Abstract
Following a pine beetle epidemic in British Columbia, Canada, we investigated the effect of fire severity on rhizosphere soil chemistry and ectomycorrhizal fungi (ECM) and associated denitrifying and nitrogen (N)-fixing bacteria in the root systems of regenerating lodgepole pine seedlings at two site types (wet and dry) and three fire severities (low, moderate, and high). The site type was found to have a much larger impact on all measurements than fire severity. Wet and dry sites differed significantly for almost all soil properties measured, with higher values identified from wet types, except for pH and percent sand that were greater on dry sites. Fire severity caused few changes in soil chemical status. Generally, bacterial communities differed little, whereas ECM morphotype analysis revealed ectomycorrhizal diversity was lower on dry sites, with a corresponding division in community structure between wet and dry sites. Molecular profiling of the fungal ITS region confirmed these results, with a clear difference in community structure seen between wet and dry sites. The ability of ECM fungi to colonize seedlings growing in both wet and dry soils may positively contribute to subsequent regeneration. We conclude that despite consecutive landscape disturbances (mountain pine beetle infestation followed by wildfire), the “signature” of moisture on chemistry and ECM community structure remained pronounced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Forest soils are subject to natural disturbances such as wildfire and insect infestations, which kill or stress trees. In recent years, the magnitude of mountain pine beetle (Dendroctonus ponderosae Hopkins) infestation of lodgepole pine (Pinus contorta var. latifolia) forests has reached epidemic proportions in British Columbia (BC), Canada, causing substantial social, economic, and ecological impacts. The mountain pine beetle (MPB) outbreak peaked in 2004 with 140 million square meters of forest attacked in that year. The current BC Provincial-Level Mountain Pine Beetle model (Walton, 2012) predicts a 58 % loss of provincial pine forest by 2021. The infestation is spreading eastward into the Canadian boreal forest, threatening major losses in lodgepole pine forests and the prospect of losses of other pine species (e.g., jack pine, Pinus banksiana) susceptible to MPB attack (Natural Resources Canada 2013). Due to unprecedented scale of this outbreak, its impacts on the future regeneration of managed and unmanaged MPB-killed stands are unknown (Burton 2006; Axelson et al. 2009; Burton 2010). In addition, climate change is both expanding the range of the MPB (Carroll et al. 2006; Mikkelson et al. 2013) and resulting in drier conditions (Hamann and Wang 2006). Stands of dead wood, killed by MPB, and dry conditions create an ideal environment for forest wildfires (Turner et al. 1999).
The effect of MPB attack on forest stands, and optimal mitigation strategies, were highlighted in recent studies (Kaufmann et al. 2008; Burton 2010; Mikkelson et al. 2013). However, the impact of MPB attack on soil chemical and biological properties is not well understood. Tree death due to insect attack has the potential to influence entire forest ecosystems. Beetle attacks have been shown to increase nitrogen (N) concentrations in organic soil horizons in several types of forest (Clow et al. 2011; Griffin et al. 2011; Kana et al. 2012; Keville et al. 2013; Rhoades et al. 2013), although these increases do not seem to result in long-term changes to soil biogeochemistry (Keville et al. 2013; Rhoades et al. 2013). The death of trees also affects the future regeneration potential of the forest through influences on mycorrhizal networks and rhizosphere processes (Simard 2009; Štursová et al. 2014). Soil microbes play crucial roles in forest nutrient cycling, carrying out key processes in the carbon, sulfur, and nitrogen cycles. In addition to microbes that inhabit bulk soil, ectomycorrhizal (ECM) associations are vital to tree growth in lodgepole pine forests, providing pine hosts with essential nutrients, such as nitrogen (N) and phosphorus (P), in return for a ready source of available carbon (C) to the fungal partner (Simard and Durall 2004). As trees die, these ECM fungi lose their C source and can be diminished or eliminated, and, as a consequence, the availability of N and P in the soil may decrease (Treu et al. 2014). A reduction in ECM fungi may also result in a decrease in associated “mycorrhizal helper bacteria” (Frey-Klett et al. 2007), many of which perform important nutrient cycling roles. For example, N-fixing bacterial genes have been found in association with ECM fungi (Burke et al. 2006; Izumi et al. 2006). Two key N-cycling processes performed by bacteria include nitrogen-fixation, which can be assayed using the gene for nitrogenase reductase (nifH) (Zehr et al. 2003), and denitrification, which can be assayed by targeting the gene encoding dinitrogenase reductase (nosZ) (Throbäck et al. 2004).
Wildfire disturbance can alter both aboveground forest properties (removal of trees and other plants) and belowground soil chemistry, microbial communities, and nutrient cycling (Yeager et al. 2005; Certini 2005; Dooley and Treseder 2011; Mataix-Solera et al. 2011; Switzer et al. 2012). Fire has been demonstrated to alter the community structure of both soil fungi (Cairney and Bastias 2007) and ECM fungi (Stendell et al. 1999; Mah et al. 2001; Dahlberg et al. 2001; Smith et al. 2005; Gundale et al. 2005; Rincón and Pueyo 2010; Kennedy and Egger 2010; Buscardo et al. 2012; Barker et al. 2013). In addition, disturbance by MPB has been documented to increase fire severity (Turner et al. 1999; Page et al. 2012), with MPB-attacked (red) stands experiencing higher severities of crown fire than might otherwise be expected (S. Taylor, CFS Forestry Officer, pers. comm.). In light of the increase in wildfire incidence following MPB infestation, it is critical to understand the impact of these disturbances on the biological component of forest soils, and the resulting impact upon natural seedling regeneration.
