Abstract
The purpose of current article is to simulate melting of NEPCM inside a cylinder with V shaped fins. Two dimensional transient formulations were modeled via finite volume method. Copper oxide nanoparticles were dispersed into paraffin. Second law treatments during melting are reported. Outputs illustrate that thermal entropy generation detracts with augment of temperature. Temperature rises as time progress due to stronger buoyancy forces.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Several scientist selected phase change materials for saving energy in heat storage units. Also, they added metallic Nano sized particles to overcome the low thermal conductivity. Selimefendigil and Oztop (2019) scrutinized CNT nanoparticles transportation due to MHD inside a corrugated cavity. Sheikholeslami et al. (2018) illustrated the unsteady solidification of NEPCM. They employed Galerkin approach to show the phase change process. Nanofluid mass transfer over step with elastic wall was scrutinized by Selimefendigil and Oztop (2018). Khan et al. (2017) demonstrated nanomaterial treatment due to radiative heating. They utilized non-Newtonian model in presence of MHD. Sheikholeslami et al. (2019) employed nanoparticles and V shaped fins to reach higher rate of solidification. They presented FEM simulation for solving unsteady problem. Heatline approach has been applied by Bondareva et al. (2016) for natural convection of nanoparticles inside a wavy enclosure with local heater. Sheremet et al. (2015) presented second law behavior of nanomaterial thorough a cavity with hot obstacle. They utilized hemogenious model (Tiwari model) for thermal conductivity. Mahian et al. (2013) scrutinized irreversibility analysis of nnaoparticles in existence of MHD impact. Tahir et al. (2017) studied the Maxwell nanofluid radiation along a sheet. They supposed uniform magnetic field to control the flow. Copper oxide migration due to MHD impact in a duct was scrutinized by Selimefendigil and Oztop (2018). They examined optimization procedure via ANFIS. Nguyen et al. (2016) demonstrated the transient nanofluid flow in an enclosure with various heaters. Moatimid et al. (2017) scrutinized nanofluid transient double diffusion flow. Characteristics of nanofluid inside a heat exchanger were examined by Qi et al. (2018). They reported stability behavior of nanofluid and its characteristics. Finding better working fluid in view of heat transfer is hot topic in recent decade (Rehman et al. 2018; Sun et al. 2017; Sheikholeslami et al. 2019; Sheikholeslami and Oztop 2016, 2017; Sheikholeslami 2018, 2019a, b; Khan et al. 2018a, b; Eldabe et al. 2018; Hayat et al. 2018; Darzi et al. 2016; Ahmed et al. 2017).
In present report, V shaped fins and NEPCM are considered as two effective methods to improve the melting rate. Simulation is done via FEM. Entropy and exergy contours at various stages are demonstrated.
2 Geometry and formulations
2.1 Enclosure with fins
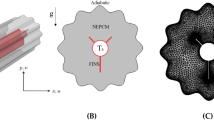
The aim of current article is to carry out numerical investigation of transient, two-dimensional melting process inside the cavity with V shaped fins. The cavity is filled with PCM based copper oxide nanoparticle. This geometry can be fins in Fig. 1. In this paper, we consider L = 0.04 m, D = 0.02 m, l = 0.01 m, t = 0.5 mm. The concentration of copper oxide is 0.05.
2.2 Problem formulations
Let us consider transient laminar 2D melting process considering Boussinesq estimation as follows:
Here we considered ɛ = 10−3, C = 105.
(ρβ)nf, ρnf, (ρL)nf and (ρCp)nf are:
knf, μnf are obtained as:
To see the properties, Table 1 has been presented.
Equation (11) and (12) can be employed to find the enthalpy:
In above equation, we considered href as enthalpy at 273 K.
In the present work, the definitions of Sgen,total, Sgen,th, Sgen,f are:
2.3 Detail of modeling
Governing equations with the boundary conditions have solution which is obtained via FVM utilizing ANSYS Fluent 18.1. The space domain is modeled using Enthalpy-porosity method (Brent et al. 1988). PRESTO model is selected for pressure correction. Velocity under-relaxation is considered 0.2 and SIMPLE algorithm has been utilized. Figures 2 and 3 demonstrate sample grid and verification (Tan et al. 2009).
Validation of code with Tan et al. (2009)
3 Results and discussion
Current section has been devoted to the analysis of melting process in an enclosure with V shaped fins. Not only melt fraction and temperature but also entropy generation and exergy have been analyzed. The system is filled with n-octadecane paraffin in which CuO has been dispersed. The solution of equations was calculated using Finite Volume Method.
