhizopogon luteolus, S. luteus, and Russula adusta showed considerably faster germination at a higher percentage when induced by roots of host woody plants, but not by those of non-host woody plants or herbs, with some exceptions for other ectomycorrhizal species (Melin 1962; Birraux and Fries 1981; Fries and Swedjemark 1986; Theodorou and Bowen 1987; Fries 1989).
Among the phenomena of flavonoid-mediated plant–microbe associations, relationships between flavonoids and nodulation by rhizobia have been most intensively studied, and flavonoids have been shown to play a role in the determination of host range through interaction with the nodD protein (Cooper 2004; Brencic and Winans 2005). Inducers and anti-inducers co-exist in root exudates of a single legume species, and inhibitory effects can be overcome by increasing the concentration of inducers (Peters and Long 1988). The hypothesis that levels of nod gene induction depend on the net outcome of positive and negative flavonoid effects has been proposed (Peters and Long 1988; Rolfe 1988). This hypothesis was supported by the findings of Peck et al (2006). A similar mechanism might operate in host-specific effects of root exudates on spore germination of ectomycorrhizal fungi, as effective and non-effective flavonoids on germination of S. bovinus spores were present (Fig. 2).
Conclusion
We showed that certain flavonoids induce basidiospore germination of the ectomycorrhizal fungus S. bovinus. This suggests the stimulant for germination of spores of ectomycorrhizal fungi to be one of the roles of flavonoids as signaling molecules in plant–microbe interactions. However, as no reports have been published on flavonoid profiles in root exudates of woody plants except for a legume woody plant, Robinia pseudoacacia (Scheidemann and Wetzel 1997), whether morin or hesperidin, which were the most effective for basidiospore germination of S. bovinus, act as stimulants in the field remains unclear. For further study, identification and quantification of flavonoids in root exudates of woody plants and evaluation of the effects of each flavonoid on spore germination of S. bovinus and other ectomycorrhizal species will contribute to further interpretation of symbiosis and co-evolution between woody plants and ectomycorrhizal fungi.
References
Aguilar JMM, Ashby AM, Richards AJM, Loake, GJ, Watson MD, Shaw CH (1988) Chemotaxis of Rhizobium leguminosarum biovar phaseoli towards flavonoid inducers of the symbiotic nodulation genes. J Gen Microbiol 134:2741–2746
Ali NA, Jackson RM (1988) Effects of plant roots and their exudates on germination of spores of ectomycorrhizal fungi. Trans Br Mycol Soc 91:253–260
Armitage JP, Gallagher A, Johnson AWB (1988) Comparison of the chemotactic behaviour of Rhizobium leguminosarum with and without the nodulation plasmid. Mol Microbiol 2:743–748
Barbour WM, Hattermann DR, Stacey G (1991) Chemotaxis of Bradyrhizobium japonicum to soybean exudates. Appl Environ Microbiol 57:2635–2639
Bauer WD, Caetano-Anollés G (1990) Chemotaxis, induced gene expression and competitiveness in the rhizosphere. Plant Soil 129:45–52
Bergemann SE, Miller SL (2002) Size, distribution, and persistence of genets in local populations of the late-stage ectomycorrhizal basidiomycete, Russula brevipes. New Phytol 156:313–320
Birraux D, Fries N (1981) Germination of Thelephora terrestris basidiospores. Can J Bot 59:2062–2064