In recent years, molecular advances have allowed ecologists using techniques such as length-heterogeneity polymerase chain reaction (LH-PCR) and terminal restriction fragment length polymorphism (T-RFLP) to quickly profile microbial populations in the environment (Hirsch et al. 2010). These “fingerprinting” techniques provide a powerful way to gain information about the richness of the targeted gene (number of genotypes), while the fragment peak height gives some indication of the relative abundance of each genotype within a soil sample. In the case of ECM, analysis of community structure by traditional morphotyping can be complemented by the use of a molecular profiling approach such as LH-PCR.
In this study, we hypothesized that wildfire would affect the ability of the soil to support tree regeneration in MPB-attacked lodgepole pine forests by (1) altering the nutrient status of the soil directly via physical effects and (2) reducing ECM fungi inoculum both directly (through fungi in the soil being consumed by fire) and indirectly (through death of any living trees remaining), which in turn could result in further decreases in available soil nutrients. We further hypothesized that losses in ECM fungi would have a negative impact upon the soil’s potential for supporting seedling regeneration, and that the more severe the fire, the greater the impact would be on soil nutrient status, ECM fungal diversity and structure, and associated N-cycling bacteria. In order to test our hypotheses, we studied rhizosphere soil and root systems of lodgepole pine seedlings regenerating in two soil types (as delineated by moisture), and three fire severity classes.
Materials and methods
Study area and site description
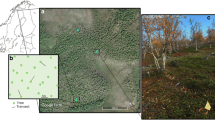
The study area was located near Kenny Dam (124° 54′ 27″ W, 53° 36′ 34″ N) in central BC, where the devastation from MPB was severe. The MPB attack occurred over many years but was considered to reach its height in 1999–2000 (Kathy Lewis (UNBC), personal communication), with evidence of attack still present in 2004/2005. The area was not devoid of under storey plants or trees following attack. Some smaller lodgepole pine had escaped the beetle attack, and a variety of other mycorrhizal host species were present in the under storey as well as over storey. These occurred randomly across sites and included, but were not restricted to Alnus crispa spp. sinuata Regel, Arctostaphylos uva-ursi L., Populus tremuloides Michx., and Pyrola asarifolia Michx. Some areas of MPB-killed stands in the study area were disturbed by a large forest fire (~10,000 ha) in June 2004. The study area was dominated by Brunisolic soil order in dry sites and Luvisolic soil order in wet sites developed on glaciolacustrine deposits (Dawson 1989). Selection of study sites in MPB-killed stands (>80 % attack, determined by surveying trees for the presence of MPB galleries) was completed in late April–May 2005. Level of MPB attack was determined in unburned stands by a visual estimate and the presence of red crowns. In burned stands using fixed radius plots in combination with MPB-enhanced aerial photography, visual identification of beetle galleries under the bark and pitch-tubes was necessary to verify MPB attack. A time domain reflectometer (TDR, Campbell Scientific) with a 12-cm probe for volumetric water content (%) was used to randomly sample target stands during field reconnaissance. The range in site moisture content across the study area was classified as dry (<20 %) and wet (20–45 %). To accommodate the wet and dry sites, severity conditions were visually aggregated into three post-fire severity classes: high, moderate, and low. The distinction was based on the amount of crown and subsequent cone consumption. A relative measure of duff consumption by depth between sites was also used in defining the classes. Classifications were as follows: high—forest floor and tree crown were completely burned; moderate—forest floor was completely burned and crown was burned but still recognizable; and low—forest floor was partially burned and crown was burned but still recognizable. The two site moisture classes (dry and wet), each with three fire severity classes (high, moderate, and low), were replicated three times for a total of 18 disturbance plots of 1 × 1 m2 each (see Scholefield 2007 for full description of plot selection). Unburned, MPB-attacked lodgepole pine plots were identified on the same soil types as burned plots. Lodgepole pine seed (100 wild seeds (BC 25110) per plot) was sown directly into all plots in May 2005. Forceps were used to insert individual seeds 5 mm beneath the soil at distances of 50 cm apart. Due to competing vegetation, lodgepole pine seedlings were unable to establish in unburned plots; therefore, data for unburned plots are included for soil analysis, but not for root-associated microbe analyses (ECM and N-cycling bacteria).
Soil sampling and analyses
In each plot, soil was collected in August 2006 from three locations at a depth of 0–12 cm; equal volumes of these soils were combined to form one composite sample (approximately 1 kg) per replicate plot. Soil samples were kept at 4 °C during transport to the laboratory; each sample was then air-dried and passed through a 2-mm sieve prior to analysis. Soils were analyzed (BC Ministry of Forests, Lands and Natural Resource Operations, Research Branch Lab, Victoria, BC) for selected soil properties (total C and N; pH (water and CaCl2); SO42 −; conductivity; exchangeable Al, Ca, Fe, K, Mg, Mn, and Na; CEC; available NH4-N; NO3-N; mineralizable NH4-N; and particle size) following procedures in Kalra and Maynard (1991).