Both first and second law analysis during process was illustrated in Figs. 4, 5, 6 and 7. In lower time, the buoyancy is not enough strong to affect the melting. In greater time, convective mechanism affects the melting front and its shape become more complex. Vortex becomes stronger as the melting front reach to outer surface. The middle up of the enclosure converts to liquid phase sooner than the middle down. The region near the down wall is the final space in which the remained solid convert to liquid. This phenomena is due to absence of rotating eddies in that region. As temperature enhances, thermal entropy generation detracts as depicted in contours. Exergy loss has same treatment, too. Liquid fraction, Sgen,f and Sgen,th are shown in Fig. 8. As time progress, average temperature enhances and melts fraction increases until it reaches to its maximum value. Thermal entropy generation detracts with rise of melt fraction. Frictional component has on maximum point in beginning times.
4 Conclusion
The NEPCM melting heat transfer inside a cylinder with V shaped fins are demonstrated. The unsteady problem has been modeled as governing PDEs which were solved via Finite Volume Method. Contours for melt fraction, entropy, isotherm and exergy are reported in various times. Melt fraction enhances as time progress which means greater convection mode and higher temperature. So, Sgen,th reduces with augment of time. At start of process, conduction has main role, thus Sgen,f has one maximum point in its profile.
Abbreviations
- g:
-
Gravity
- NEPCM:
-
Nano-enhanced PCM
- Tm :
-
Fusion temperature
- C:
-
Mushy zone constant
- k:
-
Thermal conductivity
- L f :
-
Latent heat coefficient
- ϕ :
-
Nanoparticle volume fraction
- ρ :
-
Fluid density
- α:
-
Thermal diffusivity (m2/s)
- p :
-
Particle
- s :
-
Solidus
- nf :
-
NEPCM
- l :
-
Liquidus
- nf :
-
Nanofluid
References
Ahmed N, Khan AU, Mohyud-Din ST (2017) Unsteady radiative flow of chemically reacting fluid over a convectively heated stretchable surface with cross-diffusion gradients. Int J Therm Sci 121:182–191
Bondareva NS, Sheremet MA, Oztop HF, Abu-Hamdeh N (2016) Heatline visualization of MHD natural convection in an inclined wavy open porous cavity filled with a nanofluid with a local heater. Int J Heat Mass Transf 99:872–881
Brent AD, Voller VR, Reid KJ (1988) Enthalpy-porosity technique for modeling convection-diffusion phase change: application to the melting of a pure metal. Numer Heat Transf 13:297–318
Darzi AAR, Jourabian M, Farhadi M (2016) Melting and solidification of PCM enhanced by radial conductive fins and nanoparticles in cylindrical annulus. Energy Convers Manag 118:253–263
Eldabe NT, Gabr ME, Zaher SA (2018) Two dimensional boundary layer flow with heat and mass transfer of magneto hydrodynamic non-Newtonian nanofluid through porous medium over a semi-infinite moving plate. Microsyst Technol 24:2919–2928
Hayat T, Aziz A, Muhammad T, Alsaedi A (2018) Three-dimensional flow of Prandtl fluid with Cattaneo–Christov double diffusion. Results Phys 9:290–296
Khan M, Irfan M, Khan WA, Ahmad L (2017) Modeling and simulation for 3D magneto Eyring–Powell nanomaterial subject to nonlinear thermal radiation and convective heating. Results Phys 7:1899–1906
Khan HM, Alshomrani AS, Haq RU (2018a) Investigation of dual solutions in flow of a non-Newtonian fluid with homogeneous-heterogeneous reactions: critical points. J Eur J Mech B Fluids 68:30–38
Khan MI, Dong H, Shabbir F, Shoukat R (2018b) Embedded passive components in advanced 3D chips and micro/nano electronic systems. Microsyst Technol 24:869–877
Mahian O, Pop I, Sahin AZ, Oztop HF, Wongwises S (2013) Irreversibility analysis of a vertical annulus using TiO2/water nanofluid with MHD flow effects. Int J Heat Mass Transf 64:671–679
Moatimid GM, Mohamed MAA, Hassan MA, El-Dakdoky EMM (2017) Unsteady Cattaneo–Christov double diffusion of conducting nanofluid. Sci Eng Appl 2(3):164–176
Nguyen MT, Aly AM, Lee SW (2016) Unsteady natural convection heat transfer in a nanofluid-filled square cavity with various heat source conditions. Adv Mech Eng 8(5):1–18