Bjurman J (1984) An organic acid, inhibitory to spore germination of mycorrhizal fungi, formed from agar during autoclaving. Microbios 39:109–116
Bjurman J, Fries N (1984) Purification and properties of the germination-inducing factor in the ectomycorrhizal fungus Leccinum aurantiacum (Boletaceae). Physiol Plant 62:465–471
Bowen GD, Theodorou C (1973) Growth of ectomycorrhizal fungi around seeds and roots. In: Marx GC, Kozlowski TT (eds) Ectomycorrhizae: their ecology and physiology. Academic, New York, USA, pp 107–150
Brencic A, Winans SC (2005) Detection of and response to signals involved in host–microbe interactions by plant-associated bacteria. Microbiol Mol Biol Rev 69:155–194
Burg D, Guillaume J, Tailliez R (1982) Chemotaxis by Rhizobium meliloti. Arch Microbiol 133:162–163
Caetano-Anollés G, Crist-Estes DK, Bauer WD (1988) Chemotaxis of Rhizobium meliloti to the plant flavone luteolin requires functional nodulation genes. J Bacteriol 170:3164–3169
Carlile MJ (1983) Motility, taxis, and tropism in Phytophthora. In: Erwin DC, Bartnicki-Garcia S, Tsao PH (eds) Phytophthora—its biology, taxonomy, ecology and pathology. APS, St. Paul, USA, pp 95–107
Cooper JE (2004) Multiple responses of rhizobia to flavonoids during legume root infection. Adv Bot Res 41:1–62
Dharmatilake AJ, Bauer WD (1992) Chemotaxis or Rhizobium meliloti towards nodulation gene inducer compounds from alfalfa roots. Appl Environ Microbiol 58:1153–1158
Fiore-Donno A-M, Martin F (2001) Populations of ectomycorrhizal Laccaria amethystina and Xerocomos spp. show contrasting colonization patterns in a mixed forest. New Phytol 152:533–542
Fries N (1976) Spore germination in Boletus induced by amino acids. Proc K Ned Akad Wet Ser C 79:142–146
Fries N (1978) Basidiospore germination in some mycorrhiza-forming hymenomycetes. Trans Br Mycol Soc 70:319–324
Fries N (1983a) Spore germination, homing reaction, and intersterility groups in Laccaria laccata (Agaricales). Mycologia 75:221–227
Fries N (1983b) Basidiospore germination in species of Boletaceae. Mycotaxon 18:345–354
Fries N (1984) Spore germination in the higher Basidiomycetes. Proc Indian Acad Sci Plant Sci 93:205–222
Fries N (1987) Ecological and evolutionary aspects of spore germination in the higher basidiomycetes. Trans Br Mycol Soc 88:1–7
Fries N (1988) Specific effects of diterpene resin acids on spore germination of ectomycorrhizal basidiomycetes. Experientia 44:1027–1030
Fries N (1989) The influence of tree roots on spore germination of ectomycorrhizal fungi. Agric Ecosyst Environ 28:139–144
Fries N, Birraux D (1980) Spore germination in Hebeloma stimulated by living plant roots. Experientia 36:1056–1057
Fries N, Swedjemark G (1986) Specific effects of tree roots on spore germination in the ectomycorrhizal fungus, Hebeloma mesophaeum (Agaricales). In: Gianninazzi-Pearson V, Gianninazzi S (eds) Physiological and genetical aspects of mycorrhizae. INRA, Paris, France, pp 725–730
Fries N, Serck-Hanssen K, Dimberg LH, Theander O (1987) Abietic acid, an activator of basidiospore germination in ectomycorrhizal species of the genus Suillus (Boletaceae). Exp Mycol 11:360–363
Gherbi H, Delaruelle C, Selosse M-A, Martin F (1999) High genetic diversity in a population of the ectomycorrhizal basidiomycete Laccaria amethystina in a 150-year-old beech forest. Mol Ecol 8:2003–2013
Gianinazzi-Pearson V, Branzanti B, Gianinazzi S (1989) In vitro enhancement of spore germination and early hyphal growth of a vesicular–arbuscular mycorrhizal fungus by host root exudates and plant flavonoids. Symbiosis 7:243–255