Seedling harvest and ECM characterization
Harvesting of lodgepole pine seedlings for ECM assessment occurred in August 2006, and consisted of seedlings grown from unimproved wild seed, sown in May 2005. Depending on date of germination, seedlings were approximately two growing seasons old. All seedlings were randomly selected, harvested with soil surrounding roots, bagged, and kept cool. Seedlings were stored at 4 °C until assessed. A total of 103 seedlings were harvested, 49 from dry sites and 54 from wet sites, representing all three fire severity classes. Seedling roots were gently cleaned of soil and root tips were assessed using standard microscopy techniques (Robertson et al. 2006). Ectomycorrhizal tips were initially described using a dissecting microscope and classified according to standard features such as colour, lustre, tip dimension and shape, branching pattern, and presence or absence of rhizomorphs (mycelial strands) (Agerer 1987–2008; Ingleby et al. 1990). Root tip squash mounts were subsequently examined using bright field microscopy, and descriptions of mantle features, emanating hyphae, rhizomorphs, and other characteristics were used to further categorize the different ECM morphotypes. When possible, ECMs were identified to a species, genus, or family based on their morphological features; when identification was not possible, a descriptive name was assigned. Uncolonized or lightly colonized tips that lacked identifiable, well-developed mantles were classified as one group. Identification to family, genus, or species was made based on similarities in features to published descriptions (Agerer 1987–2008; Ingleby et al. 1990). Only roots that appeared turgid with intact meristems were examined. For each seedling, all tips were assessed and the percent abundance for each morphotype was calculated from this total. A total of 18,696 root tips were characterized according to their fungal-root associations (ECM morphotypes). ECM abundances were calculated by dividing the total number of root tips of each particular morphotype found on all seedlings in a plot by the total number of mycorrhizal root tips counted in that plot. Following ECM assessment, root samples (entire seedling root systems) were frozen at −20 °C for subsequent molecular analysis.
Molecular characterization of ECM and N-cycling communities
The Ultraclean Soil DNA kit (MoBio, Carlsbad, CA, USA) was used to extract DNA from all whole seedling root systems, following the manufacturer’s recommended alternative protocol for increased yield. Before extraction, samples were frozen at −80 °C for 30 min, thawed for 30 min, frozen at −80 °C for 30 min, and thawed again for 30 min to fracture cells. Fungal communities were profiled by length-heterogeneity PCR (LH-PCR) using primers ITS3 and NLB4, while nitrogen-cycling bacterial communities were profiled by T-RFLP of nifH and nosZ genes, as previously described (Kennedy and Egger 2010).
Statistical analysis
Data from all soil samples (soil chemistry) and root tips (ECM morphotype, LH-PCR fungal genotypes, and N-cycling bacterial genotypes) analyzed in each plot were averaged in order to create a composite plot profile, resulting in n = 3 (plots) for each disturbance. Richness and evenness of morphotypes and genotypes were determined using indices described by Blackwood et al. (2007): richness (S′) and Smith and Wilson evenness (E var). All single-variable (soil chemistry, richness/evenness, and morphotype abundances) data were analyzed using Statistica version 6.1. First, data were tested for normality using the Shapiro-Wilk W test. If normal, factorial ANOVA (moisture (2 levels), fire (4 levels for soil chemistry, 3 levels for all other datasets), and their interaction) was performed, with the significance level set at P < 0.05. Post hoc Tukey HSD tests were performed to determine significant differences between means in disturbances showing significance. Some non-normal results could not be made normal by transformation and so were analyzed using nonparametric Kruskal–Wallis ANOVA. Correlations between soil chemical variables and richness/evenness/morphotype abundances were determined using linear regression.
Multivariate statistical analysis was performed using PC-ORD 5.12. First, LH-PCR (fungal) and T-RFLP (bacterial) profiles from individual seedlings in a plot were averaged together using the program RiboSort (Scallan et al. 2008) and the statistical software R. The relative abundance of genotypes in each composite sample was calculated after relativizing the fluorescent signal strength of each fragment peak to the total peak area of the sample (Osborne et al. 2006). Community structure was assessed graphically with nonmetric multidimensional scaling (NMS) (McCune et al. 2002). This was calculated on the basis of a Sørensen (Bray–Curtis) distance measure with 100 runs with real and randomized data (compared using Monte Carlo simulations) and a maximum of 250 iterations to assess stability. Pearson and Kendall correlations with soil chemistry variables were assessed, and correlations with values higher than 0.150 were overlaid as vectors on the NMS plot. Cluster analysis on ECM morphotype data was performed using two-way hierarchical cluster analysis with flexible beta linkage and Sørensen (Bray–Curtis) distance measure.
Results
The majority of soil properties (16 out of 22) were significantly influenced by moisture, with total C and N, SO4-S, conductivity, mineral NH4-N, silt and clay content, and exchangeable Al, Ca, Fe, Mg, and Na, as well as CEC values, higher in wet compared to dry soils. Soil pH (water and CaCl2) and sand content values were higher in dry sites (Table 1). Fire severity (ranging from unburned to high) did not have a significant impact on any soil properties.
Ectomycorrhizal morphotype assessment identified a total of 29 unique morphotypes, 25 from dry, and 27 from wet disturbance sites (Table 2). Seedlings had from two to six ECM morphotypes, while plots had from five to 10 each. Table 2 shows mean plot values for percent ECM abundance of each morphotype as affected by disturbance. Four ECM morphotypes showed a significant moisture preference: three to wet sites (Russulaceae 2, MRA, and Piloderma) and one to dry sites (Russulaceae Lact-like1). Most seedlings (approximately 90 %) had some level of tips considered uncolonized or lightly colonized (i.e., with too little fungal mantle for characterization); no significant difference was identified between moisture or fire severity disturbances for this uncolonized/lightly colonized group (P > 0.05, data not shown). Figure 1 shows a two-way cluster analysis of ECM morphotype data, showing some broad clustering of morphotypes according to moisture. Most dry sites clustered together at the top of the diagram, indicating that they had similar morphotype profiles, while wet sites exhibited similar clustering at the bottom.