Qi C, Liu M, Wang G, Pan Y, Liang L (2018) Experimental research on stabilities, thermophysical properties and heat transfer enhancement of nanofluids in heat exchanger systems. Chin J Chem Eng. https://doi.org/10.1016/j.cjche.2018.03.021
Rehman FU, Nadeem S, Rehman HU, Haq RU (2018) Thermophysical analysis for three-dimensional MHD stagnation-point flow of nano-material influenced by an exponential stretching surface. Results Phys 8:316–323
Selimefendigil F, Öztop HF (2016) Conjugate natural convection in a cavity with a conductive partition and filled with different nanofluids on different sides of the partition. J Mol Liq 216:67–77
Selimefendigil F, Öztop HF (2018a) Laminar convective nanofluid flow over a backward-facing step with an elastic bottom wall. J Therm Sci Eng Appl 10(4):041003
Selimefendigil F, Öztop HF (2018b) Magnetic field effects on the forced convection of CuO-water nanofluid flow in a channel with circular cylinders and thermal predictions using ANFIS. Int J Mech Sci 146:9–24
Selimefendigil F, Öztop HF (2019) Corrugated conductive partition effects on MHD free convection of CNT-water nanofluid in a cavity. Int J Heat Mass Transf 129:265–277
Sheikholeslami M (2018) Finite element method for PCM solidification in existence of CuO nanoparticles. J Mol Liq 265:347–355
Sheikholeslami M (2019a) Numerical approach for MHD Al2O3-water nanofluid transportation inside a permeable medium using innovative computer method. Comput Methods Appl Mech Eng 344:306–318
Sheikholeslami M (2019b) New computational approach for exergy and entropy analysis of nanofluid under the impact of Lorentz force through a porous media. Comput Methods Appl Mech Eng 344:319–333
Sheikholeslami M, Oztop H (2017) MHD free convection of nanofluid in a cavity with sinusoidal walls by using CVFEM. Chin J Phys 55(6):2291–2304
Sheikholeslami M, Shehzad SA, Li Z, Shafee A, Abbasi FM (2018) Time dependent conduction heat transfer during solidification in a storage system using nanoparticles. Microsyst Technol 15:16. https://doi.org/10.1007/s00542-018-4050-8
Sheikholeslami M, Haq R, Shafee A, Li Z (2019a) Heat transfer behavior of Nanoparticle enhanced PCM solidification through an enclosure with V shaped fins. Int J Heat Mass Transf 130:1322–1342
Sheikholeslami M, Gerdroodbary MB, Moradi R, Shafee A, Li Z (2019b) Application of Neural Network for estimation of heat transfer treatment of Al2O3- H2O nanofluid through a channel. Comput Methods Appl Mech Eng 344:1–12
Sheremet MA, Oztop HF, Pop I, Abu-Hamdeh N (2015) Analysis of entropy generation in natural convection of nanofluid inside a square cavity having hot solid block: Tiwari and Das’ model. Entropy 18(1):9
Sun F, Yao Y, Li X, Zhao L, Ding G, Zhang X (2017) The mass and heat transfer characteristics of superheated steam coupled with non-condensing gases in perforated horizontal wellbores. J Petrol Sci Eng 156:460–467
Tahir F, Gul T, Islam S, Shah Z, Khan A, Khan W, Ali L (2017) Flow of a nano-liquid film of maxwell fluid with thermal radiation and magneto hydrodynamic properties on an unstable stretching sheet. J Nanofluids 6:1–10
Tan FL, Hosseinizadeh SF, Khodadadi JM, Fan L (2009) Experimental and computational study of constrained melting of phase change materials (PCM) inside a spherical capsule. Int J Heat Mass Transf 52:3464–3472
Acknowledgements
This paper was supported by the National Sciences Foundation of China (NSFC) (No. U1610109), Australia ARC DECRA (No. DE190100931) and Taishan Scholar of Shandong. Besides, the authors acknowledge the funding support of Babol Noshirvani University of Technology through Grant program No. BNUT/390051/97.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sheikholeslami, M., Jafaryar, M., Shafee, A. et al. Analyze of entropy generation for NEPCM melting process inside a heat storage system. Microsyst Technol 25, 3203–3211 (2019). https://doi.org/10.1007/s00542-019-04301-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00542-019-04301-w