Gould KS, Lister C (2005) Flavonoid functions in plants. In: Andersen ØM, Markham KR (eds) Flavonoids—chemistry, biochemistry and applications. Taylor & Francis, Boca Raton, USA, pp 397–441
Gryta H, Debaud J-C, Effosse A, Gay G, Marmeisse R (1997) Fine-scale structure of populations of the ectomycorrhizal fungus Hebeloma cylindrosporum in coastal sand dune forest ecosystems. Mol Ecol 6:353–364
Hartwig UA, Joseph CM, Phillips DA (1991) Flavonoids released naturally from alfalfa seeds enhance growth rate of Rhizobium meliloti. Plant Physiol 95:797–803
Horio T, Kawabata Y, Takayama T, Tahara S, Kawabata J, Fukushi Y, Nishimura H, Mizutani J (1992) A potent attractant of zoospores of Aphanomyces cochlioides isolated from its host, Spinacia oleracea. Experientia 48:410–414
Huai W-X, Guo L-D, He W (2003) Genetic diversity of an ectomycorrhizal fungus Tricholoma terreum in a Larix principis-rupprechtii stand assessed using random amplified polymorphic DNA. Mycorrhiza 13:265–270
Iwase K (1992) Induction of basidiospore germination by gluconic acid in the ectomycorrhizal fungus Tricholoma robustum. Can J Bot 70:1234–1238
Kape R, Parniske M, Werner D (1991) Chemotaxis and nod gene activity of Bradyrhizobium japonicum in response to hydroxycinnamic acids and isoflavonoids. Appl Environ Microbiol 57:316–319
Kikuchi K, Matsushita N, Suzuki K (2006) Germination of Suillus bovinus spores in vitro. Nippon Kingakukai Kaiho 47:37–40 (in Japanese with summary in English)
Kope HH, Fortin JA (1990) Germination and comparative morphology of basidiospores of Pisolithus arhizus. Mycologia 82:350–357
Lagrange H, Jay-Allgmand C, Lapeyrie F (2001) Rutin, the phenolglycoside from eucalyptus root exudates, stimulates Pisolithus hyphal growth at picomolar concentrations. New Phytol 149:349–355
Liang Y, Guo L, Ma K (2005) Population genetic structure of an ectomycorrhizal fungus Amanita manginiana in a subtropical forest over two years. Mycorrhiza 15:137–142
Melin E (1962) Physiological aspects of mycorrhizae of forest trees. In: Kozlowski TT (ed) Tree growth. Ronald Press, New York, USA, pp 247–263
Miller SL, Torres P, McClean TM (1993) Basidiospore viability and germination in ectomycorrhizal and saprotrophic basidiomycetes. Mycol Res 97:141–149
Mo Y, Nagel C, Taylor LP (1992) Biochemical complementation of chalcone synthase mutants defines a role for flavonoids in functional pollen. Proc Natl Acad Sci U S A 89:7213–7217
Morris PF, Ward EWB (1992) Chemoattraction of zoospores of the soybean pathogen, Phytophthora sojae, by isoflavones. Physiol Mol Plant Pathol 40:17–22
Nagahashi G, Douds DD Jr (1999) Rapid and sensitive bioassay to study signals between root exudates and arbuscular mycorrhizal fungi. Biotechnol Tech 13:893–897
Ohta A (1986) Basidiospore germination of Tricholoma matsutake (I). Effects of organic acids on swelling and germination of the basidiospores. Trans Mycol Soc Jpn 27:167–173
Ohta A (1988) Effects of butyric acid and related compounds on basidiospore germination of some ectomycorrhizal fungi. Trans Mycol Soc Jpn 29:375–381
Oort AJP (1974) Activation of spore germination in Lactarius species by volatile compounds of Ceratocystis fagacearum. Proc K Ned Akad Wet Ser C 77:301–307
Parke D, Rivelli M, Ornston LN (1985) Chemotaxis to aromatic and hydroaromatic acids: comparison of Bradyrhizobium japonicum and Rhizobium trifolii. J Bacteriol 163:417–422