Significant differences were not found for richness or evenness values of ECM (fungal morphotypes (richness 8.3–11.3; evenness 0.27–0.40) or LH-PCR genotypes (richness 7.7–13.3; evenness 0.62–0.72) or denitrifying (richness 6.3–11.3; evenness 0.41–0.53) or N-fixing (richness 15.3–20.7; evenness 0.59–0.74) bacteria with respect to moisture or fire severity (all P > 0.05; data not shown). However, ECM morphotype richness was consistently, but not significantly, higher from the wet (all fire severities) compared to dry sites. Significant correlations were observed between ECM morphotype richness and soil SO4 (positive, P = 0.01, r 2 = 0.34), and between ECM morphotype evenness and C:N ratios (positive, P = 0.01, r 2 = 0.34). There was also a significant correlation between denitrifier richness and available NO3 (positive, P = 0.03, r 2 = 0.26).
Nonmetric multidimensional scaling ordination of fungal ECM morphotype profiles exhibited clustering according to moisture (Fig. 2a), with wet sites clustering on the left side of the plot, while dry sites clustered to the right. Overlay of the plot with soil chemical properties indicated pH, total C and N, and mineral NH4 were significantly correlated with ECM morphotype profiles. Axis 1 was most highly correlated with pH (r 2 = 0.524), while axis 2 was most highly correlated with mineral NH4 (r 2 = 0.292). Similar clustering according to moisture was evident in the NMS ordination plot of LH-PCR fungal genotypes (Fig. 2b). As in the ECM morphotype plot, there were significant correlations between LH-PCR genotype profiles and pH, total C and N, and mineral NH4. The impact of moisture on fungal community structure was confirmed by PERMANOVA, which showed both ECM morphotype and LH-PCR genotype profiles of wet sites differed significantly (Table 3) from dry sites. In contrast to the strong effect of moisture, NMS plots showed no clustering due to fire severity, and PERMANOVA analysis found no significant differences between fungal communities in soils from different fire severities. Denitrifying (Supplemental Fig. 1) and N-fixing (Supplemental Fig. 2) bacterial genotypes did not exhibit any clustering due to moisture or fire severity. However, there were significant correlations between N-fixing genotype profiles and C:N and pH.
NMS plots of fungal community structure. r 2 values represent the variance explained by each axis. Line vectors show correlations of profiles with soil chemistry data. a NMS plot of fungal ECM morphotype profiles. Stress = 23.9, instability = 0.00001. b NMS plot of fungal LH-PCR profiles. Stress = 23.0, instability = 0.01037. Site type (dry, wet); fire severity (low, moderate, high)
Discussion
Wet and dry sites had distinct soil chemistries
Following examination of the full suite of soil chemistry data, it became evident that wet and dry sites were different in almost every aspect of soil chemistry, not just moisture, and as expected, represented two discrete soil types. Interestingly, despite consecutive disturbances (mountain pine beetle infestation and wildfire), moisture remained the most important factor influencing soil chemistry. The lower pH of wet sites was likely due to increased moisture leading to increased biological activity, which in turn can lead to production of organic acids (Prescott et al. 2000). Similarly, greater microbial biomass in wet sites may also have resulted in higher levels of mineralizable ammonium. This was supported by higher levels of available nitrate and ammonium in wet soils than dry soils, although these were not significant. The sandy nature of dry soil sites could result in increased leaching on these sites, leading to lower levels of nitrate. Although nitrate levels were higher in wet soils, they were still considered low compared to typical levels (Ryan and Waring 1992; Cullings and New 2003; Douglas et al. 2005). This may be due to the higher fungal biomass in wet soils capturing nitrate (Martin et al. 2000).
Soil type strongly influenced ECM community structure
Our molecular and morphological results showed a clear distinction between ECM fungal communities from wet versus dry sites. The use of both approaches confirmed the trends seen in the data, as each approach has different advantages and limitations. Morphotyping has been extensively used to describe ECM communities, but is time-consuming and requires advanced expertise; in addition, it is possible that one morphotype can have more than one genetic identity. Molecular fingerprinting techniques, such as LH-PCR, are rapid and high-throughput and may be able to resolve species that morphotyping cannot; however, caution must be used in analyzing profiling data due to possible interspecies ITS heterogeneity and PCR amplification bias (Dickie and FitzJohn 2007). In this study, both approaches revealed the same overall trends in ECM community structure, thus confirming one another’s results. Complementarity of ECM morphotype and fingerprinting data was also noted by Burke et al. (2005), who found that genotypes detected with TRFLP accounted for 93 % of their colonized root tips.
The cluster analysis revealed the influence of soil type on individual morphotypes. Although some morphotypes occurred in both site types, there were four morphotypes that were restricted to, or found in greater abundance in, either wet or dry sites. The distribution of ECM morphotypes between soil types indicated three broad groups: “ubiquitous/cosmopolitan,” “wet-preferring,” and “dry-tolerating.” Ubiquitous morphotypes, including Cenococcum and E-strain 1, were found in almost all sites. These ascomycetes have been previously described from a wide diversity of habitats and are known to associate with a broad range of coniferous hosts (e.g. Mah et al. 2001; Douglas et al. 2005; Robertson et al. 2006; DeBellis et al. 2006). In particular, the E-strain morphotype has been described as a dominant member of post-fire ECM communities (reviewed in Jones et al. 2003). Morphotypes such as MRA, Piloderma, and Russulaceae 2 were preferentially found in wet sites and were largely absent from dry sites, while dry-tolerating types, such as Russulaceae-Lact-like1 and Asco-like, were present in both wet and dry sites. These differences in distribution likely represent individual species adaptations to soil moisture (or chemical changes associated with soil moisture) that allow for survival (or, in some instances, for ECM fungi to thrive) in diverse habitats. A fourth group of morphotypes exhibited a patchy, pattern-less distribution, representing a small component of the root community.
Our findings are supported by other studies which have noted significant effects of soil conditions on forest ECM communities. Soil moisture and temperature levels were found to impact ECM community structure to a larger extent than soil nutrient status on lodgepole pine roots (Cullings and New 2003). Field studies of ECM associated with black spruce (Robertson et al. 2006) and subalpine fir (Kranabetter et al. 2009) also found variations in ECM community structure due to differences in moisture. A study of ECM communities from pinyon pine trees growing in different soils noted that soil type was linked to fungal community composition (Gehring et al. 1998). A recent study (Karst et al. 2011) found that Douglas-fir seedlings from low-moisture soils had higher levels of ectomycorrhizal colonization than those from medium and high moisture soils.
Fire severity had limited impact on soil chemistry or ECM communities
In comparison to the significant influence of soil type, our study found fire severity had a relatively small impact on soil chemistry and root-associated microbial communities. These results contrast with other studies that have found dramatic changes in soil properties due to fire. In a review of the effects of fire on soil aggregation (a measure of soil resilience), Mataix-Solera et al. (2011) found that aggregation depended upon fire severity and soil type, with high-severity fires having the greatest impact. Certini (2005) conducted a review and found that fire had severe negative effects on forest soil properties, including removal of organic matter, loss of soil nutrients, decreased microbial biomass, and changes in the composition of soil microbiota. A recent meta-analysis on microbial responses to fire found that fire decreased microbial biomass by an average of 33 % and fungal biomass by an average of 47 % (Dooley and Treseder 2011).
Several studies have looked at the impact of fire on forest ECM communities in particular (see Cairney and Bastias (2007) for review). Varied responses of ECM communities to fire have been described, with some studies finding post-fire decreases in ECM biomass/diversity on Scots pine (Dahlberg et al. 2001) and ponderosa pine stands (Stendell et al. 1999; Smith et al. 2005) in Sweden, California, and Oregon, respectively. Other studies have found fire altered ECM community structure in Douglas-fir (Kennedy and Egger 2010), ponderosa pine (Gundale et al. 2005), and spruce (Mah et al. 2001) forests, without necessarily causing large changes in diversity or biomass, while a study on Scots pine forests in Sweden did not find any consistent change in ECM richness or composition following wildfire (Jonsson et al. 1999). These varying responses of ECM to fire may indicate that local conditions (such as moisture in our study) can strongly influence the response of ECM to wildfire.
Previous studies that have included sites burned at different severities suggest the impact of fire severity on ECM communities is difficult to estimate. An assessment of maritime pine (Pinus pinaster) in Portugal indicated that soil chemistry and ECM communities in areas burned at different fire return intervals were more similar to each other than to unburned controls (Buscardo et al. 2012). Similarly, Rincón and Pueyo (2010) examined fire severity on ECM communities of a maritime pine forest in Spain and found that fire severity did not affect ECM richness or diversity, while Barker et al. (2013) found the rate of ECM colonization of Douglas-fir seedlings in interior BC forests was unaffected by wildfire severity. In contrast, an earlier study on Scots pine forests in Sweden found ECM abundance and diversity decreased with increased fire severity (Dahlberg et al. 2001). Their study also emphasized the impact of burning on forest floor soil properties, noting that even low-severity burns killed mycorrhizal fungi in the organic layer of the soil. Other studies focused on the mineral layer of soil have noted that this layer acts as reservoir of resistant propagules of ECM, such as spores and sclerotia, which can rapidly colonize seedling roots post-fire (Stendell et al. 1999; Grogan et al. 2000; Bruns et al. 2002; Rincón and Pueyo 2010; Barker et al. 2013). This suggests that all fire severities in our study may have been hot enough to kill mycorrhizal fungi in the organic layer, allowing colonization of regenerating seedlings from the mineral layer, and resulting in an evenness of effect across fire intensities with respect to both chemical and microbial impacts.
N-cycling bacteria were unaffected by soil type or fire severity
In contrast to the large impact of moisture on fungal communities, there was no significant impact of moisture (or fire severity) on two types of N-cycling bacterial communities. This concurs with results for burned Douglas-fir pine forests in southern British Columbia, where fire affected ECM community structure, but not the structure of denitrifying or N-fixing bacterial communities (Kennedy and Egger 2010). Similarly, Štursová et al. (2014) found that insect infestation affected forest soil fungal communities more than bacterial communities. The few studies that have been conducted specifically on root-associated N-cycling communities in forests have noted low diversity of nitrogen-fixing bacteria (Burke et al. 2006; Izumi et al. 2006), which is consistent with our results. However, the inability of lodgepole pine to establish on unburned control plots may have prevented our ability to identify an effect of fire severity (at any intensity) on N-cycling bacterial communities. It is also possible that the differences between the fire severities tested were too small to be detected in community analyses that were used. This is supported by the results of Yeager et al. (2005), which showed few significant differences between nitrogen-fixing and ammonia-oxidizing bacterial profiles from moderately and severely-burned mixed conifer forest soils. We did note significant correlations between pH and C:N ratio and N-fixing communities, which was consistent with previous reports that changes in C:N ratio (Deslippe et al. 2005; Coelho et al. 2008) and pH (Hayden et al. 2010) can influence soil nifH profiles.