Peck MC, Fisher RF, Long SR (2006) Diverse flavonoids stimulate NodD1 binding to nod gene promoters in Sinorhizobium meliloti. J Bacteriol 188:5417–5427
Peters NK, Long SR (1988) Alfalfa root exudate and compounds which promote or inhibit induction of Rhizobium meliloti nodulation genes. Plant Physiol 88:396–400
Peters NK, Frost JW, Long SR (1986) A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science 233:977–980
R Development Core Team (2005) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Redecker D, Szaro TM, Bowman RJ, Bruns TD (2001) Small genets of Lactarius xanthogalactus, Russula cremoricolor and Amanita fracheti in late-stage ectomycorrhizal successions. Mol Ecol 10:1025–1034
Redmond JW, Batley M, Djordjevic MA, Innes RW, Kuempel PL, Rolfe BG (1986) Flavones induce expression of nodulation genes in Rhizobium. Nature 323:632–635
Rolfe BG (1988) Flavones and isoflavones as inducing substances of legume nodulation. Biofactors 1:3–10
Ruan Y, Kotraiah V, Straney DC (1995) Flavonoids stimulate spore germination in Fusarium solani pathogenic on legumes in a manner sensitive to inhibitors of cAMP-dependent protein kinase. Mol Plant Microbe Interact 8:929–938
Scervino JM, Ponce MA, Erra-Bassells R, Vierheilig H, Ocampo JA, Godeas A (2005) Flavonoids exhibit fungal species and genus specific effects on the presymbiotic growth of Gigaspora and Glomus. Mycol Res 109:789–794
Scheidemann P, Wetzel A (1997) Identification and characterization of flavonoids in the root exudate of Robinia pseudoacacia. Trees 11:316–321
Sekizaki H, Yokosawa R (1988) Studies on zoospore-attracting activity. I. Synthesis of isoflavones and their attracting activity to Aphanomyces euteiches zoospore. Chem Pharm Bull 36:4876–4880
Sekizaki H, Yokosawa R, Chinen C, Adachi H, Yamane Y (1993) Studies on zoospore attracting activity. II. Synthesis of isoflavones and their attracting activity to Aphanomyces euteiches zoospore. Biol Pharm Bull 16:698–701
Spaink HP, Kondorosi A, Hooykaas PJJ (eds) (1998) The rhizobiaceae. Kluwer, Dordrecht, Netherlands
Theodorou C, Bowen GD (1987) Germination of basidiospores of mycorrhizal fungi in the rhizosphere of Pinus radiata D. Don. New Phytol 106:217–223
Uren NC (2001) Types, amounts, and possible functions of compounds released into the rhizosphere by soil-grown plants. In: Pinton R, Varanini Z, Nannipieri, P (eds) The rhizosphere. Marcel Decker, New York, USA, pp 19–40
Vierheilig H, Bago B, Albrecht C, Poulin M-J, Piché Y (1998) Flavonoids and arbuscular-mycorrhizal fungi. In: Manthey J, Buslig B (eds) Flavonoids in the living system. Plenum, New York, USA, pp 9–33
Ylstra B, Touraev A, Bentino Moreno RM, Stöger E, van Tunen AJ, Vicente O, Mol JNM, Heberle-Bors E (1992) Flavonols stimulate development, germination, and tube growth of tobacco pollen. Plant Physiol 100:902–907
Yokosawa R, Kuninaga S, Sekizaki H (1986) Aphanomyces euteiches zoospore attractant isolated from pea root: prunetin. Ann Phytopathol Soc Jpn 52:809–816
Acknowledgments
We thank Mr. Masahiro Kake and Mr. Yasuharu Kake of Nisshin Ringyo Ltd. for kindly providing the research site. This research was financially supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kikuchi, K., Matsushita, N., Suzuki, K. et al. Flavonoids induce germination of basidiospores of the ectomycorrhizal fungus Suillus bovinus . Mycorrhiza 17, 563–570 (2007). https://doi.org/10.1007/s00572-007-0131-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-007-0131-8