A lack of response to soil type and fire severity by N-cycling bacteria may also indicate greater resilience in bacterial compared to fungal communities. A review of soil microbe responses to fire found that fire reduced fungal biomass more than overall microbial biomass (Dooley and Treseder 2011). Similarly, fungal communities (especially mycorrhizal fungi) were shown to be more sensitive than bacterial communities to tree harvesting disturbance (Hartmann et al. 2012).
Conclusions
Our most striking finding was that soil type influenced ECM community much more than fire severity. Similarly, results showed that significant differences in soil chemistry parameters were due to soil type rather than to fire severity, leading us to conclude that local soil chemical variables were controlling ECM community structure. In particular, moisture appeared to be the most important influence on both soil chemistry and ECM composition, with the “signature” of moisture remaining pronounced even after consecutive landscape disturbances. Neither a major insect outbreak nor wildfire could eliminate this signature.
In summary, our results indicate that local soil conditions had a greater impact than fire severity on ECM communities on the roots of regenerating seedlings in post-beetle forests. This has implications for forest management in that it may be more important to consider soil chemistry and local soil moisture regime than fire history of sites selected for “restoration” (Burton 2006) through planting of seedlings.
References
Agerer R (ed) (1987–2008) Colour atlas of ectomycorrhizae. 1st–14th edns. Einhorn-Verlag Eduard Dietenberger, Schwäbisch Gmünd
Axelson JN, Alfaro RI, Hawkes BC (2009) Influence of fire and mountain pine beetle on the dynamics of lodgepole pine stands in British Columbia, Canada. For Ecol Manag 257:1874–1882
Barker JS, Simard SW, Jones MD, Durall DM (2013) Ectomycorrhizal fungal community assembly on regenerating Douglas-fir after wildfire and clearcut harvesting. Oecologia 172:1179–1189
Blackwood CB, Hudleston D, Zak DR, Buyer JS (2007) Interpreting ecological diversity indices applied to terminal restriction fragment length polymorphism data: insights from simulated microbial communities. Appl Environ Microbiol 73:5276–5283
Bruns T, Tan J, Bidartondo M, Szaro T, Redecker D (2002) Survival of Suillus pungens and Amanita francheti ectomycorrhizal genets was rare or absent after a stand-replacing wildfire. New Phytol 155:517–523
Burke DJ, Kretzer AM, Rygiewicz PT, Topa M (2006) Soil bacterial diversity in a loblolly pine plantation: influence of ectomycorrhizas and fertilization. FEMS Microbiol Ecol 57:409–419
Burke D, Martin K, Rygiewicz P, Topa M (2005) Ectomycorrhizal fungi identification in single and pooled root samples: terminal restriction fragment length polymorphism (TRFLP) and morphotyping compared. Soil Biol Biochem 37:1683–1694
Burton P (2006) Restoration of forests attacked by mountain pine beetle: misnomer, misdirected, or must-do? BC J Ecosyst Manag 7:1–10
Burton PJ (2010) Striving for sustainability and resilience in the face of unprecedented change: the case of the mountain pine beetle outbreak in British Columbia. Sustainability 2:2403–2423
Buscardo E, Rodríguez-Echeverría S, Barrico L, García MÁ, Freitas H, Martín MP, De Angelis P, Muller LAH, De Angelis P (2012) Is the potential for the formation of common mycorrhizal networks influenced by fire frequency? Soil Biol Biochem 46:136–144
Cairney JWG, Bastias BA (2007) Influences of fire on forest soil fungal communities. Can J For Res 37:207–215
Carroll A, Régnière J, Logan J, Taylor S, Bentz B, Powell J (2006) Impacts of climate change on range expansion by the mountain pine beetle. Mountain Pine Beetle Initiative Working Paper 2006-14. Natural Resources Canada, Victoria, BC
Certini G (2005) Effects of fire on properties of forest soils: a review. Oecologia 143:1–10
Clow DW, Rhoades C, Briggs J, Caldwell M, Lewis WM (2011) Responses of soil and water chemistry to mountain pine beetle induced tree mortality in Grand County, Colorado, USA. Appl Geochem 26:S174–S178
Coelho MRR, de Vos M, Carneiro NP, Marriel IE, Paiva E, Seldin L (2008) Diversity of nifH gene pools in the rhizosphere of two cultivars of sorghum (Sorghum bicolor) treated with contrasting levels of nitrogen fertilizer. FEMS Microbiol Lett 279:15–22
Cullings K, New M (2003) Effects of litter addition on ectomycorrhizal associates of a lodgepole pine (Pinus contorta) stand in Yellowstone National Park. Appl Environ Microbiol 69:3772–3776
Dahlberg A, Schimmel J, Taylor AFS, Johannesson H (2001) Post-fire legacy of ectomycorrhizal fungal communities in the Swedish boreal forest in relation to fire severity and logging intensity. Biol Conserv 100:151–161
Dawson AB (1989) Soils of the Prince George-McLeod Lake area. Ministry of Environment, BC
DeBellis T, Kernaghan G, Bradley R, Widden P (2006) Relationships between stand composition and ectomycorrhizal community structure in boreal mixed-wood forests. Microb Ecol 52:114–126
Deslippe J, Egger KNE, Henry G (2005) Impacts of warming and fertilization on nitrogen-fixing microbial communities in the Canadian High Arctic. FEMS Microbiol Ecol 53:41–50
Dickie IA, FitzJohn RG (2007) Using terminal restriction fragment length polymorphism (T-RFLP) to identify mycorrhizal fungi: a methods review. Mycorrhiza 17:259–270
Dooley SR, Treseder KK (2011) The effect of fire on microbial biomass: a meta-analysis of field studies. Biogeochemistry 109:49–61
Douglas R, Parker V, Cullings K (2005) Belowground ectomycorrhizal community structure of mature lodgepole pine and mixed conifer stands in Yellowstone National Park. For Ecol Manag 208:303–317
Frey-Klett P, Garbaye J, Tarkka M (2007) The mycorrhiza helper bacteria revisited. New Phytol 176:22–36
Gehring C, Theimer T, Whitham T, Keim P (1998) Ectomycorrhizal fungal community structure of pinyon pines growing in two environmental extremes. Ecology 79:1562–1572
Griffin JM, Turner MG, Simard M (2011) Nitrogen cycling following mountain pine beetle disturbance in lodgepole pine forests of Greater Yellowstone. For Ecol Manag 261:1077–1089
Grogan P, Baar J, Bruns TD (2000) Below-ground ectomycorrhizal community structure in a recently burned bishop pine forest. J Ecol 88:1051–1062
Gundale MJ, DeLuca TH, Fiedler CE, Ramsey PW, Harrington MG, Gannon JE (2005) Restoration treatments in a Montana ponderosa pine forest: effects on soil physical, chemical and biological properties. For Ecol Manag 213:25–38
Hamann A, Wang T (2006) Potential effects of climate change on ecosystem and tree species distribution in British Columbia. Ecology 87:2773–2786
Hartmann M, Howes CG, Vaninsberghe D, Yu H, Bachar D, Christen R, Henrik Nilsson R, Hallam SJ, Mohn WW (2012) Significant and persistent impact of timber harvesting on soil microbial communities in Northern coniferous forests. ISME J 6:2199–2218
Hayden HL, Drake J, Imhof M, Oxley APA, Norng S, Mele PM (2010) The abundance of nitrogen cycle genes amoA and nifH depends on land-uses and soil types in South-Eastern Australia. Soil Biol Biochem 42:1774–1783
Hirsch PR, Mauchline TH, Clark IM (2010) Culture-independent molecular techniques for soil microbial ecology. Soil Biol Biochem 42:878–887
Ingleby K, Mason PA, Last FT, Fleming LV (1990) Identification of Ectomycorrhizae. Institute of Terrestrial Ecology, Natural Environment Research Council, London
Izumi H, Anderson IC, Alexander IJ, Killham K, Moore ERB (2006) Diversity and expression of nitrogenase genes (nifH) from ectomycorrhizas of Corsican pine (Pinus nigra). Environ Microbiol 8:2224–2230
Jones MD, Durall DM, Cairney JWG (2003) Ectomycorrhizal fungal communities in young forest stands regenerating after clearcut logging. New Phytol 157:399–422
Jonsson L, Dahlberg A, Nilsson M, Zackrisson O, Kårén O (1999) Ectomycorrhizal fungal communities in late-successional Swedish boreal forests, and their composition following wildfire. Mol Ecol 8:205–215
Kalra YP, Maynard DG (1991) Methods manual for forest soil and plant analysis (Vol. 319). Information report NOR-X-319. Forestry Canada, Edmonton
Kana J, Tahovska K, Kopacek J (2012) Response of soil chemistry to forest dieback after bark beetle infestation. Biogeochemistry 113:369–383
Karst J, Hoeksema JD, Jones MD, Turkington R (2011) Parsing the roles of abiotic and biotic factors in Douglas-fir seedling growth. Pedobiologia 54:273–280
Kaufmann M, Aplet G, Babler M, Baker W, Bentz B, Harrington M, Huckaby LS, Jenkins M, Kashian D, Keane RE, Kulakowski C, McHugh C, Negron J, Popp W, Romme T, Schoennagel W, Shepperd W, Smith F, Sutherland EK, Tinker D, Veblen T (2008) The status of our scientific understanding of lodgepole pine and mountain pine beetles—a focus on forest ecology and fire behavior. The Nature Conservancy, Arlington
Kennedy N, Egger KN (2010) Impact of wildfire intensity and logging on fungal and nitrogen-cycling bacterial communities in British Columbia forest soils. For Ecol Manag 260:787–794
Keville MP, Reed SC, Cleveland CC (2013) Nitrogen cycling responses to mountain pine beetle disturbance in a high elevation whitebark pine ecosystem. Plos One 8(6):e65004. doi:10.1371/journal.pone.0065004
Kranabetter JM, Durall DM, MacKenzie WH (2009) Diversity and species distribution of ectomycorrhizal fungi along productivity gradients of a southern boreal forest. Mycorrhiza 19:99–111
Mah K, Tackaberry LE, Egger KN, Massicotte HB (2001) The impacts of broadcast burning after clear-cutting on the diversity of ectomycorrhizal fungi associated with hybrid spruce seedlings in central British Columbia. Can J For Res 31:224–235
Martin FM, Perotto S, Bonfante P (2000) Mycorrhizal fungi: a fungal community at the interface between soil and roots. In: Pinton R, Varanini Z, Nannipieri P (eds) The rhizosphere: biochemistry and organic substances at the soil-plant interface. Marcel Dekker Inc., New York, pp 263–296
Mataix-Solera J, Cerdà A, Arcenegui V, Jordán A, Zavala L (2011) Fire effects on soil aggregation: a review. Earth-Sci Rev 109:44–60
McCune B, Grace JB, Urban DL (2002) Analysis of ecological communities. MjM Software Design, Gleneden Beach, Oregon
Mikkelson KM, Bearup LA, Maxwell RM, Stednick JD, McCray JE, Sharp JO (2013) Bark beetle infestation impacts on nutrient cycling, water quality and interdependent hydrological effects. Biogeochemistry 115:1–21
Natural Resources Canada (2013) The threat of mountain pine beetle to Canada’s boreal forest. http://www.nrcan.gc.ca/forests/insects-diseases/13381. Accessed on February 16, 2014
Osborne CA, Rees GN, Bernstein Y, Janssen PH (2006) New threshold and confidence estimates for terminal restriction fragment length polymorphism analysis of complex bacterial communities. Appl Environ Microbiol 72:1270
Page WG, Jenkins MJ, Runyon JB (2012) Mountain pine beetle attack alters the chemistry and flammability of lodgepole pine foliage. Can J For Res 42:1631–1647
Prescott CE, Maynard DG, Laiho R (2000) Humus in northern forests: friend or foe? For Ecol Manag 133:23–36
Rhoades CC, McCutchan JH, Cooper LA, Clow D, Detmer TM, Briggs JS, Stednick JD, Veblen TT, Ertz RM, Likens GE, Lewis WM (2013) Biogeochemistry of beetle-killed forests: explaining a weak nitrate response. Proc Natl Acad Sci U S A 110:1756–1760
Rincón A, Pueyo JJ (2010) Effect of fire severity and site slope on diversity and structure of the ectomycorrhizal fungal community associated with post-fire regenerated Pinus pinaster Ait. seedlings. For Ecol Manag 260:361–369
Robertson SJ, Tackaberry LE, Egger KN, Massicotte HB (2006) Ectomycorrhizal fungal communities of black spruce differ between wetland and upland forests. Can J For Res 985:972–985
Ryan M, Waring R (1992) Maintenance respiration and stand development in a subalpine lodgepole pine forest. Ecology 73:2100–2108
Scallan U, Liliensiek A, Clipson N, Connolly J (2008) Ribosort: a program for automated data preparation and exploratory analysis of microbial community fingerprints. Mol Ecol Resour 8:95–98
Scholefield SR (2007) Regeneration of lodgepole pine after wildfire in mountain pine beetle-killed stands in north-central British Columbia. Dissertation, University of Northern British Columbia. 89 pp
Simard SW (2009) Mycorrhizal networks and complex systems: contributions of soil ecology science to managing climate change effects in forested ecosystems. Can J Soil Sci 89:369–382
Simard SW, Durall DM (2004) Mycorrhizal networks: a review of their extent, function, and importance. Can J Bot 82:1140–1165
Smith JE, McKay D, Brenner G, McIver J, Spatafora JW (2005) Early impacts of forest restoration treatments on the ectomycorrhizal fungal community and fine root biomass in a mixed conifer forest. J Appl Ecol 42:526–535
Stendell E, Horton T, Bruns T (1999) Early effects of prescribed fire on the structure of the ectomycorrhizal fungus community in a Sierra Nevada ponderosa pine forest. Mycol Res 103:1353–1359
Štursová M, Snajdr J, Cajthaml T, Bárta J, Santrůčková H, Baldrian P (2014) When the forest dies: the response of forest soil fungi to a bark beetle-induced tree dieback. ISME J 8:1920–1931
Switzer JM, Hope GD, Grayston SJ, Prescott CE (2012) Changes in soil chemical and biological properties after thinning and prescribed fire for ecosystem restoration in a Rocky Mountain Douglas-fir forest. For Ecol Manag 275:1–13
Throbäck IN, Enwall K, Jarvis A, Hallin S (2004) Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol Ecol 49:401–417
Treu R, Karst JD, Randall M, Pec GJ, Cigan PW, Simard S, Cook JEK, Erbilgin N, Cahill JF (2014) Decline of ectomycorrhizal fungi following a mountain pine beetle epidemic. Ecology 95:1096–1103
Turner MG, Romme WH, Gardner RH (1999) Prefire heterogeneity, fire severity, and early postfire plant reestablishment in subalpine forests of Yellowstone National Park, Wyoming. Int J Wildland Fire 9:21–36
Walton A (2012) Provincial-Level Projection of the Current Mountain Pine Beetle Outbreak: Update of the infestation projection based on the Provincial Aerial Overview Surveys of Forest Health conducted from 1999 through 2011 and the BCMPB model (Year 9). Ministry of Forests, Lands and Natural Resource Operations, Victoria
Yeager CM, Northup DE, Grow CC, Barns SM, Kuske CR (2005) Changes in nitrogen-fixing and ammonia-oxidizing bacterial communities in soil of a mixed conifer forest after wildfire. Appl Environ Microbiol 71:2713
Zehr JP, Jenkins BD, Short SM, Steward GF (2003) Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ Microbiol 5:539–554
Acknowledgments
This project was funded by the Government of Canada through the Mountain Pine Beetle Initiative (administered by Natural Resources Canada, Canadian Forest Service) as well as a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) to HBM. We are grateful to Ashley Gosselin, Jennifer Dupuis, Jordan Koopmans, Mark Thompson, Patrick McMechan, and Dana O’Bryan for field and laboratory assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
(GIF 77 kb)
Supplemental Fig. 2
(GIF 79 kb)
Rights and permissions
About this article
Cite this article
Kennedy, N.M., Robertson, S.J., Green, D.S. et al. Site properties have a stronger influence than fire severity on ectomycorrhizal fungi and associated N-cycling bacteria in regenerating post-beetle-killed lodgepole pine forests. Folia Microbiol 60, 399–410 (2015). https://doi.org/10.1007/s12223-014-0374-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-014-0374-7